Introduction
The unique red blood cell (RBC) morphology plays an important role in the optimal function of RBC as an oxygen transporter. This biconcave disc-shape offers a high surface area to volume ratio, allowing for efficient diffusion of oxygen molecules into and out of the RBCs. Any abnormality in red blood cell (RBC) morphology can significantly impact this process, potentially leading to oxygen deficiency in the body’s tissues.
Approach to Anemia: A Brief Overview
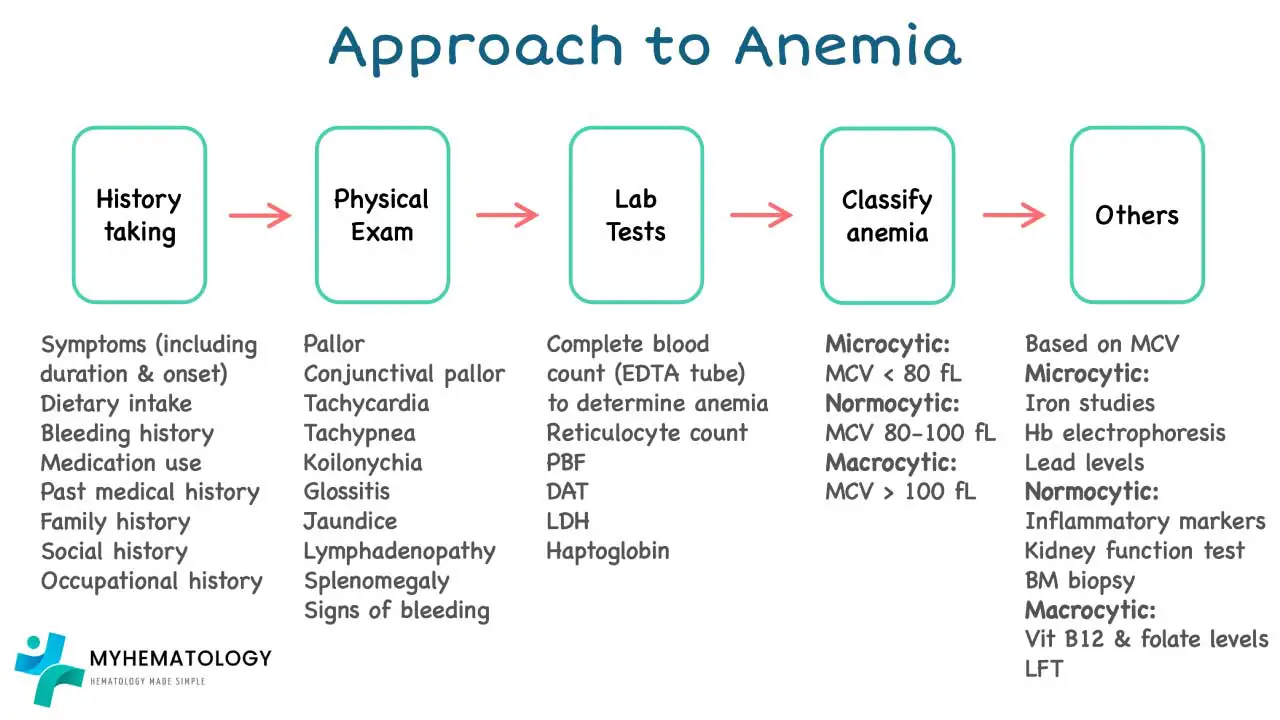
Anemia is a condition characterized by a deficiency in red blood cells (RBCs) or hemoglobin, the oxygen-carrying protein within them. This deficiency leads to reduced oxygen delivery to tissues, causing symptoms like fatigue, weakness, and shortness of breath. Diagnosing anemia involves a multi-step approach, and RBC morphology examination plays a vital role in pinpointing the underlying cause. A more detailed article regarding the systemic approach to anemia can be viewed here.
Complete Blood Count (CBC)
The CBC serves as the cornerstone of anemia evaluation. It provides a quantitative assessment of RBC count, hemoglobin concentration, hematocrit (percentage of blood volume occupied by RBCs), and other cellular parameters like Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC).
History, Physical Examination, and Additional Tests
A detailed medical history and physical examination can provide valuable clues. These include:
- Dietary habits (pointing towards potential iron or vitamin deficiencies)
- Menstrual history (heavy periods can contribute to iron deficiency)
- Family history (certain anemias are hereditary)
- Presence of chronic illnesses (chronic inflammatory diseases can suppress RBC production)
- Medication use (some medications can cause drug-induced anemia)
Depending on the initial findings, further investigations may be warranted:
- Iron studies: Serum iron, ferritin, transferrin saturation, and total iron binding capacity (TIBC) help differentiate between iron deficiency anemia and other causes.
- Vitamin B12 and folate levels: Assessed to rule out deficiencies that can lead to macrocytic anemia.
- Autoimmune workup: Certain autoimmune conditions can target RBCs, requiring specific tests for confirmation.
- Bone marrow examination: In complex cases, a bone marrow biopsy or aspirate might be necessary to evaluate RBC production.
RBC Morphology Examination
A peripheral blood smear offers a wealth of information about red blood cell (RBC) morphology. By examining the size, shape, and color of RBCs, you can gain valuable insights into the underlying cause.
Diagnosis and Treatment
By analyzing the CBC results, medical history, physical examination findings, additional tests, and crucially, the RBC morphology, we can arrive at a specific diagnosis. This paves the way for targeted treatment, which may involve:
- Iron supplementation: The mainstay of therapy for iron deficiency anemia.
- Vitamin B12 or folate replacement: For deficiencies leading to megaloblastic anemia.
- Supportive care: Blood transfusions in severe cases or for specific hemolytic anemias.
- Treating underlying conditions: Addressing chronic illnesses or autoimmune disorders that contribute to anemia.
Key RBC Characteristics Assessed in Morphology
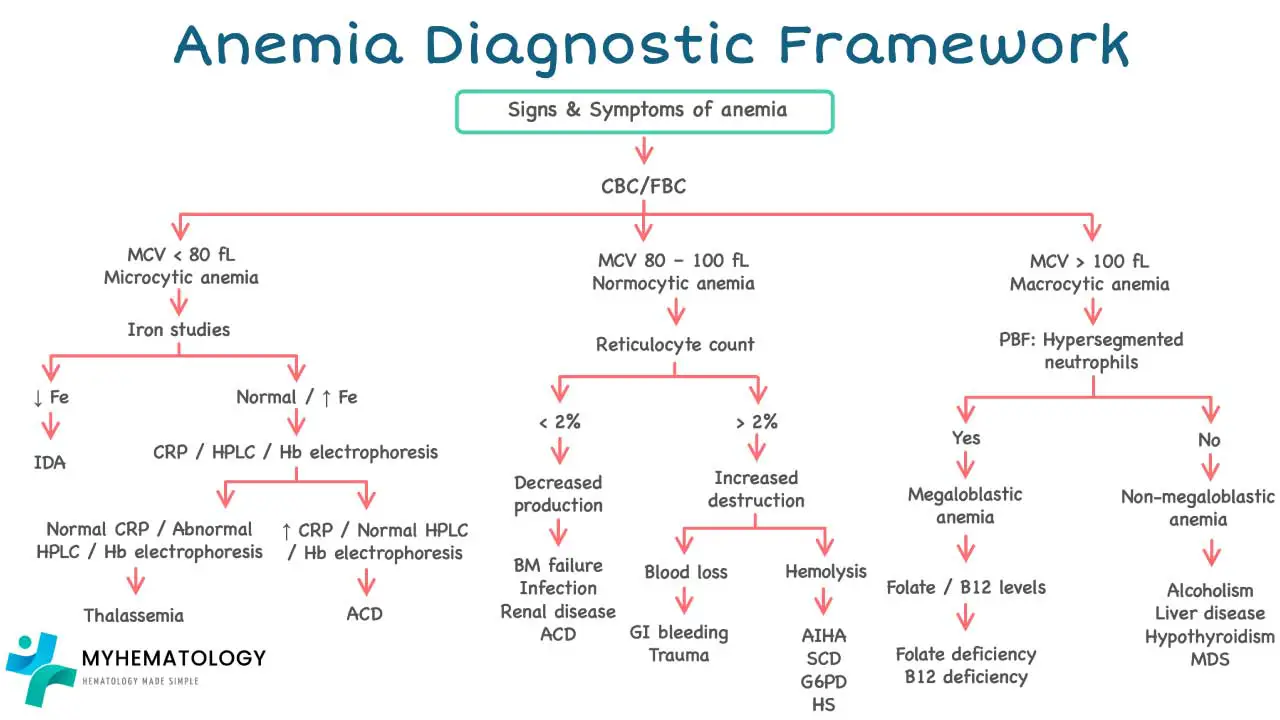
The peripheral blood smear, a simple yet powerful tool, serves as the gold standard for evaluating red blood cell (RBC) morphology. This readily available and cost-effective test provides valuable information about the size, shape, and color of RBCs, offering crucial clues for diagnosing various RBC disorders, particularly anemias. A trained medical professional, typically a laboratory technician or hematopathologist, examines the stained smear for RBC morphology under a microscope. By analyzing the RBCs under high magnification, the examiner can identify various RBC morphology features, including:
- Size (Normocytosis, Microcytosis, Macrocytosis): A healthy red blood cell (RBC) would fall between 7.2 and 7.9 microns across. This ideal size allows RBCs to squeeze through tiny blood vessels and deliver oxygen efficiently. Mean Cell Volume (MCV) is an average measurement of the volume of RBCs in a blood sample. The MCV helps assess the overall size of RBCs.
- Normocytic RBCs (80 < MCV < 100 fL): Normocytic RBCs are red blood cells with a normal size. Healthy RBCs fall within this range. However, normocytic RBCs can also be present in certain types of hemolytic anemia.
- Microcytic RBCs (MCV < 80 fL): These are smaller than normal cells. This can be a sign of iron deficiency anemia or other conditions that affect hemoglobin production.
- Macrocytic RBCs (MCV > 100 fL): These are larger than red cells normal cells in red blood cell (RBC) morphology. This can indicate vitamin B12 or folate deficiency, among other causes.
- Color (Normochromia, Hypochromia, Polychromia): Healthy RBCs appear round with a pink-stained rim. This pink color comes from hemoglobin, the protein inside RBCs that carries oxygen. The center of the cell is typically more pale, is called central pallor. The size of this pale area can tell us about the amount of hemoglobin present in the RBC. A normal central pallor is about ⅓ in size of the RBC. Mean corpuscular hemoglobin (MCH) is a measurement of the average amount of hemoglobin within a single RBC. A normal MCH range is 27 – 32 pg.
- Hypochromic Cells: If an RBC has less hemoglobin than normal, it will have a larger central pallor. These pale cells are called hypochromic (MCH less than 27 pg). This can be a sign of iron deficiency anemia.
- Polychromatic cells: These are usually young, immature RBCs (reticulocytes) that are slightly larger than mature RBCs and may have a slight bluish tint due to residual RNA. They are normally present in very small numbers (less than 1%) in the bloodstream.
- Shape (Discocyte, Spherocyte, Elliptocyte, Stomatocyte etc.): The typical biconcave disc shape allows for optimal oxygen transport. Abnormalities like sickle cells, spherocytes, elliptocytes, and fragmented RBCs (schistocytes) can be indicative of specific diseases.
- Distribution: Normally, red blood cells (RBCs) are spread out evenly across the blood smear. This allows for clear examination of the RBC morphology including individual size, shape, and color. However, sometimes RBCs can clump together in abnormal patterns:
- Rouleaux formation: This refers to RBCs stacked together in long rows, resembling a pile of coins. It’s a non-specific finding and can be seen in conditions like increased blood protein levels, particularly in the presence of abnormal proteins called paraproteins. These abnormal proteins can act like a bridge between RBCs, causing them to stick together.
- Cold agglutination: This is a specific type of clumping where RBCs aggregate due to cold temperatures or the presence of cold agglutinins, which are antibodies that target RBCs at cooler temperatures. This can be a sign of an underlying autoimmune disorder.
Common RBC Morphology Seen in Disorders
Normal Red Blood Cell (RBC) Morphology
The red blood cell (RBC) is approximately 7.2 – 7.9 μm in diameter with a pinkish hue cytoplasm. The central pallor is approximately 1/3 the size of the red cell. The cells are almost all the same shape and size. The red cells are slightly smaller than the small lymphocyte in size.
