Introduction
This article provides an overview of key findings in abnormal white blood cell morphology observed during a peripheral blood smear analysis. Understanding these abnormal white blood cell morphological changes can aid in diagnosing various underlying conditions.
Role of WBCs
White blood cells (WBCs), also known as leukocytes, are the body’s defense system cells, playing a critical role in the immune response. They circulate throughout the bloodstream and tissues, constantly surveying for and eliminating pathogens (disease-causing organisms) and debris. There are five main types of WBCs, each with specialized functions.
- Neutrophils: These are the most abundant WBCs, acting as the first responders to infections by engulfing and destroying bacteria through a process called phagocytosis. They are also the first to migrate to sites of inflammation.
- Lymphocytes: These are further divided into B and T cells. B cells produce antibodies, which are proteins that specifically target and neutralize pathogens. T cells come in various subtypes, some of which directly attack infected cells or regulate the immune response by helping other immune cells function.
- Monocytes: They mature into macrophages in tissues. Macrophages are like bigger, stronger phagocytes that engulf debris, pathogens, and even cancer cells. They also process and present antigens (foreign molecules) to lymphocytes, helping them recognize and target the invaders.
- Eosinophils: They are involved in allergic reactions and defense against parasitic infections. Eosinophils release chemicals that damage parasites and contribute to inflammatory responses.
- Basophils: These are the least common WBCs, releasing histamine and other chemicals during allergic reactions and inflammation. Histamine causes vasodilation (widening of blood vessels) and increased permeability, leading to allergy symptoms like runny nose and itchy eyes.
WBC Morphology
WBC morphology plays a crucial role in blood smear analysis for several reasons:
1. Detailed Information Beyond Cell Count: Some complete blood count (CBC) for example a 3-part full blood count analyzer provides the total WBC count, but it doesn’t differentiate between the types of WBCs. Examining the morphology of WBCs in a blood smear allows for a differential white blood cell count (WBC diff). This diff reveals the percentages of each WBC type, which can be highly informative. For example, an elevated eosinophil count on the diff might suggest an allergic reaction or parasitic infection, even with a normal total WBC count.
2. Identification of Abnormal White Blood Cells: The morphology of healthy WBCs has specific characteristics. Deviations from these characteristics (abnormal white blood cell morphology), like the presence of toxic granulation or Dohle bodies, can indicate underlying conditions like infections or myeloproliferative disorders. Early detection of these abnormal white blood cells can prompt further investigation and lead to a faster diagnosis.
3. Supporting Diagnosis: Abnormal white blood cell morphology findings can support diagnoses suggested by other clinical findings or laboratory tests. For instance, hypersegmented neutrophils are abnormal white blood cells and might point towards vitamin B12 deficiency, while May-Hegglin anomaly strengthens the suspicion of May-Hegglin syndrome when giant platelets are also observed.
4. Guiding Treatment Decisions: Identifying the specific type of WBC involved in an elevated count can guide treatment decisions. For example, an elevated neutrophil count suggests a bacterial infection, prompting antibiotic therapy, whereas an elevated eosinophil count might point towards an allergy, requiring a different treatment approach.
5. Monitoring Treatment Response: WBC morphology can be used to monitor the effectiveness of treatment. For example, a decrease in abnormal white blood cells following treatment for an infection indicates a positive response.
Cytoplasmic Inclusions
Toxic Granulation in Neutrophils
Toxic granulation refers to the presence of abnormal granules within neutrophils observed during a blood smear analysis. These abnormal white blood cell granules have a distinct appearance compared to normal neutrophil granules.
Appearance on Blood Smear
- Size and Color: These abnormal white blood cell granules are larger and more coarse compared to the fine, evenly distributed pink or lilac granules in mature neutrophils.
- Staining: These abnormal white blood cell granules stain a darker blue or purple with traditional blood stains (e.g., Wright-Giemsa stain) due to a higher concentration of enzymes.