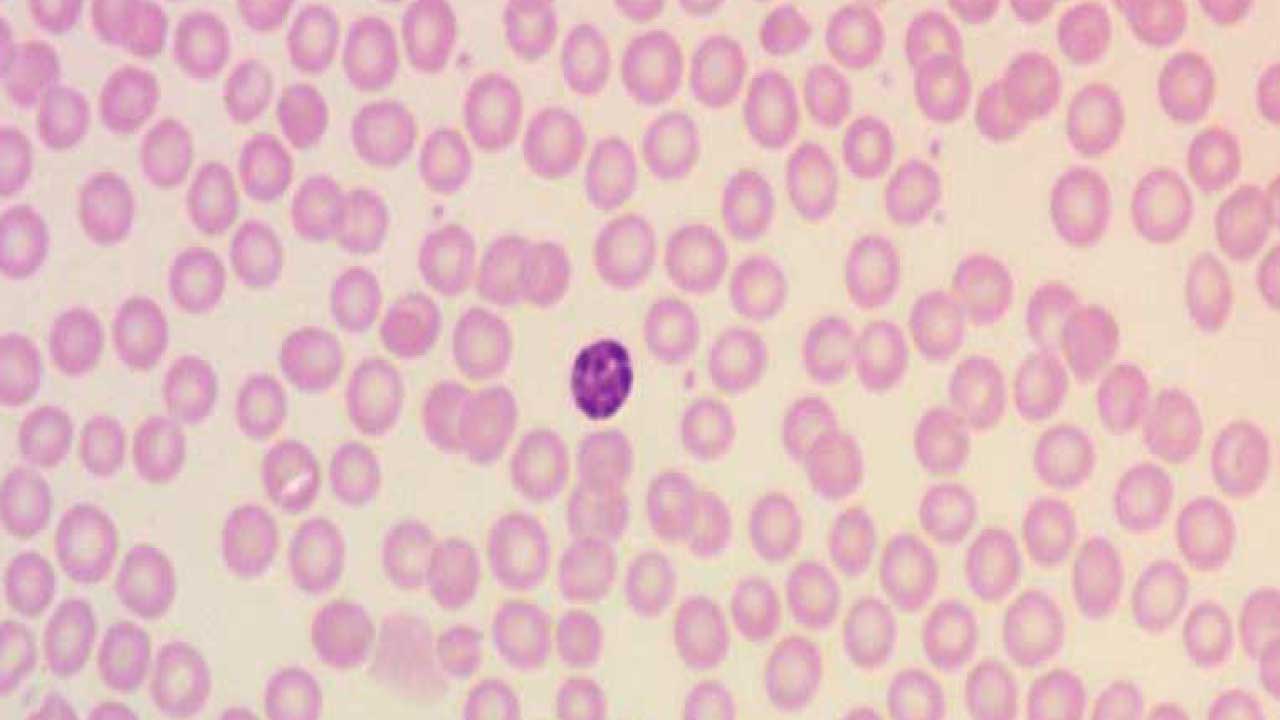
Microcytosis (Microcytes)
Microcytic red blood cells (microcytes) are smaller than their healthy counterparts in terms of RBC morphology. Typically, their diameter falls below 7.2 µm.
RBC Morphology Appearance on the Blood Smear
- Smaller in size: Compared to normal RBCs, microcytes have a noticeably reduced diameter.
- Maintain biconcave shape: Despite their smaller size, microcytic RBCs usually retain the characteristic biconcave disc shape of healthy RBCs.
- Increased central pallor: Due to potentially reduced hemoglobin content, the central pale area of the cell may appear larger than usual (more than ⅓ in diameter).
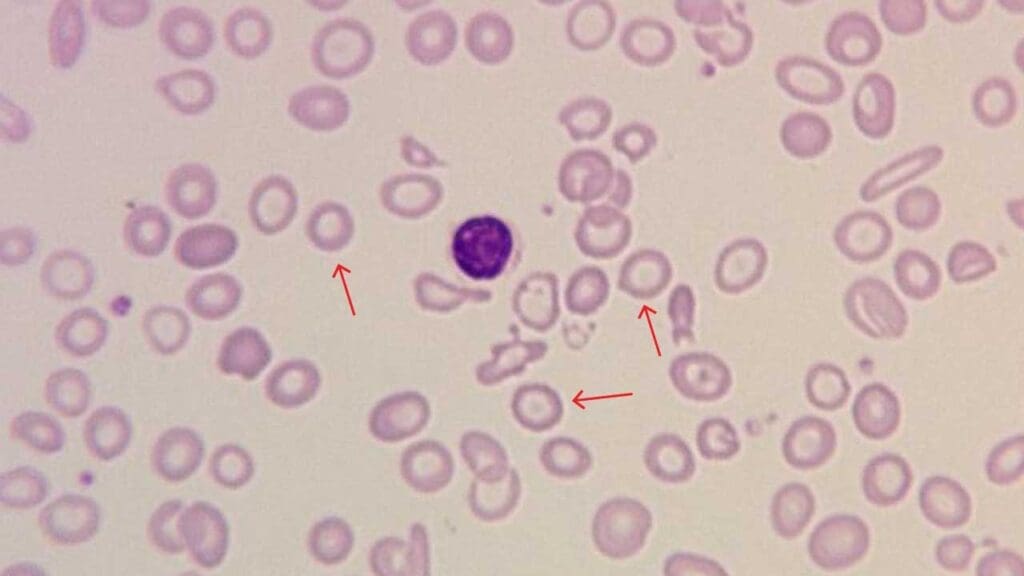
Causes of Microcytic Anemia
Microcytic RBC morphology (microcytes) are a hallmark feature of microcytic anemia, a condition where the body produces abnormally small red blood cells. Several factors can contribute to this:
- Iron deficiency anemia: This is the most common cause of microcytic anemia. It occurs when the body doesn’t have enough iron, a crucial component of hemoglobin. Without sufficient iron, the body produces smaller RBCs with less hemoglobin, leading to reduced oxygen-carrying capacity. While IDA is the most common cause of microcytic anemia globally, its presence on a blood smear immediately prompts a clinician to investigate the source of iron loss. This often involves looking for chronic blood loss (e.g., gastrointestinal bleeding, heavy menstrual periods) or assessing dietary intake. The morphology guides further laboratory tests like serum ferritin, iron, and total iron-binding capacity (TIBC) to confirm the diagnosis and differentiate it from other microcytic anemias.
- Thalassemia: Unlike iron deficiency, microcytosis in thalassemia is a genetic disorder affecting hemoglobin production. A key clinical point is that patients with thalassemia traits or minor forms are not iron deficient, and therefore, iron supplementation is generally contraindicated as it can lead to iron overload. The presence of microcytosis without iron deficiency often leads to further testing like hemoglobin electrophoresis to identify the specific type of thalassemia.
- Chronic inflammation: Long-term inflammatory conditions can suppress red blood cell production and lead to microcytic anemia. Microcytosis in the context of chronic inflammation (e.g., autoimmune diseases, chronic infections, cancer) is common, especially in hospitalized patients. Here, the microcytosis is due to impaired iron utilization rather than a true deficiency. Clinically, differentiating anemia of chronic inflammation from IDA is crucial because treatment focuses on managing the underlying inflammatory condition, not necessarily iron supplementation, unless a co-existing iron deficiency is confirmed.
- Sideroblastic anemia: This is a rarer group of disorders where the body has sufficient iron, but it cannot be properly incorporated into hemoglobin, leading to iron accumulation in the mitochondria of red blood cell precursors (ring sideroblasts). While microcytosis is a feature, its presence on a smear, especially if other common causes are ruled out, might prompt a bone marrow biopsy to look for these characteristic ring sideroblasts, indicating a more complex underlying bone marrow dysfunction.
- Lead poisoning: Microcytosis due to lead poisoning is a toxic cause and is often accompanied by basophilic stippling (small, dark blue granules) on the peripheral blood smear. Recognizing this combination is a critical clinical clue that should immediately trigger a lead level test, especially in at-risk populations (e.g., children, occupational exposures). Early diagnosis and removal of the lead source are vital to prevent severe neurological and developmental complications.
Macrocytosis (Macrocytes)
Macrocytic red blood cells (macrocytes) are larger than their healthy counterparts in terms of RBC morphology. Typically, their diameter falls above 100 µm. On a peripheral blood smear, they can exhibit varying characteristics.
RBC Morphology Appearance on the Blood Smear
- Increased size: The most striking feature is their noticeably larger diameter compared to normal RBCs.
- Shape variations: Macrocytes may retain the biconcave disc shape of healthy RBCs, but they can also appear more oval-shaped or even teardrop-shaped giving rise to the abnormal RBC morphology.
- Variable central pallor: The size of the central pale area can vary depending on the underlying cause. In some cases, it might be similar to normal RBCs, while in others, it may appear larger due to a potential hemoglobin deficiency.
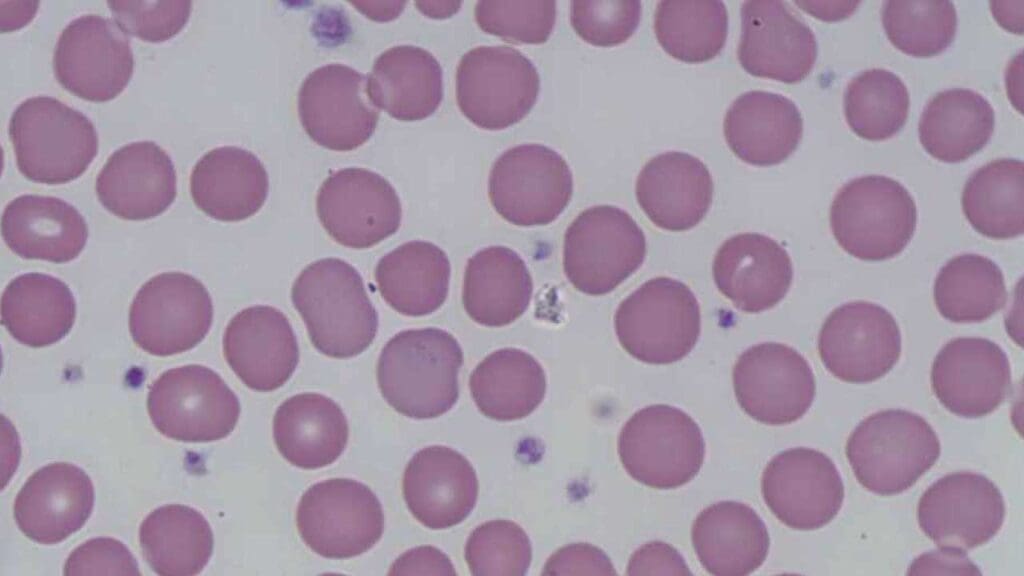
Causes of Macrocytic Anemia
Macrocytic RBCs (macrocytes) are a hallmark feature of macrocytic anemia, a condition where the body produces abnormally large red blood cells. Several factors can contribute to this:
- Vitamin B12 deficiency: Vitamin B12 is essential for DNA synthesis, which is crucial for proper RBC production. A deficiency can lead to problems with RBC maturation, resulting in macrocytic RBCs. Macrocytosis due to Vitamin B12 deficiency often presents with characteristic oval macrocytes and hypersegmented neutrophils on the peripheral blood smear. Clinically, this finding is critical because B12 deficiency can lead to severe neurological complications (e.g., peripheral neuropathy, cognitive impairment) that may become irreversible if not treated promptly. The presence of macrocytosis prompts immediate investigation of serum B12 levels and, if confirmed, requires B12 supplementation, often lifelong, to prevent or reverse both hematological and neurological symptoms.
- Folate deficiency: Folate, like vitamin B12, plays a vital role in DNA synthesis. Similar to B12 deficiency, folate deficiency also causes macrocytic anemia with oval macrocytes and hypersegmented neutrophils. However, unlike B12 deficiency, it typically does not cause neurological symptoms. Clinically, it’s crucial to differentiate between B12 and folate deficiency because treating B12 deficiency with folate alone can mask the B12 deficiency and allow neurological damage to progress. Folate deficiency is often linked to poor diet, malabsorption, or increased demand (e.g., pregnancy), guiding dietary advice or specific supplementation.
- Alcohol abuse: Chronic alcohol consumption can interfere with vitamin B12 absorption and metabolism, leading to macrocytosis in the RBC morphology. Alcohol can also directly damage bone marrow, further affecting RBC production. Chronic alcohol abuse is a very common cause of macrocytosis, even in the absence of overt liver disease or nutritional deficiencies. The mechanism is often multifactorial, involving direct toxic effects on bone marrow, folate deficiency (due to poor diet or impaired absorption), and liver disease. Clinically, recognizing alcohol as the cause is vital as it directs patient counseling and intervention for alcohol cessation, which can often reverse the macrocytosis.