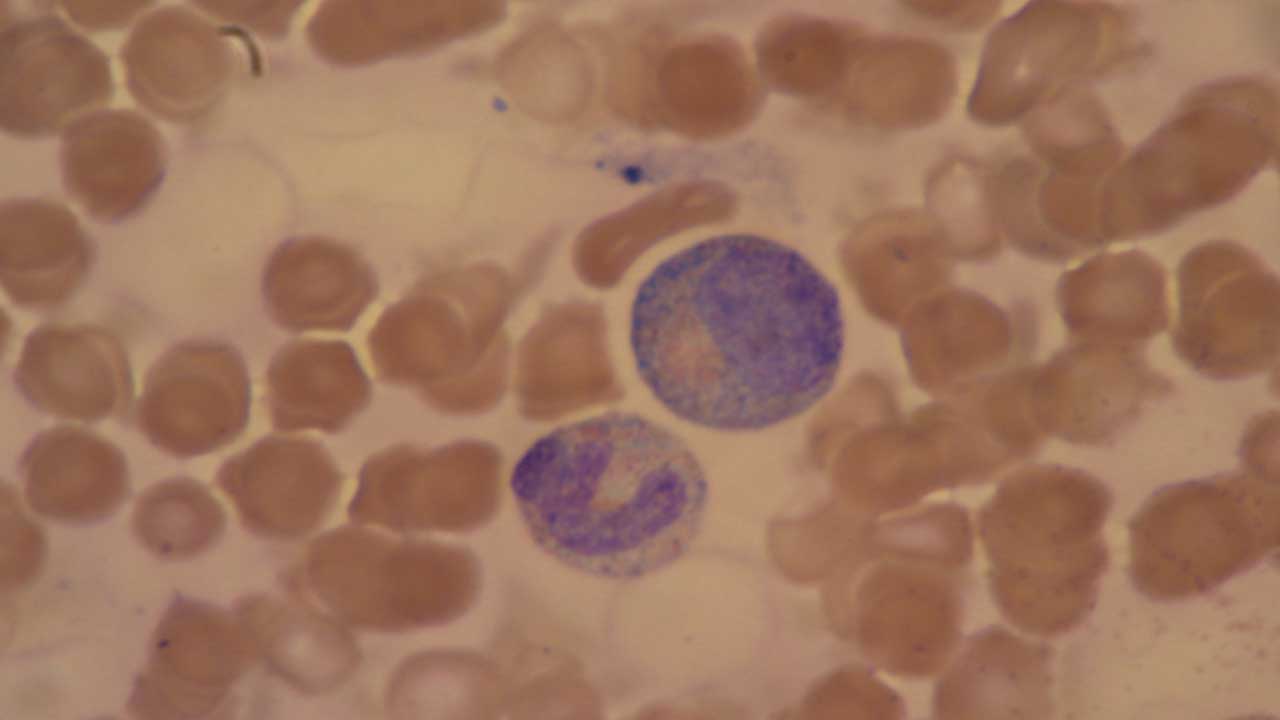
Causes of Toxic Granulation in Neutrophils
While toxic granulation is a strong indicator of an inflammatory response, it can be caused by various conditions.
- Bacterial Infections: This is the most common cause for this abnormal white blood cell morphology. The presence of bacteria triggers the release of cytokines, which stimulate neutrophils to mature more rapidly particularly in severe bacterial infections like sepsis, pneumonia, or meningitis. This rapid maturation leads to incomplete packaging of enzymes within the granules, resulting in their coarse and hyper-staining appearance.
- Viral Infections: Some viral infections, particularly severe ones, can also induce toxic granulation in neutrophils.
- Severe Burns and Trauma: Extensive tissue damage triggers an inflammatory response, leading to the release of cytokines and potential toxic granulation.
- Inflammatory Bowel Disease (IBD): Active phases of IBD involve significant inflammation, which can be reflected in the blood smear by the presence of toxic granulation as abnormal white blood cell morphology.
- Myeloproliferative Disorders: These are a group of bone marrow diseases characterized by abnormal growth of myeloid cells. Some types of myeloproliferative disorders can cause toxic granulation for example chronic neutrophilic leukemia.
- Drug Reactions: Certain medications can cause toxic granulation as a side effect for example chemotherapy or granulocyte colony-stimulating factor (G-CSF), can stimulate neutrophil production and lead to toxic granulation.