- Liver disease: Macrocytosis in liver disease is typically due to altered lipid metabolism, leading to increased red blood cell membrane surface area, often resulting in round macrocytes. It can also be exacerbated by associated folate deficiency or bone marrow suppression. The presence of macrocytosis in a patient with known or suspected liver disease reinforces the severity of the hepatic dysfunction and prompts further evaluation of liver function and potential complications like portal hypertension.
- Certain medications: A variety of medications can cause macrocytosis, often by interfering with DNA synthesis (e.g., chemotherapy agents like hydroxyurea, methotrexate, azathioprine) or by affecting folate metabolism (e.g., trimethoprim). Clinically, it’s essential to review a patient’s medication list when macrocytosis is identified. If a causative drug is found, the clinician must weigh the benefits of the medication against the severity of the macrocytosis, potentially adjusting the dose, switching medications, or providing concurrent supplementation (e.g., leucovorin with methotrexate).
- Rare bone marrow disorders: When common causes of macrocytosis (B12/folate deficiency, alcohol, liver disease, medications) have been ruled out, the presence of persistent macrocytosis, especially if accompanied by other cytopenias (low white blood cells or platelets) or dysplastic features on the blood smear (e.g., abnormal neutrophil granulation, abnormal platelet morphology), raises concern for a primary bone marrow disorder like Myelodysplastic Syndrome (MDS). This finding is clinically significant as it often necessitates a bone marrow biopsy for definitive diagnosis and guides the management towards supportive care, growth factors, or more aggressive therapies depending on the MDS subtype and risk stratification.
Normochromic Normocytic Red Cells
Normochromic normocytic RBCs appear pink or red, similar to healthy RBC morphology. The central pallor (pale area) is also typically within the normal range. This indicates that they contain a typical amount of hemoglobin, the protein responsible for carrying oxygen. However, their normal color and size doesn’t necessarily rule out anemia.
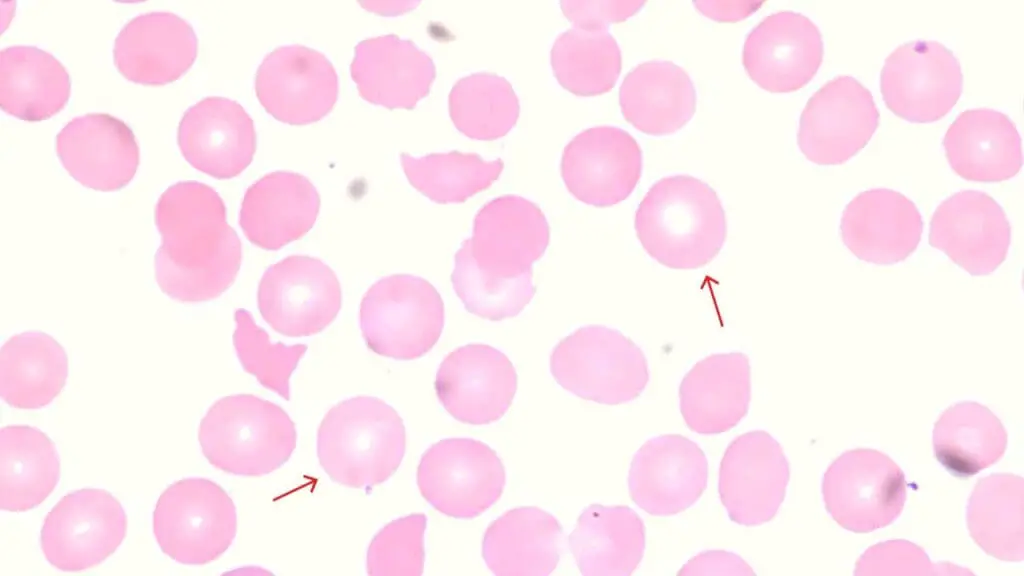
Causes of Normochromic Normocytic Anemia
Several factors can contribute to normochromic normocytic anemia RBC morphology:
- Chronic Inflammatory Disease: Normocytic anemia is a common finding in patients with chronic inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease, or chronic infections. The anemia arises from the body’s inflammatory response, which impairs iron utilization and red blood cell production. Long-term inflammation can suppress red blood cell production in the bone marrow, leading to normochromic anemia. Clinically, it’s crucial to identify and manage the underlying inflammatory condition, as treating the inflammation often improves the anemia. Iron supplementation is generally ineffective unless there’s a co-existing iron deficiency, and excessive iron can be harmful. This diagnosis often prompts a search for the source of inflammation.
- Chronic Kidney Disease: Kidneys play a role in red blood cell production. Anemia is almost universally present in advanced chronic kidney disease (CKD). The primary mechanism is reduced production of erythropoietin by the kidneys, a hormone essential for red blood cell production in the bone marrow. Clinically, the severity of anemia often correlates with the stage of kidney disease. Management typically involves erythropoiesis-stimulating agents (ESAs) and careful iron management, as iron deficiency can limit the effectiveness of ESAs. Recognizing this link is vital for integrated care of CKD patients.
- Blood Loss: In the immediate aftermath of acute blood loss (e.g., trauma, gastrointestinal hemorrhage), the red blood cells that remain are still of normal RBC morphology, leading to normocytic anemia. It’s only later, as the bone marrow attempts to regenerate red cells and iron stores are depleted, that the anemia might become microcytic. Clinically, acute blood loss is a medical emergency requiring rapid assessment of hemodynamic stability, identification and control of the bleeding source, and often immediate blood transfusions. The normocytic RBC morphology finding on initial labs is a critical clue to recent, significant blood loss.
- Bone Marrow Disorders: Certain bone marrow disorders like aplastic anemia or myelodysplastic syndrome (MDS) can disrupt red blood cell production, causing normochromic anemia.
- Aplastic Anemia: This is a severe condition where the bone marrow fails to produce enough blood cells, including red blood cells. The normocytic anemia is often accompanied by low white blood cells and thrombocytopenia (pancytopenia). Clinically, this is a life-threatening diagnosis requiring urgent investigation, often a bone marrow biopsy, to confirm the hypocellular marrow. Treatment can range from immunosuppressive therapy to stem cell transplantation.
- Myelodysplastic Syndromes (MDS): These are a group of disorders where the bone marrow produces abnormal, ineffective blood cells. While MDS can cause macrocytic anemia, it can also present as normocytic anemia, often with other cytopenias and dysplastic features (abnormal shapes/sizes) in other cell lines (e.g., neutrophils, platelets) on the peripheral smear. Clinically, MDS is a pre-leukemic condition, and its diagnosis requires a bone marrow biopsy. The normocytic finding in RBC morphology, especially when other common causes are ruled out, should prompt a deeper look into bone marrow function.
- Nutritional Deficiencies (early stages or mixed deficiencies): While iron, B12, and folate deficiencies typically cause microcytic or macrocytic anemias, respectively, in their very early stages, or in cases of mixed deficiencies, the anemia might initially present as normocytic RBC morphology. For example, a patient with early iron deficiency might still have normocytic red cells before they become overtly microcytic. Clinically, this emphasizes the need for a comprehensive nutritional assessment and specific laboratory tests (e.g., iron studies, B12, folate levels) even when the MCV is normal, especially if other clinical signs of deficiency are present.
Hypochromic Red Cells
Hypochromic red blood cells (RBCs) appear paler than normal on a peripheral blood smear in terms of RBC morphology. This pale appearance signifies a reduced concentration of hemoglobin within the cell. Hemoglobin, the protein responsible for carrying oxygen, gives healthy RBCs their characteristic pink color. When there’s less hemoglobin, the RBCs appear fainter.
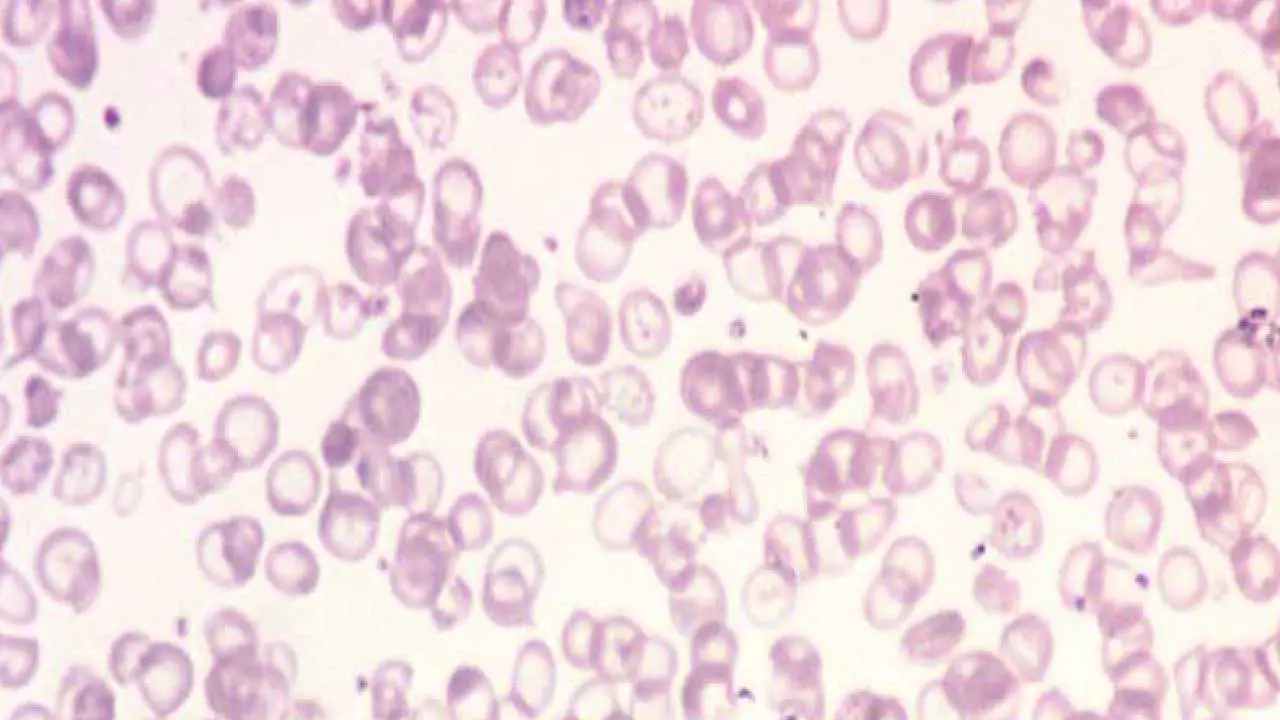
RBC Morphology Appearance on the Blood Smear
- Pale Color: The most striking feature is the overall paler appearance compared to healthy RBC morphology. The central pallor, the pale area in the center of the cell, appears significantly larger than normal (more than ⅓ in diameter). In severe cases, the entire cell might appear almost colorless.
- Possible Size Variations: Hypochromic anemia can sometimes co-occur with microcytes. This combination is frequently seen in iron deficiency anemia. However, the size of hypochromic RBCs can be variable depending on the underlying cause.
Causes of Hypochromic Anemia
The most common cause of hypochromic anemia is
- Iron deficiency anemia: This occurs when the body doesn’t have enough iron, a crucial component of hemoglobin. Without sufficient iron, red blood cells become smaller and paler due to reduced hemoglobin content.
Other potential causes include
- Chronic blood loss: Long-term blood loss, due to conditions like peptic ulcers or heavy menstruation, can deplete iron stores and lead to hypochromic anemia.
- Chronic inflammatory disease: Long-term inflammation can disrupt iron metabolism and contribute to hypochromic anemia.
- Certain inherited blood disorders: Conditions like thalassemia can affect hemoglobin production and lead to hypochromic anemia.
Polychromatic Red Cells (Polychromasia)
Polychromatic red blood cells (polychromasia) appear slightly different on a blood smear compared to their mature counterparts in terms of RBC morphology. These immature cells, also known as reticulocytes, offer a glimpse into red blood cell production within the bone marrow.