Döhle Bodies in Neutrophils
Döhle bodies are another type of abnormal white blood cell morphology. They are pale blue-gray, oval-shaped inclusions found within the cytoplasm of neutrophils, typically near the cell membrane.
Appearance on Blood Smear
- Size: Döhle bodies measure 1-3 micrometers in diameter, which is relatively small compared to a neutrophil.
- Color: They appear pale blue or gray due to their composition of rough endoplasmic reticulum (RER) remnants.
- Shape: Döhle bodies are usually oval or round, but they can also be crescent-shaped or elongated.
- Location: These inclusions are most commonly seen close to the neutrophil’s cell membrane.
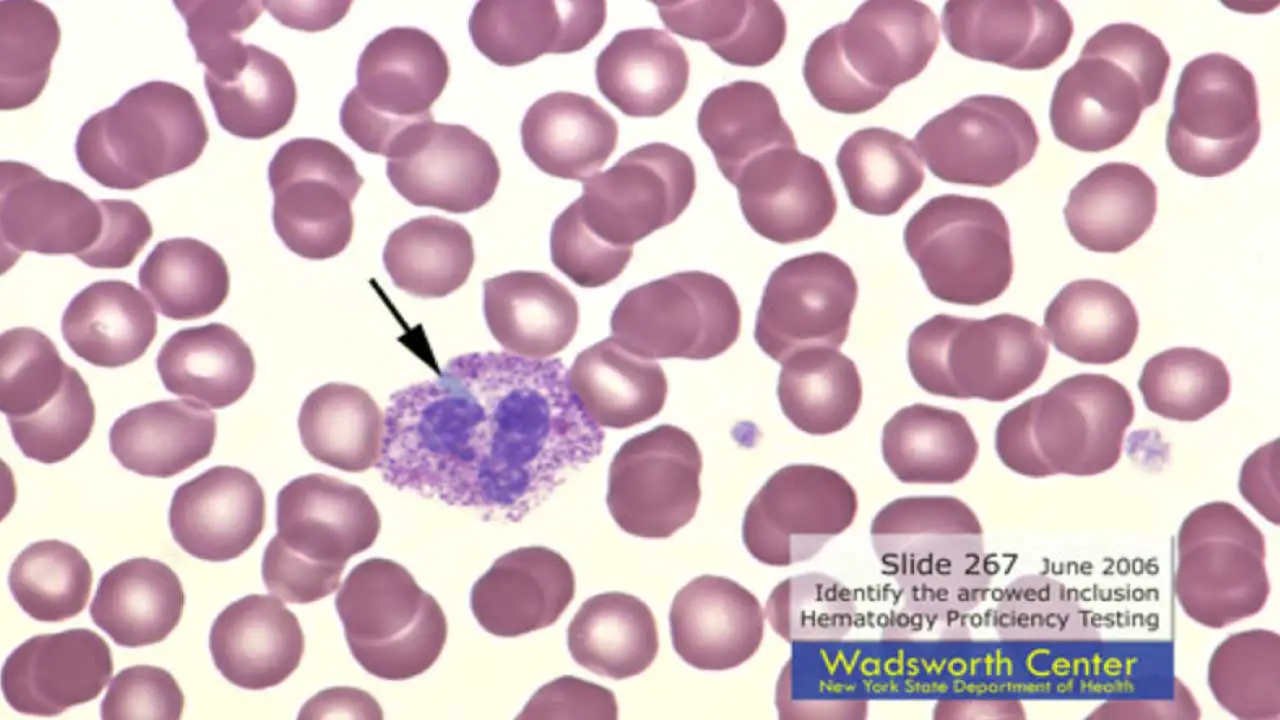
Causes of Döhle Bodies in Neutrophils
The presence of Döhle bodies can be due to various factors:
- Infections: Döhle bodies are often associated with viral infections, particularly severe ones like cytomegalovirus (CMV) or influenza. They can also be seen in some bacterial infections.
- Increased Endoplasmic Reticulum Activity: Conditions that stimulate the endoplasmic reticulum (ER) in neutrophils, like drug reactions or deficiencies (vitamin B12, folate), can lead to Döhle body formation.