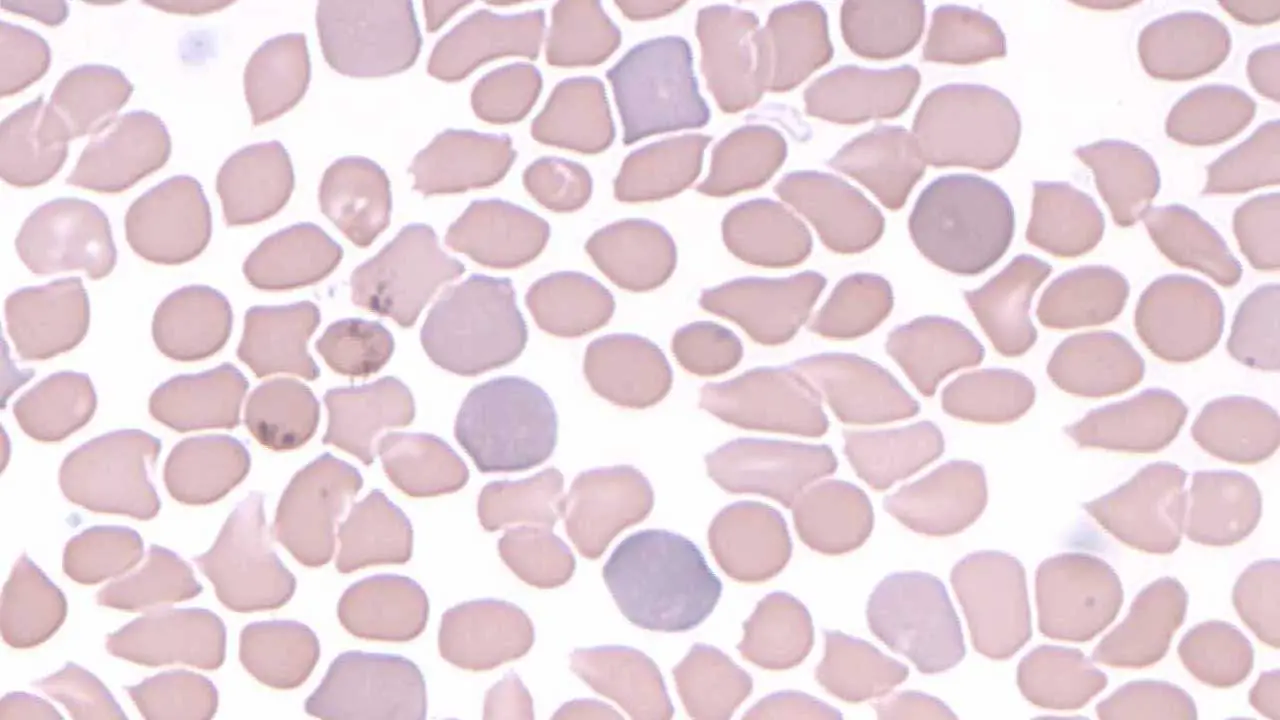
RBC Morphology Appearance on the Blood Smear
- Size Variations: Polychromatic RBCs can be slightly larger than mature RBCs. However, the size variation is usually not as significant as seen in microcytic or macrocytic anemia.
- Bluish Hue: When stained with certain dyes used in blood smears, polychromatic RBCs may exhibit a faint bluish tint. This bluish color is due to the presence of residual ribosomal RNA (ribonucleic acid) within the cells. Mature RBCs typically lack this RNA and appear solely pink due to hemoglobin.
Causes of Polychromatic Red Cells
The presence of polychromatic RBCs in a blood smear is not necessarily a cause for concern. A small percentage (typically less than 1%) of these immature cells are normally present in the bloodstream. However, an increased number of polychromatic RBCs (reticulocytosis) can indicate:
- Increased red blood cell production:
- Blood loss: Polychromasia in the RBC morphology indicates that the bone marrow is actively producing and releasing new red blood cells into circulation at an accelerated rate. When seen after acute blood loss (e.g., from trauma or gastrointestinal bleeding), it’s a positive sign that the bone marrow is responding appropriately to the anemia by trying to replenish the lost red cells. Clinically, this helps confirm that the anemia is due to blood loss rather than a bone marrow production problem, and it guides the clinician to focus on identifying and stopping the bleeding source.
- Hemolytic anemia: In hemolytic anemias, red blood cells are prematurely destroyed in the bloodstream or spleen. The presence of polychromasia in the RBC morphology signifies that the bone marrow is attempting to compensate for this destruction by ramping up red blood cell production. This finding is a key indicator of a hemolytic process and prompts further investigation into the cause of hemolysis (e.g., autoimmune conditions, inherited red cell defects, drug-induced hemolysis). The degree of polychromasia often correlates with the severity of hemolysis.
- Early recovery from anemia: Following treatment for anemia, the body might be actively producing new RBCs, leading to an elevated reticulocyte count.
Target Cells
Target cells, also known as codocytes, have a distinctive appearance on a peripheral blood smear in terms of RBC morphology.
RBC Morphology Appearance on the Blood Smear
- Bulls-eye Pattern: The defining feature of a target cell is its resemblance to a shooting target. It has three distinct zones in the RBC morphology:
- Central Dense Area: A dark, centrally located disc containing a normal or slightly increased amount of hemoglobin.
- Pale Ring: A clear or pale zone encircling the central area, lacking sufficient hemoglobin.
- Peripheral Rim: A darker outer rim containing hemoglobin, similar in intensity to the central area.
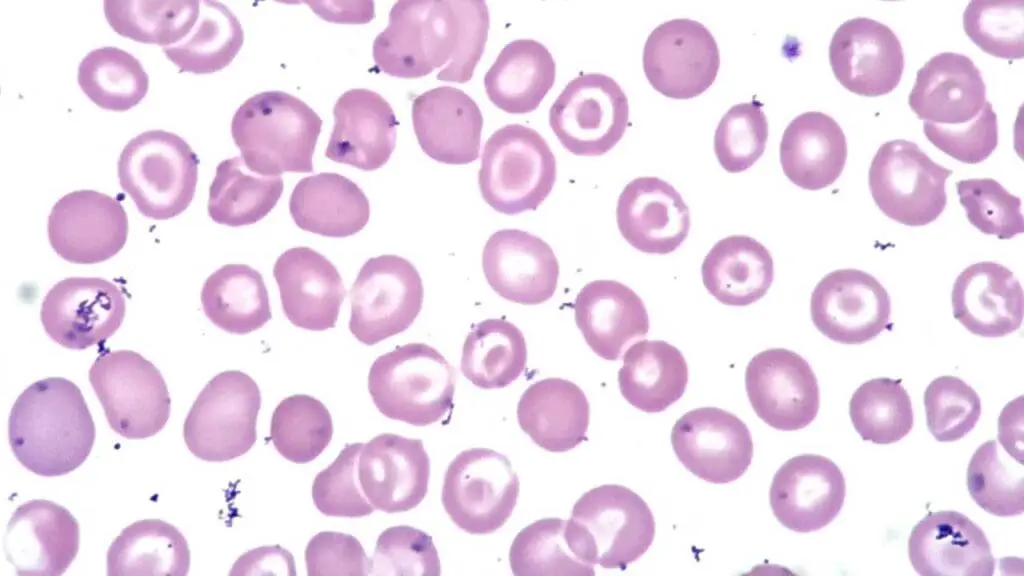
Causes of Target Cells
The presence of target cells on a blood smear can be associated with several conditions:
- Liver Disease: Target cells are commonly seen in various liver diseases, particularly obstructive jaundice and severe hepatocellular disease. Their presence is due to an increase in red blood cell membrane surface area relative to the cell volume, often caused by altered lipid metabolism. Clinically, finding target cells in a patient with suspected liver issues reinforces the diagnosis of hepatic dysfunction and may prompt further evaluation of liver enzymes, bilirubin, and liver synthetic function.
- Hemoglobinopathies: Target cells are a classic feature of many hemoglobinopathies, especially thalassemia syndromes, where there’s an imbalance in globin chain synthesis. In these genetic conditions, the red blood cells have reduced hemoglobin content and excess membrane, leading to the target cell appearance in the RBC morphology. Clinically, their presence, particularly in individuals from specific ethnic backgrounds or with a family history of anemia, should immediately raise suspicion for a hemoglobinopathy and prompt further diagnostic tests like hemoglobin electrophoresis or genetic analysis.
- Iron Deficiency Anemia: While severe iron deficiency typically causes microcytic and hypochromic red cells, mild iron deficiency can sometimes present with target cells. This occurs as the red blood cells attempt to compensate for reduced hemoglobin by spreading out their remaining hemoglobin. Clinically, if target cells are seen, iron studies (ferritin, iron, TIBC) are crucial to rule out or confirm iron deficiency, guiding appropriate iron supplementation.
- Splenectomy: The spleen normally removes excess red blood cell membrane and abnormal cells. After a splenectomy, the absence of this “pitting” function allows red blood cells with excess membrane, including target cells, to persist in circulation. Clinically, finding target cells in a patient who has undergone splenectomy is an expected and benign finding, confirming the functional asplenia.
- Lead Poisoning: Lead poisoning can interfere with heme synthesis, leading to various RBC morphology abnormalities, including target cells. While less common as a primary cause of target cells compared to other conditions, their presence, especially alongside basophilic stippling, should raise suspicion for lead toxicity. Clinically, this finding warrants immediate investigation of blood lead levels, as lead poisoning requires urgent intervention to prevent severe systemic effects.
Stomatocytes
Stomatocytes are a type of red blood cell (RBC) with a distinctive appearance on a peripheral blood smear in terms of RBC morphology.
RBC Morphology Appearance on the Blood Smear
- Mouth-Shaped Indentation: The defining feature of a stomatocyte is a centrally located, slit-like indentation that resembles a small mouth. This indentation gives the cell its name (stoma means “mouth” in Greek).
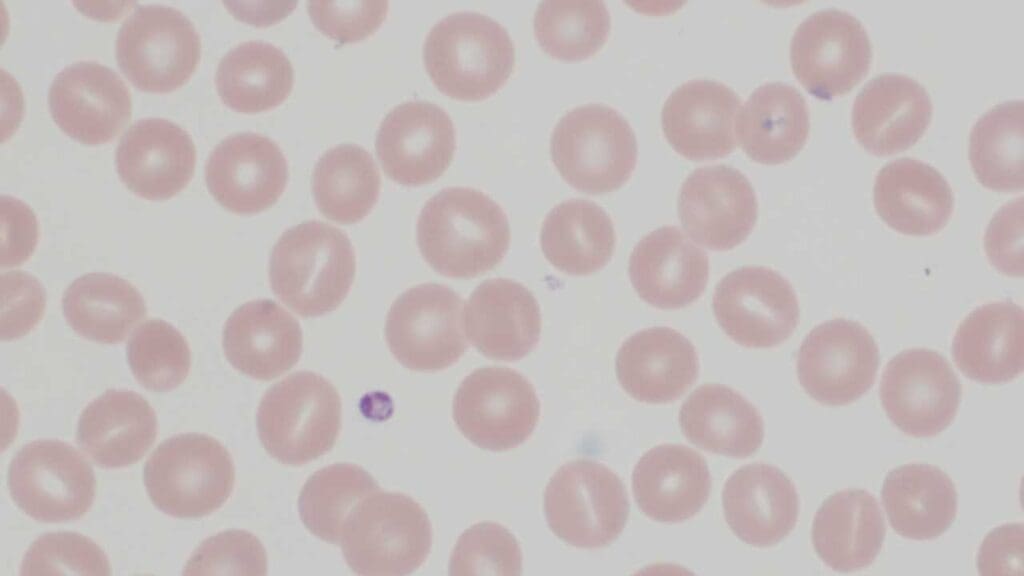
Causes of Stomatocytes
The presence of stomatocytes on a blood smear can be associated with several conditions:
- Hereditary Stomatocytosis: This is a rare group of inherited disorders where the genes responsible for maintaining the structure and function of the RBC membrane are defective. This defect leads to the formation of stomatocytes. Further specialized tests, such as osmotic fragility testing or genetic analysis, may be required to confirm the diagnosis and guide management, which might include splenectomy in some cases.
- Liver Disease: Stomatocytes can be seen in various forms of liver disease, particularly alcoholic liver disease. The mechanism often involves changes in the red blood cell membrane’s lipid composition due to altered liver metabolism. Clinically, their presence in a patient with liver dysfunction reinforces the diagnosis of hepatic impairment and may prompt further evaluation of liver function, as well as assessment for underlying causes like alcohol abuse or viral hepatitis.
- Certain drugs: The exact mechanisms by which some drugs induce stomatocytosis are not fully established. Some medications known to be associated with drug-induced stomatocytosis including phenolthiazine and chlorpromazine.
- Alcoholism: Chronic alcohol abuse can lead to stomatocytosis, often alongside macrocytosis, due to direct toxic effects on red blood cell membranes and bone marrow, as well as associated liver disease or nutritional deficiencies. Clinically, recognizing stomatocytes in a patient with a history of alcohol use should prompt comprehensive assessment for alcohol-related complications and intervention for alcohol cessation, which can often resolve the red blood cell abnormality.
Pencil cells
Pencil cells are a type of abnormally elongated thin-shaped red blood cells (RBCs) morphology seen on a peripheral blood smear.
RBC Morphology Appearance on the Blood Smear
- Elongated Shape: The defining feature of a pencil cell is its markedly elongated shape, resembling a pencil lead in terms of RBC morphology. The length of the cell can be several times greater than its width.
- Microcytic Tendencies: Pencil cells are often, but not always, microcytic. This means they are smaller than normal red blood cells in diameter despite their elongated form.
- Pale Color: In some cases, pencil cells may also appear pale, suggesting a reduced hemoglobin concentration (hypochromic). This is particularly common when they occur due to iron deficiency.
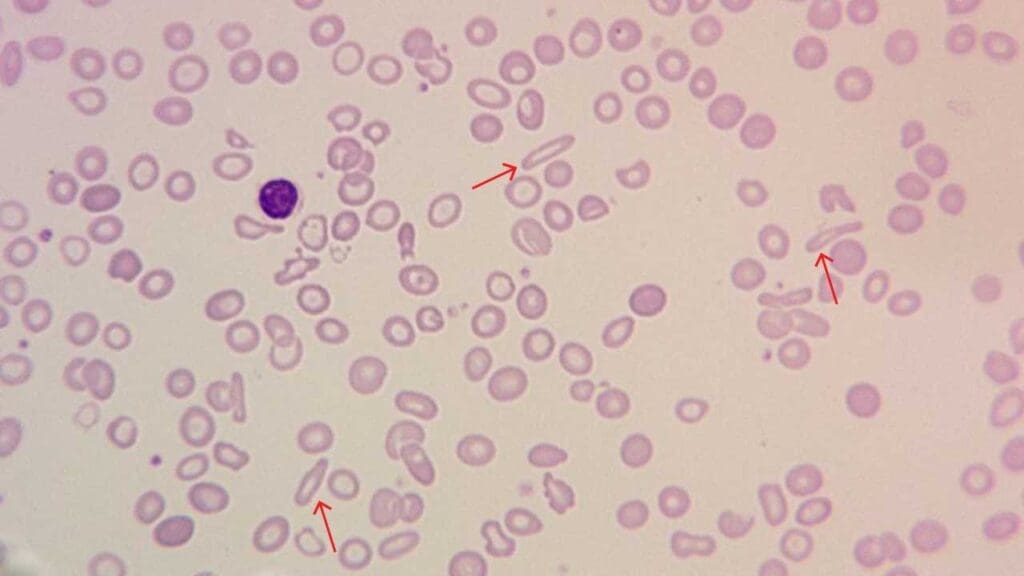
Causes of Pencil Cells
The presence of pencil cells on a blood smear is most commonly associated with:
- Iron Deficiency Anemia: This is the most frequent cause of pencil cells. When the body lacks sufficient iron, a crucial component of hemoglobin, it produces smaller and more elongated red blood cells. In severe iron deficiency, these cells can take on the characteristic pencil shape.
Other potential, less common causes include
- Thalassemia: A group of inherited blood disorders where there’s a defect in globin protein production. Globin chains combine with iron to form hemoglobin. A deficiency in globin production, independent of iron stores, can sometimes lead to pencil cell formation.
- Lead Poisoning: Exposure to lead can interfere with normal red blood cell development and contribute to the formation of pencil cells, along with other abnormalities.
- Sideroblastic Anemia: This is a rare group of anemias where iron gets trapped inside bone marrow cells and isn’t readily available for hemoglobin production. In some cases, pencil cells can be seen.
Echinocytes (Burr or Crenated Cells)
Echinocytes are a type of red blood cell (RBC) morphology with spiky projections appearance on a peripheral blood smear.
RBC Morphology Appearance on the Blood Smear
- Spiky Projections: The defining feature of an echinocyte is the presence of numerous small, evenly spaced projections on the cell surface. These projections resemble the spikes on a sea urchin (echinoderm) or the burrs on a plant, hence the nicknames.
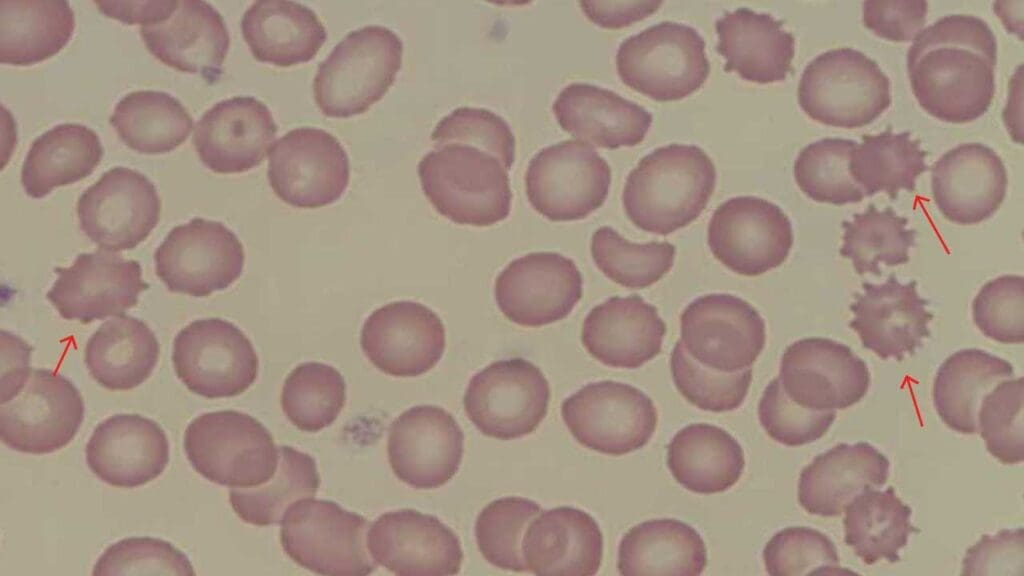
Causes of Echinocytes
The presence of echinocytes on a blood smear can be associated with several conditions:
- Artificially Induced: In some cases, echinocytes can appear due to technical issues during blood smear preparation. This is why proper blood collection and smear processing are crucial for accurate interpretation. Clinically, if echinocytes are the only abnormality seen, and there are no other clinical signs of disease, the first step is often to request a fresh blood smear to rule out an artifact. This prevents unnecessary and costly investigations.
- Kidney Disease: chinocytes can be a true finding in patients with severe kidney disease, particularly uremia (accumulation of waste products in the blood). The altered metabolic environment and accumulation of toxins can affect the red blood cell membrane, leading to this morphology. Clinically, their presence in a patient with renal failure reinforces the severity of kidney dysfunction and may prompt closer monitoring of renal parameters and consideration of dialysis.
- Liver Disease: Similar to kidney disease, significant liver dysfunction can also lead to the formation of echinocytes due to changes in plasma lipids affecting the red blood cell membrane. Clinically, finding echinocytes in the RBC morphology of a patient with liver disease adds another piece of evidence to the overall picture of hepatic impairment and may prompt further evaluation of liver function and underlying causes.
- Other conditions: While less frequent, echinocytes can also be seen in conditions like severe burns, sepsis, or certain electrolyte imbalances. Clinically, if echinocytes are present and artifacts have been ruled out, their appearance should prompt a thorough clinical assessment for these underlying systemic conditions, as they often indicate significant physiological stress or disease.
Acanthocytes
Acanthocytes, also known as thorn cells, are a type of red blood cell (RBC) morphology with spiky projections.
RBC Morphology Appearance on the Blood Smear
- Irregular Spikes: The defining feature of an acanthocyte is the presence of numerous, irregularly spaced, and often pointed projections on the cell surface in the RBC morphology. These spikes are larger and more prominent compared to the smaller, evenly spaced projections seen in echinocytes (burr cells).
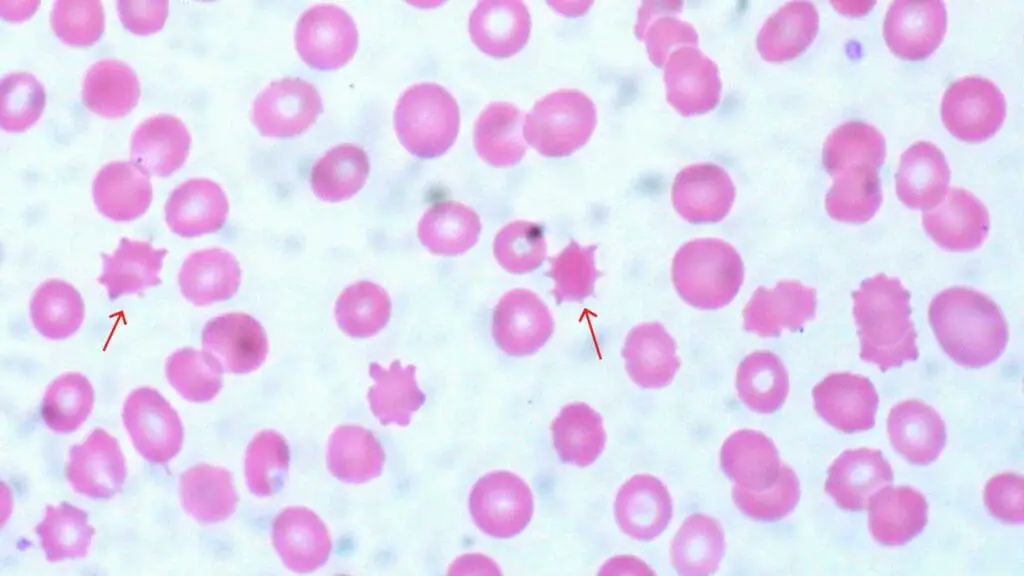
Causes of Acanthocytes
The presence of acanthocytes on a blood smear can be associated with several conditions:
- Abetalipoproteinemia: This is a rare, inherited disorder characterized by the inability to absorb dietary fats, leading to severe vitamin deficiencies (especially fat-soluble vitamins A, D, E, K) and neurological symptoms. The presence of acanthocytes is a classic and highly specific finding in abetalipoproteinemia, prompting immediate investigation of lipid profiles and vitamin levels. Early diagnosis is crucial to initiate high-dose vitamin supplementation to prevent progressive neurological damage.
- Liver Disease: Acanthocytes can be a significant finding in severe, often end-stage, liver disease (e.g., alcoholic cirrhosis). Their formation is due to abnormal lipid metabolism, leading to an excess of cholesterol in the red blood cell membrane. Clinically, their presence indicates severe hepatic impairment and may be associated with a poor prognosis, often seen in patients being evaluated for liver transplantation.
- Neuroacanthocytosis Syndromes: This is a group of rare, inherited neurological disorders characterized by progressive neurodegeneration (e.g., chorea, dystonia, seizures) combined with the presence of acanthocytes. Clinically, when acanthocytes are observed in a patient with unexplained neurological symptoms, these syndromes should be strongly considered. Diagnosis often involves specialized genetic testing, as there is no specific treatment, and management is supportive.
- Certain Medications: Some medications, particularly high doses of drugs like phenytoin (anti-seizure medication) or misoprostol, can induce acanthocyte formation.
Spherocytes
Spherocytes are a type of red blood cell (RBC) morphology with spherical shape seen on a peripheral blood smear.
RBC Morphology Appearance on the Blood Smear
- Spherical Shape: The defining feature of a spherocyte is its near-spherical shape, resembling a ball. Healthy RBCs are typically disc-shaped with a central indentation.
- Reduced Size (Usually): Spherocytes are often, but not always, smaller than normal red blood cells (microcytic). This is because the abnormal shape reduces the surface area of the cell.