- Myeloproliferative Disorders: These are bone marrow disorders characterized by abnormal overproduction of blood cells. Some myeloproliferative disorders, like chronic myeloid leukemia (CML), may be associated with Döhle bodies.
- May-Hegglin Anomaly: This is a rare inherited condition where Döhle bodies as abnormal white blood cell morphology, are consistently present along with giant platelets in the blood smear.
Important Considerations
- A small number of Döhle bodies in healthy individuals, particularly cats and horses, might not be a cause for concern.
- The presence of Döhle bodies alone is not diagnostic of any specific condition. It should be evaluated in conjunction with other clinical findings and laboratory tests.
- The number and prominence of Döhle bodies may offer some clues about the underlying cause. For example, a larger number of Döhle bodies might suggest a more severe condition.
Additional Notes
- Döhle bodies are sometimes confused with toxic granulation, but they differ significantly in appearance and cause. Toxic granules are coarse, basophilic, and associated with inflammation, while Döhle bodies are pale, composed of RER remnants, and linked to viral infections or increased ER activity.
Nuclear Abnormalities
Hypersegmented Neutrophils
Hypersegmented neutrophils are mature neutrophils with an abnormally high number of nuclear lobes. Typically, a healthy neutrophil has 2-5 nuclear lobes connected by thin strands of chromatin, however, hypersegmented neutrophils are abnormal white blood cells with 6 or more nuclear lobes.
Appearance on Blood Smear
- Number of Lobes: Has six or more distinct lobes as the abnormal white blood cell morphology.
- Shape: The individual lobes can be round, oval, or even horseshoe-shaped.
- Chromatin Connections: The lobes may be connected by thin chromatin strands, but sometimes appear more separated.

Causes of Hypersegmented Neutrophils
Hypersegmented neutrophils as abnormal white blood cells can be a sign of several underlying conditions.
- Megaloblastic Anemia: This is a type of anemia caused by vitamin B12 or folate deficiency. These deficiencies disrupt DNA synthesis, leading to the formation of mature neutrophils with more nuclear divisions, resulting in hypersegmentation.
- Myelodysplastic Syndromes (MDS): These are a group of bone marrow disorders characterized by abnormal blood cell production. In some cases of MDS, hypersegmented neutrophils as abnormal white blood cells might be observed due to altered nuclear maturation.
- Liver Disease: Severe liver disease can sometimes lead to hypersegmentation of neutrophils.
- Hydroxyurea Therapy: This medication is used to treat certain cancers like chronic myeloid leukemia (CML). As a side effect, it can cause hypersegmentation of neutrophils as abnormal white blood cells.
Additional Notes
- Hypersegmented neutrophils (abnormal white blood cells) are often accompanied by macrocytosis (larger than normal red blood cells) in megaloblastic anemia due to the same underlying cause of DNA synthesis disruption.
- Recognizing hypersegmented neutrophils (abnormal white blood cells) can be a valuable clue for identifying potential vitamin B12 or folate deficiencies, prompting further investigation for early intervention.
Pelger-Huët Anomaly
Pelger-Huët anomaly (PHA) is a benign, inherited condition affecting the morphology of neutrophils and sometimes eosinophils giving rise to abnormal white blood cells.
Appearance on Blood Smear
- Nuclear Shape: Neutrophils exhibit bilobed nuclei instead of the usual segmented (3-5 lobes) appearance. These bilobed nuclei can be:
- Dumbbell-shaped: Resembling two connected spheres, resembling a dumbbell.
- Peanut-shaped: Oval with a central constriction, similar to a peanut.
- Chromatin Condensation: The abnormal white blood cell’s nuclear chromatin may appear condensed and coarse compared to a healthy neutrophil.
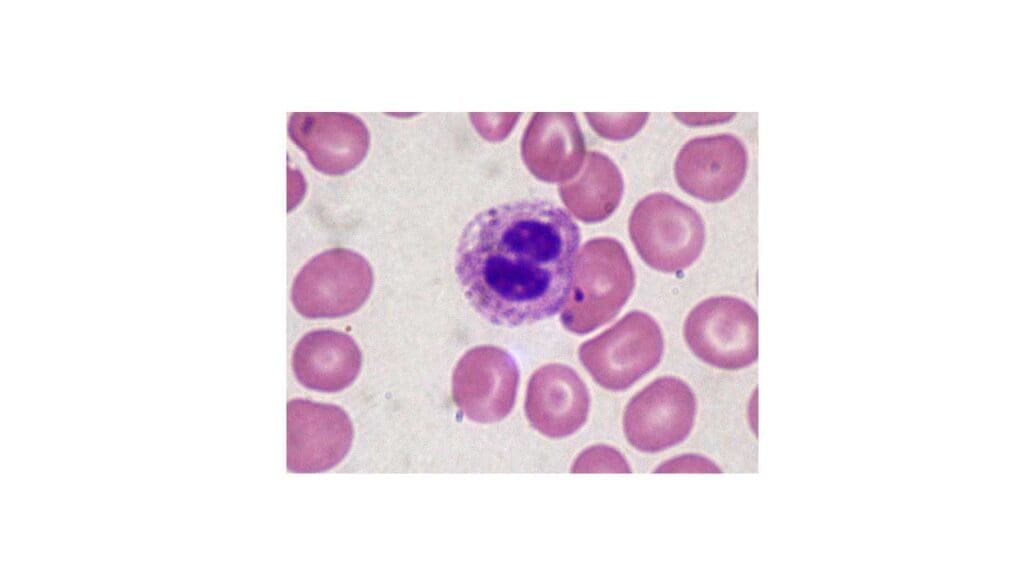
Cause of Pelger-Huët anomaly (PHA)
PHA is caused by mutations in the lamin B receptor (LBR) gene, which plays a role in nuclear envelope formation during neutrophil maturation. It’s inherited in an autosomal dominant pattern, meaning only one copy of the mutated gene is needed for the condition to manifest. PHA is a benign condition and does not affect the function or lifespan of neutrophils. While PHA itself doesn’t require treatment, recognizing it can prevent unnecessary investigations prompted by the abnormal white blood cell morphology.
It’s important to distinguish PHA from other abnormal white blood cells with similar morphology.
- Hyposegmented Neutrophils: These neutrophils have fewer than normal lobes (usually one) and can be mistaken for Pelger-Huët anomaly. However, hyposegmented neutrophils typically have a more rounded or horseshoe-shaped nucleus and are often associated with conditions like myelodysplastic syndromes, which PHA is not.
- Left-Shifted Maturation: In severe infections, immature neutrophils with fewer nuclear lobes may be released into the bloodstream. While these abnormal white blood cell’s immature forms might appear somewhat bilobed, they lack the characteristic coarse chromatin condensation of PHA and are accompanied by other signs of infection in the blood smear.
Important Considerations
- PHA typically affects all granulocytes (neutrophils, eosinophils, basophils) to some degree, but the most prominent visual changes are usually seen in neutrophils.
Hypogranularity in WBCs
Hypogranularity refers to a decrease in the number and/or staining intensity of granules within the cytoplasm of white blood cells (WBCs). This abnormal white blood cell morphology can be observed in various types of WBCs, including neutrophils, eosinophils, and basophils.
Appearance on Blood Smear
- Reduced Granules: Abnormal white blood cells exhibit a noticeable decrease in the number of visible granules compared to healthy cells. The cytoplasm appears relatively clear or pale due to the reduced granularity.
- Fainter Staining: In some cases, the remaining granules might also stain less intensely with specific dyes used in blood smears, further contributing to the pale appearance in the abnormal white blood cells.