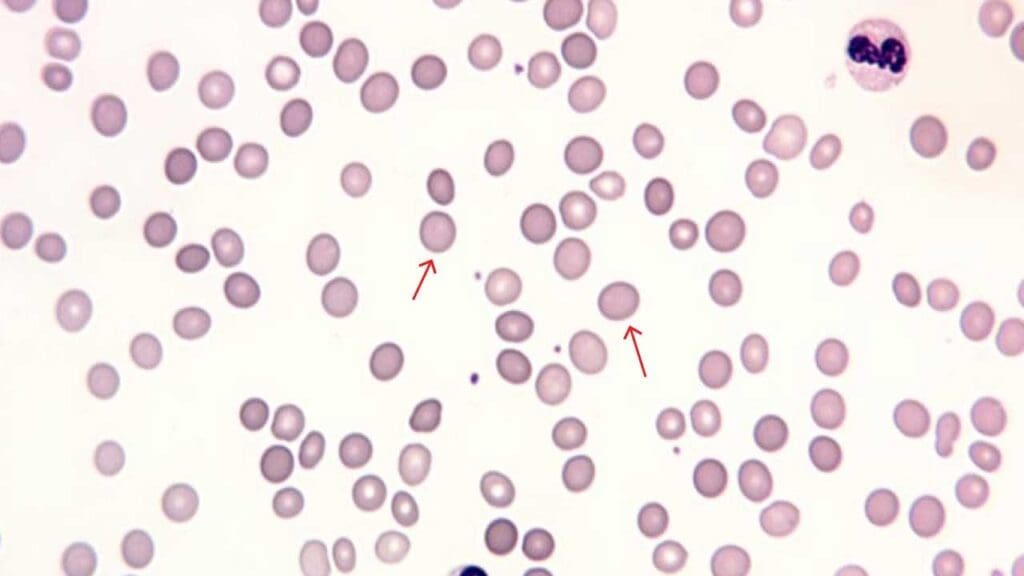
Causes of Spherocytosis
The presence of spherocytes on a blood smear can be caused by two main factors:
- Hereditary Spherocytosis: This is a common inherited disorder of the red blood cell membrane, leading to increased fragility and premature destruction of red blood cells, primarily in the spleen. Clinically, finding spherocytes, especially in a patient with a family history of anemia, jaundice, or splenomegaly, strongly suggests this diagnosis. Further tests like osmotic fragility or flow cytometry are used for confirmation, and splenectomy may be considered to reduce hemolysis.
- Acquired Spherocytosis: This is less common and can be caused by:
- Autoimmune hemolytic anemia: In AIHA, the body’s immune system mistakenly produces antibodies that coat red blood cells, leading to their destruction, often by macrophages in the spleen. This process results in the formation of spherocytes. Clinically, the presence of spherocytes along with signs of hemolysis (e.g., elevated bilirubin, LDH, low haptoglobin) should prompt a direct antiglobulin test (DAT or Coombs test) to confirm the autoimmune nature. Treatment typically involves immunosuppressive therapy.
- Hemolytic transfusion reactions: Spherocytes can appear rapidly following an incompatible blood transfusion, where recipient antibodies attack donor red blood cells, leading to acute hemolysis. Clinically, this is a medical emergency. The presence of spherocytes, along with symptoms like fever, chills, and back pain post-transfusion, necessitates immediate cessation of the transfusion and investigation for ABO incompatibility.
- Red cell fragmentation disorders (e.g. microangiopathic hemolytic anemia): While schistocytes are the primary finding in MAHA, spherocytes can also be present due to severe red cell damage and fragmentation within the microvasculature. Clinically, their presence in this context, alongside schistocytes, indicates a severe underlying condition (e.g., TTP, HUS, DIC) requiring urgent diagnosis and specific treatment to prevent organ damage and save lives.
Schistocytes (Fragmented Cells)
Schistocytes, also known as fragmented red blood cells (RBCs), have a distinct appearance on a peripheral blood smear. Their fragmented RBC morphology can offer clues about potential underlying conditions that damage or shear RBCs as they travel through the bloodstream.
RBC Morphology Appearance on the Blood Smear
- Fragmented Cell Pieces: The defining feature of a schistocyte is its fragmented appearance. The cell is broken into pieces of various sizes and shapes, resembling triangles, helmets, or teardrops.
- Variable Size: The fragmented pieces of schistocytes can be small or large depending on the severity of the damage.
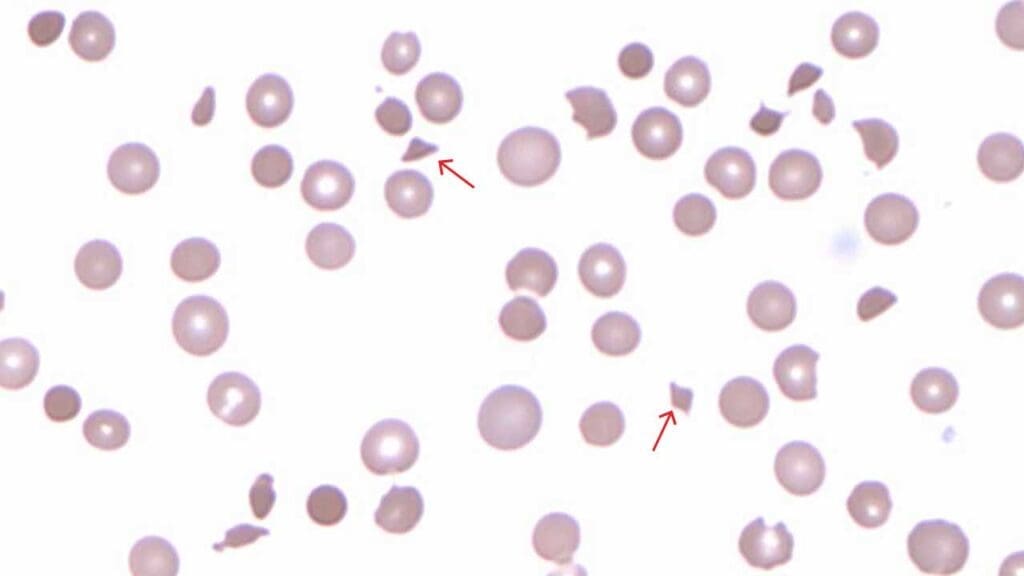
Causes of Schistocytes
The presence of schistocytes on a blood smear can be associated with several conditions that put physical stress on RBCs:
- Microangiopathic Hemolytic Anemia (MAHA): This is a general term for a group of conditions where RBCs are damaged and destroyed as they squeeze through narrowed or damaged blood vessels. This can occur in:
- Thrombotic thrombocytopenic purpura (TTP): A serious condition where blood clots form in small blood vessels throughout the body.
- Hemolytic uremic syndrome (HUS): A complication of certain infections or kidney problems that can damage blood vessel walls.
- Preeclampsia: A pregnancy complication characterized by high blood pressure and potential damage to blood vessels.
- Heart Valve Abnormalities: Mechanical heart valves, especially older models, can cause shear stress on red blood cells as they pass through, leading to fragmentation and the formation of schistocytes. Severe aortic stenosis can also cause similar mechanical trauma. Clinically, if schistocytes are seen in a patient with a prosthetic heart valve or severe valvular disease, it indicates mechanical hemolysis, which may require monitoring for anemia and, in severe cases, consideration of valve repair or replacement.
- Severe Burns: Extensive and severe burns can cause direct thermal damage to red blood cells, leading to their fragmentation and the appearance of schistocytes. Clinically, this finding in burn patients contributes to the overall assessment of injury severity and potential complications, including acute hemolytic anemia, which may necessitate blood transfusions and supportive care.
- Aortic Stenosis: Narrowing of the aortic valve can increase shear stress on RBCs as they pass through the narrowed opening.
- Sickle Cell Disease: While sickle cells are the primary RBC morphology, schistocytes can also be present, particularly during vaso-occlusive crises or in patients with severe pulmonary hypertension. Their presence indicates significant red blood cell destruction and can be a marker of disease severity or complications, prompting closer monitoring and management of the underlying sickle cell crisis.
Elliptocytes
Elliptocytes are a type of red blood cell (RBC) with an elongated oval appearance on a peripheral blood smear in terms of RBC morphology.
RBC Morphology Appearance on the Blood Smear
- Oval Shape: The defining feature of an elliptocyte is its elliptical shape, resembling an oval or elongated ellipse. Unlike oval-shaped footballs, elliptocytes typically lack the pointed ends and have a smoother, more elongated oval form.
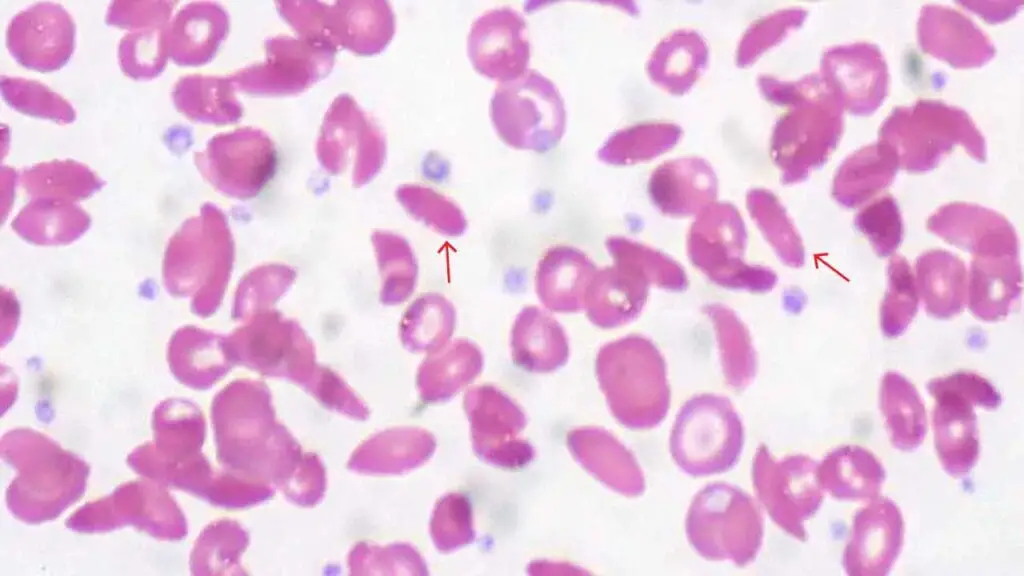
Causes of Elliptocytes
The presence of elliptocytes RBC morphology on a blood smear can be associated with two main categories of causes:
- Hereditary Elliptocytosis: This is a group of inherited disorders where there’s a defect in the genes responsible for maintaining the shape and structure of the RBC membrane. This defect leads to the production of oval-shaped RBCs. The severity of elliptocytosis can vary depending on the specific genetic mutation. While often asymptomatic, some individuals may experience chronic hemolytic anemia, which might warrant further specialized tests (e.g., osmotic fragility, genetic testing) and, rarely, splenectomy.
- Acquired Elliptocytosis: This is more common and can be caused by:
- Iron deficiency anemia: In some cases, mild iron deficiency can be associated with the presence of a few elliptocytes on the blood smear. This is thought to be due to changes in RBC membrane composition.
Tear Drop Poikilocytes (Tear Drop Cells)
Tear drop poikilocytes are also known as dacrocytes, with a teardrop shape on the peripheral blood smear in terms of RBC morphology.
RBC Morphology Appearance on the Blood Smear
- Teardrop Shape: The defining feature of a tear drop poikilocyte is its resemblance to a teardrop. One end of the cell narrows and comes to a point, while the other end is wider and rounder.
- Blunt versus Sharp Ends: It’s important to note that not all pointed RBCs are tear drop poikilocytes. Artificially induced poikilocytosis during blood smear preparation can sometimes create pointed RBCs with sharp, spiky ends. True tear drop poikilocytes typically have blunt or rounded points at the narrowed end.