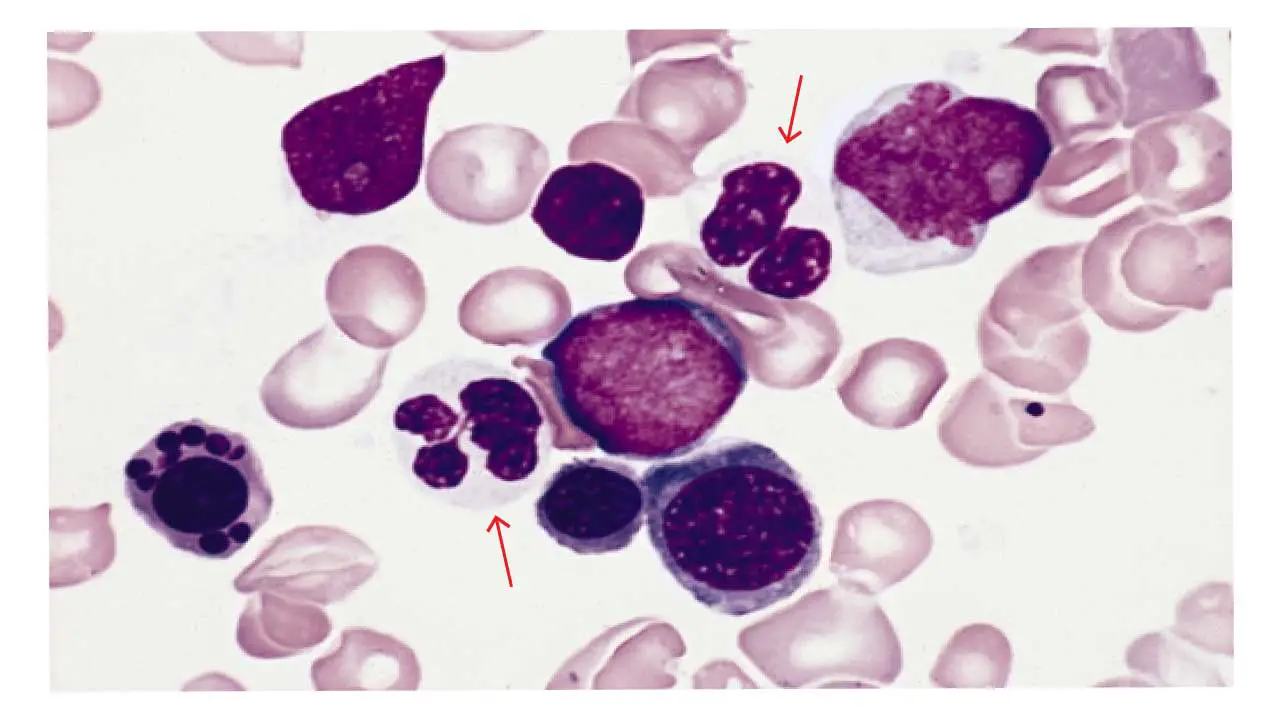
Causes of Hypogranularity in Abnormal White Blood Cells
The presence of hypogranularity in abnormal white blood cells necessitates considering several potential causes:
- Immature Cells: During maturation in the bone marrow, WBCs develop and acquire their characteristic granules. The release of immature WBCs into the bloodstream due to conditions like infections or bone marrow stress can lead to hypogranularity, as these precursors have fewer or less developed granules.
- Vitamin B12 or Folate Deficiency: Deficiencies in vitamin B12 or folate are essential for healthy DNA synthesis and cell division. These deficiencies can disrupt the proper maturation of WBCs, leading to the release of hypogranular forms with abnormal white blood cell morphology into the bloodstream.
- Myelodysplastic Syndromes (MDS): These are a group of bone marrow disorders characterized by abnormal blood cell production. In some cases of MDS, hypogranularity in neutrophils or other WBCs might be observed due to disrupted maturation processes.
- Pelger-Huët Anomaly: This is a benign, inherited condition primarily affecting neutrophils. While the hallmark feature in the abnormal white blood cell is bilobed nuclei, some individuals with Pelger-Huët anomaly might also exhibit a slight decrease in the number and staining intensity of their neutrophil granules.
Vacuolation in Abnormal White Blood Cells
Vacuoles in abnormal white blood cells appear as clear, round, or oval spaces within the cytoplasm. They lack the staining properties of normal cellular components and typically appear empty or light blue due to the presence of fluid in the abnormal white blood cells.
Appearance on Blood Smear
- Size: The size of vacuoles in abnormal white blood cells can vary depending on the underlying cause. They can range from small and inconspicuous to large and prominent, occupying a significant portion of the cytoplasm.