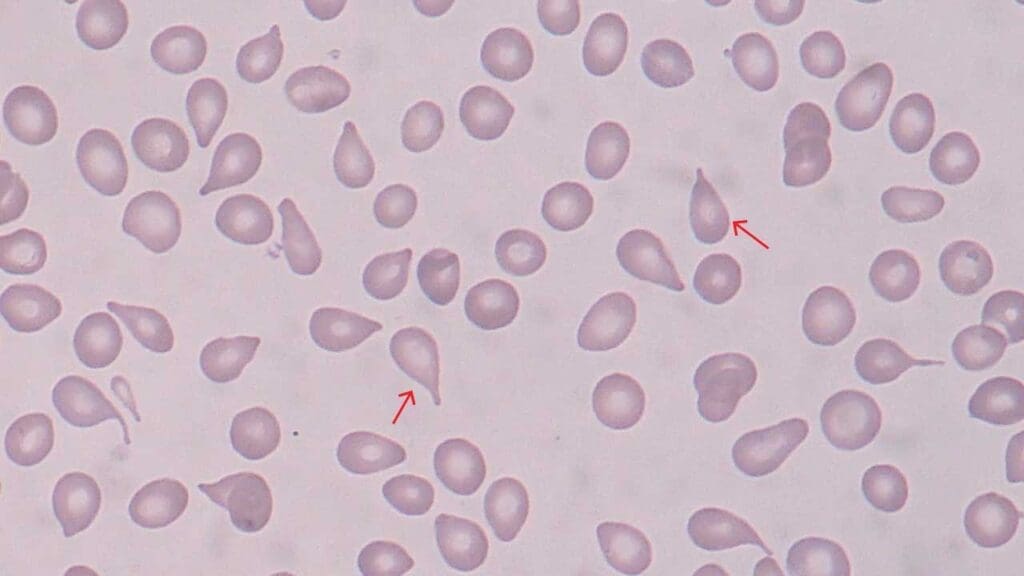
Causes of Tear Drop Poikilocytes
The presence of tear drop poikilocytes RBC morphology on a blood smear can be associated with several conditions:
- Bone Marrow Infiltration: Conditions that infiltrate or replace bone marrow with abnormal cells, such as myelofibrosis, tumors, or granulomas, can disrupt normal RBC production and lead to the formation of tear drop poikilocytes. Clinically, finding teardrop cells should immediately prompt a bone marrow biopsy to investigate the underlying marrow pathology, as it can indicate serious conditions like myelofibrosis or metastatic cancer.
- Vitamin B12 or Folate Deficiency: Deficiencies in these vitamins, crucial for healthy RBC production, can sometimes lead to the formation of tear drop poikilocytes. Clinically, if tear drop cells are seen alongside macrocytosis and hypersegmented neutrophils, it points towards a megaloblastic process, necessitating urgent assessment of B12 and folate levels to guide specific and often life-saving treatment.
Bite Cells
Bite cells, also known as degmacytes, have the characteristic “bite” appearance on the red cells on a peripheral blood smear in terms of RBC morphology.
RBC Morphology Appearance on the Blood Smear
- Circular Depressions: The defining feature of a bite cell is one or more round, well-defined depressions on the cell surface. These depressions resemble bites taken out of a cookie, hence the name.
- Smooth or Irregular Edges: The edges of the bite can be smooth or slightly irregular.
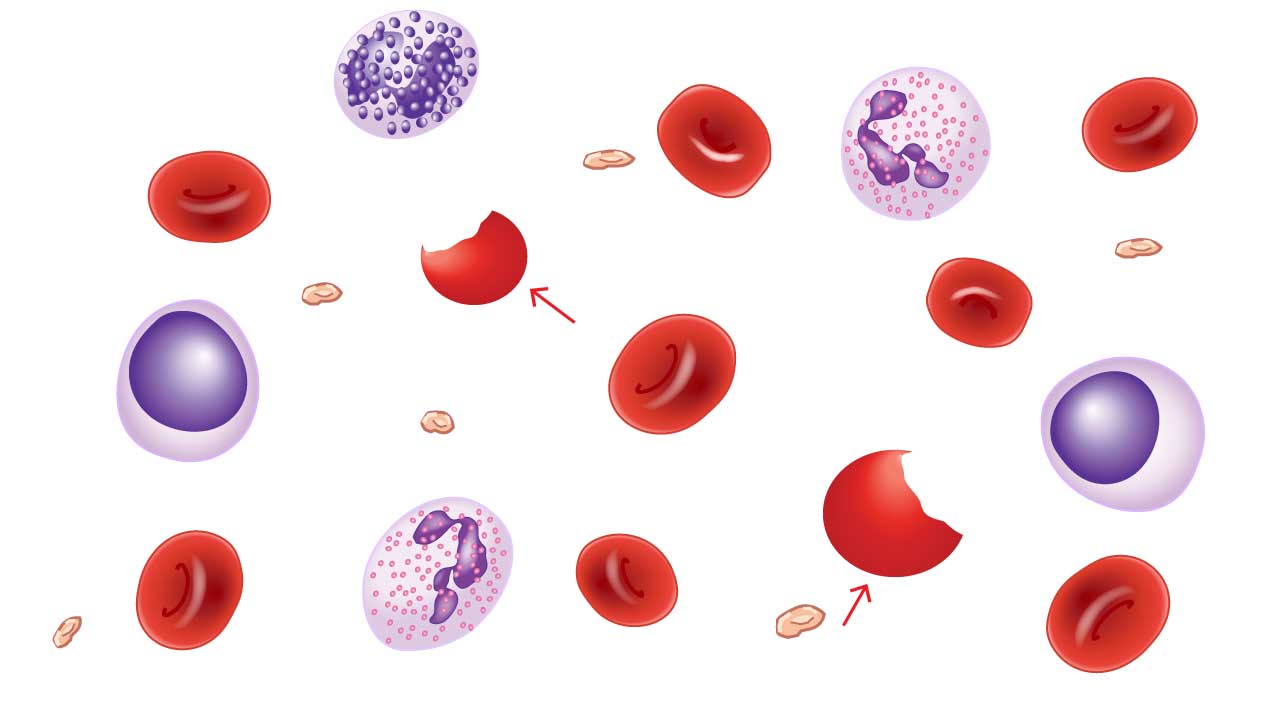
Causes of Bite Cells
The presence of bite cells RBC morphology on a blood smear can be associated with several conditions that cause oxidative stress on RBCs:
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency: This is a common inherited enzyme deficiency that hinders the RBC’s ability to protect itself from oxidative damage. The resulting stress can lead to the formation of Heinz bodies (clumps of denatured hemoglobin) within the RBC. The spleen, a filtering organ, then removes these Heinz bodies, leaving behind the characteristic “bite” on the cell surface.
- Other enzyme deficiencies: Deficiencies in other enzymes involved in the pentose phosphate pathway, which plays a role in RBC metabolism, can also contribute to bite cell formation.
- Oxidative drugs and chemicals: Certain medications or exposure to chemicals can generate excessive free radicals, leading to oxidative stress on RBCs and potential bite cell formation. Examples include dapsone (antibiotic) and sulfasalazine (anti-inflammatory).
- Unstable hemoglobins: Some inherited hemoglobin disorders can make RBCs more susceptible to oxidative damage and Heinz body formation, leading to bite cells.
Sickle Cells
Sickle cells are a hallmark feature of Sickle Cell Disease (SCD), a group of inherited blood disorders affecting red blood cells (RBCs).
RBC Morphology Appearance on the Blood Smear
- C-Shaped Elongation: The defining feature of a sickle cell is its characteristic sickle or crescent moon shape. This abnormal shape occurs when deoxygenated hemoglobin molecules inside the RBC polymerize (stick together) and form rigid rods. These rods distort the normally round RBC into a C-shaped cell.
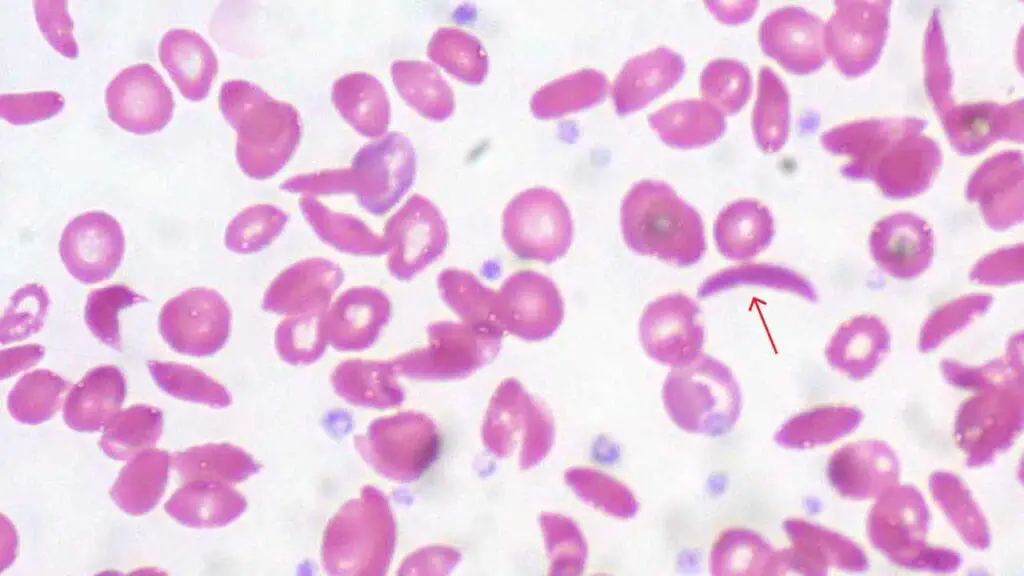
Causes of Sickle Cells
The presence of sickle cells is a direct consequence of Sickle Cell Disease, which is caused by a genetic mutation in the beta-globin gene. This mutation alters the structure of hemoglobin, the oxygen-carrying protein within RBCs. When the mutated hemoglobin loses oxygen, it clumps together, forming rigid rods that distort the RBC morphology into the characteristic sickle shape. Clinically, recognizing sickle cells is critical because it indicates a lifelong condition prone to painful vaso-occlusive crises, chronic hemolytic anemia, and various organ complications (e.g., stroke, acute chest syndrome, kidney disease). The diagnosis prompts comprehensive genetic counseling, specialized management to prevent crises, and monitoring for complications.
Anisopoikilocytosis
Anisopoikilocytosis isn’t a single cell abnormality, but rather a term used to describe the presence of both anisocytosis and poikilocytosis in a blood smear.
Anisocytosis: This refers to a variation in the size of red blood cells (RBCs) on the smear.
Poikilocytosis: This refers to a variation in the shape of RBCs on the smear.
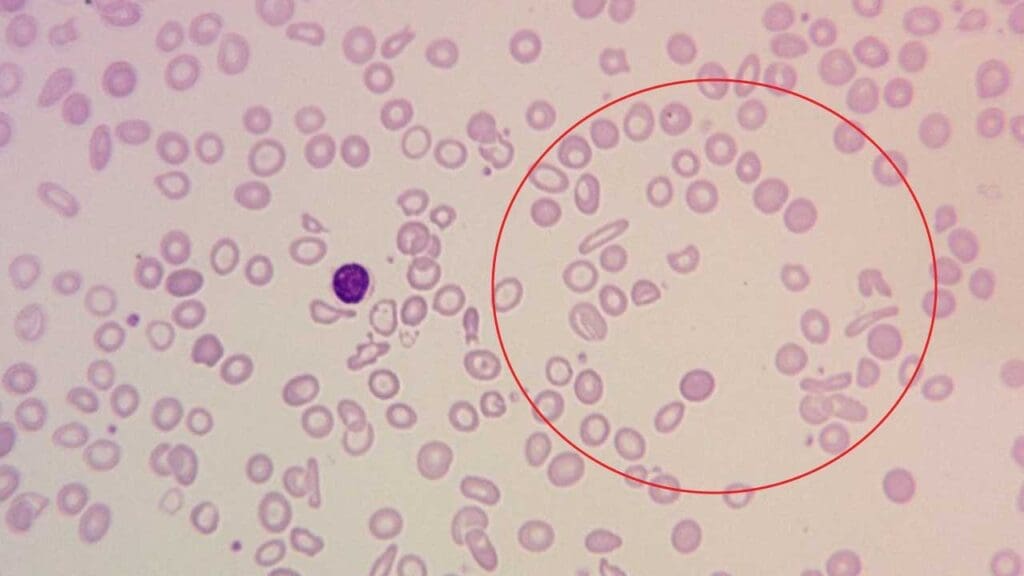
Causes of Anisopoikilocytosis
The presence of anisopoikilocytosis indicates an underlying condition affecting RBC production or lifespan causing variable size and shape in RBC morphology. Here are some potential causes:
- Nutritional deficiencies: Deficiencies in iron, vitamin B12, or folate, all crucial for healthy RBC production, can lead to anisopoikilocytosis.
- Bone marrow disorders: Conditions like myelofibrosis or aplastic anemia that affect bone marrow function can disrupt normal RBC production and lead to size and shape variations.
- Hemoglobinopathies: Inherited disorders like thalassemia or sickle cell disease cause abnormal hemoglobin production, leading to misshapen and sometimes different sized RBCs.
- Liver disease: Severe liver dysfunction can impair RBC production and contribute to anisopoikilocytosis.
- Splenomegaly: An enlarged spleen can trap and damage RBCs, leading to fragmentation (schistocytes) and other shape abnormalities.
- Blood loss: Acute or chronic blood loss can trigger the bone marrow to produce RBCs rapidly, leading to a mix of immature and mature cells with varying sizes and shapes.
Dimorphic Picture
A dimorphic blood picture refers to a peripheral blood smear that shows two distinct populations of red blood cells (RBCs) in terms of size and sometimes, color in relation to RBC morphology.
RBC Morphology Appearance on the Blood Smear
- Two Distinct Populations: The key feature is the presence of two separate populations of RBCs with clear differences in size and sometimes, color. Here are some possibilities:
- Macrocytosis and Microcytosis: One population might consist of macrocytes (larger than normal RBCs), while the other consists of microcytes (smaller than normal RBCs).
- Normochromia and Hypochromia: In some cases, there might also be a difference in color saturation. One population could be normochromic (normal pink), while the other is hypochromic (pale), suggesting a hemoglobin deficiency.
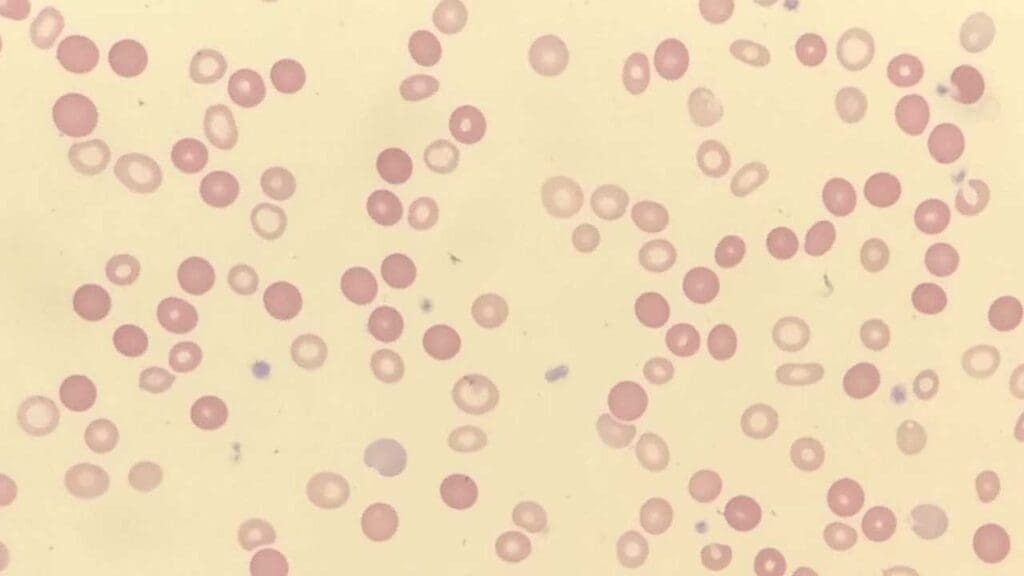
Causes of Dimorphic Blood Picture
Several conditions can lead to a dimorphic blood picture:
- Nutritional Deficiencies: Deficiencies in iron, vitamin B12, or folate, all crucial for healthy RBC production, can manifest as a dimorphic picture. For example, iron deficiency can lead to microcytic, hypochromic cells (small and pale), while a concurrent folate deficiency might also cause macrocytic, normochromic cells (large and pink).
- Bone Marrow Disorders: Conditions like myelodysplastic syndromes (MDS) or aplastic anemia can disrupt normal RBC production, leading to a mix of immature and mature cells with varying sizes and sometimes colors.
- Transfusion in Hemoglobinopathies: The presence of these two distinct populations of RBCs – the patient’s microcytic, hypochromic cells and the larger, normochromic donor cells – creates a dimorphic picture on the blood smear.
Rouleaux Formation
Rouleaux (singular: rouleau) is a term used to describe the stacking or aggregation of red blood cells (RBCs) seen on a peripheral blood smear in terms of RBC morphology. This stacking resembles a stack of coins, hence the name.
RBC Morphology Appearance on the Blood Smear
- Stacks of RBCs: Rouleaux appear as elongated rows of RBCs stacked face-to-face, resembling stacks of coins in the RBC morphology. The length of these stacks can vary, with some containing just a few cells and others forming longer chains.
- Variable RBC Distribution: In a normal smear, RBCs are typically evenly distributed with some space between them. However, with rouleaux formation, the RBCs appear clumped together, leaving clear areas devoid of cells in between the stacks.
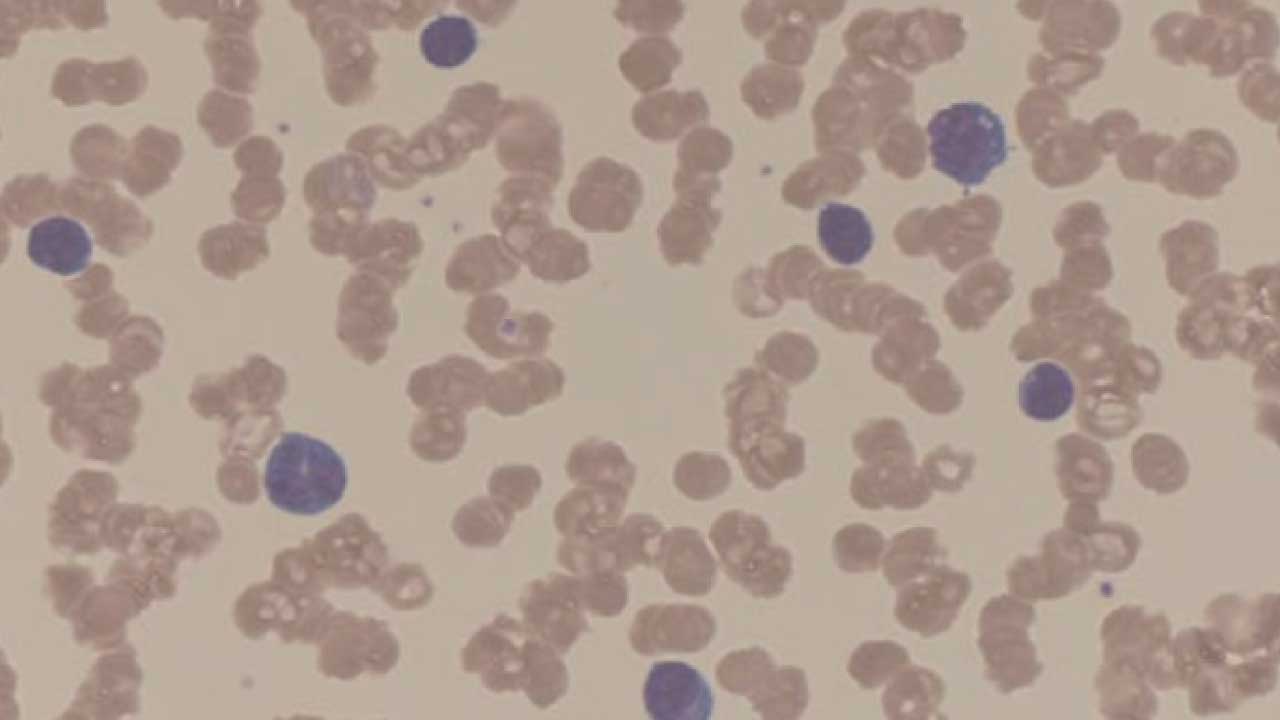
Causes of Rouleaux Formation
Rouleaux formation is primarily caused by an increase in plasma protein concentration, particularly fibrinogen, which acts like a “glue” between RBCs. Here are some conditions that can lead to increased rouleaux:
- Increased Plasma Protein Concentration: Rouleaux formation is primarily caused by an increase in plasma proteins, particularly acute phase reactants like fibrinogen or abnormal immunoglobulins. These proteins reduce the repulsive forces between red blood cells, allowing them to stack. Clinically, this finding is a non-specific indicator of inflammation or infection, as fibrinogen levels rise in these conditions. Crucially, it also strongly suggests the presence of a monoclonal gammopathy, such as Multiple Myeloma, where excessive abnormal antibodies are produced. This prompts further investigation into inflammatory markers (e.g., ESR, CRP) and, significantly, serum protein electrophoresis (SPEP) to identify and quantify abnormal proteins.
- Inflammatory Conditions: Inflammation triggers the liver to produce more acute-phase proteins, including fibrinogen. This can lead to rouleaux formation on the blood smear.
- Dehydration: When the body is dehydrated, the blood becomes more concentrated, including an increase in plasma protein levels. This can contribute to rouleaux formation.
- Autoimmune Diseases: Some autoimmune diseases can lead to increased production of certain proteins, including fibrinogen, potentially causing rouleaux. Clinically, this finding in a patient with suspected or known autoimmune disease supports the presence of systemic inflammation or dysproteinemia and may prompt further immunological workup.
- Multiple Myeloma: This is a key clinical association. Multiple myeloma is a plasma cell malignancy characterized by the overproduction of a monoclonal immunoglobulin (M-protein). This excessive protein significantly increases plasma viscosity and reduces the zeta potential, leading to prominent rouleaux formation. Clinically, the presence of marked rouleaux on a peripheral blood smear should immediately raise strong suspicion for multiple myeloma, even before other symptoms appear. This finding necessitates urgent investigation with serum protein electrophoresis, immunofixation, and a bone marrow biopsy for definitive diagnosis.
Red Cell Clumping (Agglutination)
Agglutination describes the clumping together of red blood cells (RBCs) on a peripheral blood smear. Unlike rouleaux formation, where RBCs stack in a specific pattern, agglutination appears as irregular, dense clusters of clumped cells. This abnormal clumping RBC morphology indicates the presence of antibodies or other molecules coating the RBC surface, causing them to stick together.
RBC Morphology Appearance on the Blood Smear
- Irregular Clumps: Agglutination manifests as irregular-shaped aggregates of RBCs, often appearing denser and more haphazardly arranged compared to rouleaux formation.
- Variable Size and Distribution: The size of the agglutinates can vary significantly, ranging from small clumps to large aggregates involving numerous RBCs. The distribution of these clumps is also uneven, leaving clear areas devoid of cells in between.
- Loss of Individual Cell Details: Due to the dense clumping, it can be difficult to discern individual RBC morphology (shape) or color on a smear with agglutination.
Causes of Red Blood Cell Agglutination
Agglutination occurs when antibodies or other molecules bind to specific antigens (proteins or sugars) on the RBC surface. This binding triggers the RBCs to clump together. Here are some potential causes:
- Autoimmune Hemolytic Anemia: Agglutination is a hallmark of AIHA, where the body’s immune system produces antibodies (autoantibodies) that bind to red blood cells, causing them to clump together and be prematurely destroyed. Clinically, the presence of agglutination, along with signs of hemolysis (e.g., anemia, jaundice, elevated bilirubin, LDH, low haptoglobin), should immediately prompt a direct antiglobulin test (DAT or Coombs test) to confirm the presence of antibodies on the red cell surface. This is crucial for initiating appropriate immunosuppressive treatment.
- Blood Transfusion Reactions: If a blood transfusion is incompatible (blood types are not properly matched), the recipient’s immune system can develop antibodies against the donor’s RBCs, causing agglutination in the RBC morphology.
- Infections: Certain infections, particularly those caused by Mycoplasma pneumoniae (walking pneumonia) or Epstein-Barr virus (EBV), can trigger the production of cold agglutinins – antibodies that cause RBC agglutination at cooler temperatures.
- Cold Agglutinin Disease: This is a rare autoimmune disorder characterized by the presence of cold agglutinins that cause RBC agglutination, especially in the extremities exposed to cold temperatures. Clinically, patients may experience acrocyanosis (bluish discoloration of extremities) or Raynaud’s phenomenon in cold weather. The presence of agglutination that resolves upon warming the blood sample is a key diagnostic clue, guiding further workup for cold agglutinins and underlying conditions (e.g., lymphoproliferative disorders) that may be associated with it.
- Certain Medications: Some medications, like quinine or certain cephalosporin antibiotics, can bind to RBCs and induce agglutination RBC moprhology in rare cases. Clinically, if agglutination is observed without other clear causes, a thorough review of the patient’s medication list is essential. Discontinuation of the offending agent is often necessary, and the patient may require supportive care for drug-induced hemolysis
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Bandaru SS, Killeen RB, Gupta V. Poikilocytosis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562141/
- Tyrrell, L., Rose, G., Shukri, A., & Kahwash, S. B. (2021). Morphologic changes in red blood cells: An illustrated review of clinically important light microscopic findings. The Malaysian journal of pathology, 43(2), 219–239.
- Red blood cell morphology. Clinical Hematology Atlas: A Pictorial Guide for the Hematology Laboratory (Taylor and Doty). Oregon Institute of Technology.