- Number: The number of vacuoles in abnormal white blood cells can also be variable. Some abnormal white blood cells might have a single, isolated vacuole, while others might exhibit multiple vacuoles scattered throughout the cytoplasm.
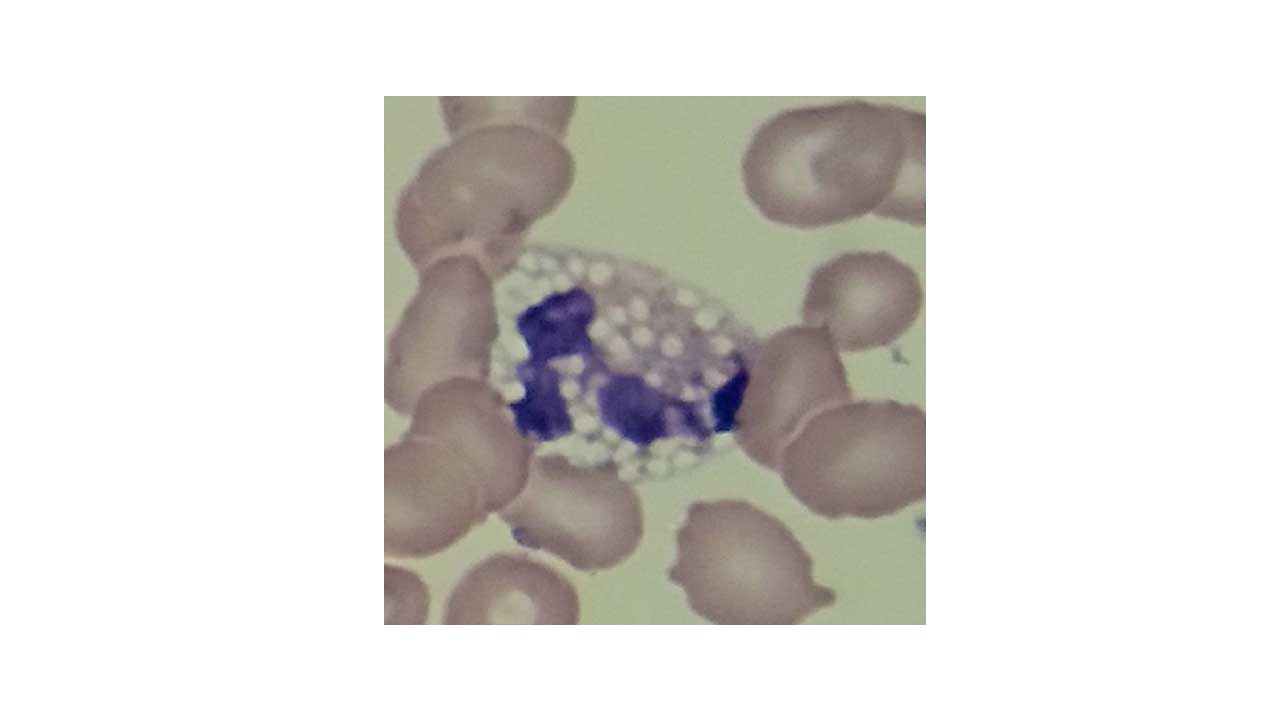
Causes of Vacuolation in Abnormal White Blood Cells
The presence of vacuoles in abnormal white blood cells necessitates considering several potential causes.
| Cause | Description | Additional Notes |
| Physiological Lipid Storage (Monocytes) | In some cases, particularly in monocytes, small vacuoles might represent the storage of excess lipids within the cell. This is a normal physiological process and doesn’t necessarily indicate pathology. | The vacuoles in abnormal white blood cells are typically small and may not be a prominent feature. |
| Impaired Phagocytosis | When WBCs, particularly neutrophils, engulf foreign material (bacteria, debris) through phagocytosis, they normally break it down within lysosomes. If lysosomal function is impaired (e.g., Chediak-Higashi syndrome), undigested material can accumulate, forming vacuoles. | The vacuoles in abnormal white blood cells may be larger and more prominent compared to physiological lipid storage. |
| Infectious Diseases | Certain viral or parasitic infections can trigger the formation of vacuoles within WBCs. The mechanism can vary depending on the specific pathogen. | The type and size of vacuoles might offer clues about the causative agent. Additional tests would be needed for definitive diagnosis. |
| Drug Toxicity | Some medications, particularly chemotherapy drugs, can have a toxic effect on WBCs, leading to vacuolization as a side effect. | The presence of vacuoles along with other abnormal white blood cell morphological changes and a history of recent medication use can be suggestive of drug toxicity. |
| Vitamin Deficiencies | Deficiencies in vitamins like B12 or folate can disrupt the normal maturation and function of WBCs, potentially leading to vacuolization. | Vacuoles might be accompanied by other abnormal white blood cell morphologies suggestive of a deficiency state. |
| Storage Disorders | Inherited metabolic disorders that affect how cells store specific molecules can cause vacuole formation within WBCs due to the accumulation of unprocessed materials. Examples include Gaucher disease and Niemann-Pick disease. | The specific type of storage disorder can be identified through further investigations based on clinical presentation and additional laboratory tests. |
Alder Anomaly
Alder anomaly is a rare benign finding in blood smear analysis characterized by the presence of abnormal white blood cell morphology such as coarse, basophilic granules within the cytoplasm of lymphocytes and, less commonly, neutrophils.
Appearance on Blood Smear
- Granules: These granules are:
- Basophilic: They stain dark blue with basophilic dyes used in blood smears.
- Coarse: They are larger and more prominent compared to the fine, azurophilic granules typically seen in lymphocytes.
- Variable number: The number of basophilic granules can vary within lymphocytes, with some cells having just a few and others exhibiting a more prominent display.
- Lymphocyte Morphology: While lymphocytes with Alder anomaly have abnormal granules, their overall size and nuclear morphology typically remain within the normal range.
Cause of Alder Anomaly
The exact cause of Alder anomaly remains unclear. Some theories suggest it might be related to altered ribosome function or abnormal protein synthesis within lymphocytes. Alder anomaly is a benign finding and doesn’t necessarily indicate any underlying medical condition. It’s more frequently observed in individuals with a history of myeloproliferative disorders, but it can also occur in healthy individuals without any associated disease.
It’s crucial to differentiate Alder anomaly from other conditions that might exhibit basophilic granules.
- Toxic Granulation: This abnormal white blood cells morphology is more commonly associated with neutrophils and presents with coarse, basophilic granules, but they are typically more numerous and evenly distributed throughout the cytoplasm compared to the more localized and variable distribution seen in Alder anomaly.
- Chronic Myeloproliferative Neoplasms (CMPN): These are bone marrow disorders that can cause abnormal basophilic granules in precursors of various blood cells, including lymphocytes. However, CMPN typically presents with other abnormal white blood cell morphologies in blood smear analysis and additional clinical features that differentiate it from Alder anomaly.
- Mast Cell Activation Syndrome (MCAS): This condition can sometimes lead to the release of basophilic granules from mast cells into the bloodstream, which might be mistaken for abnormal basophilia in lymphocytes on a blood smear. However, the presence of mast cell fragments or atypical cells can help differentiate MCAS from Alder anomaly.
Drumsticks in Neutrophils
Drumsticks are a specific type of nuclear appendage observed in neutrophils during blood smear analysis. They are named for their characteristic drumstick-like shape and are considered a marker of sex chromatin in females.This is because the drumstick represents the condensed, inactive X chromosome (Barr body) in females (inactivation of one X chromosome).
Appearance on Blood Smear
- Shape: They resemble a small drumstick with a:
- Head: A rounded or oval-shaped terminal portion.
- Stalk: A thin, thread-like connection to the main body of the neutrophil nucleus.
- Size: Drumsticks are typically smaller than the main nucleus but can vary slightly in size.
- Location: They can be attached to any lobe of the neutrophil nucleus but are most commonly seen near the periphery.
- Frequency: In healthy females, the drumstick appearance is usually seen in only a small percentage (1-2%) of circulating neutrophils.
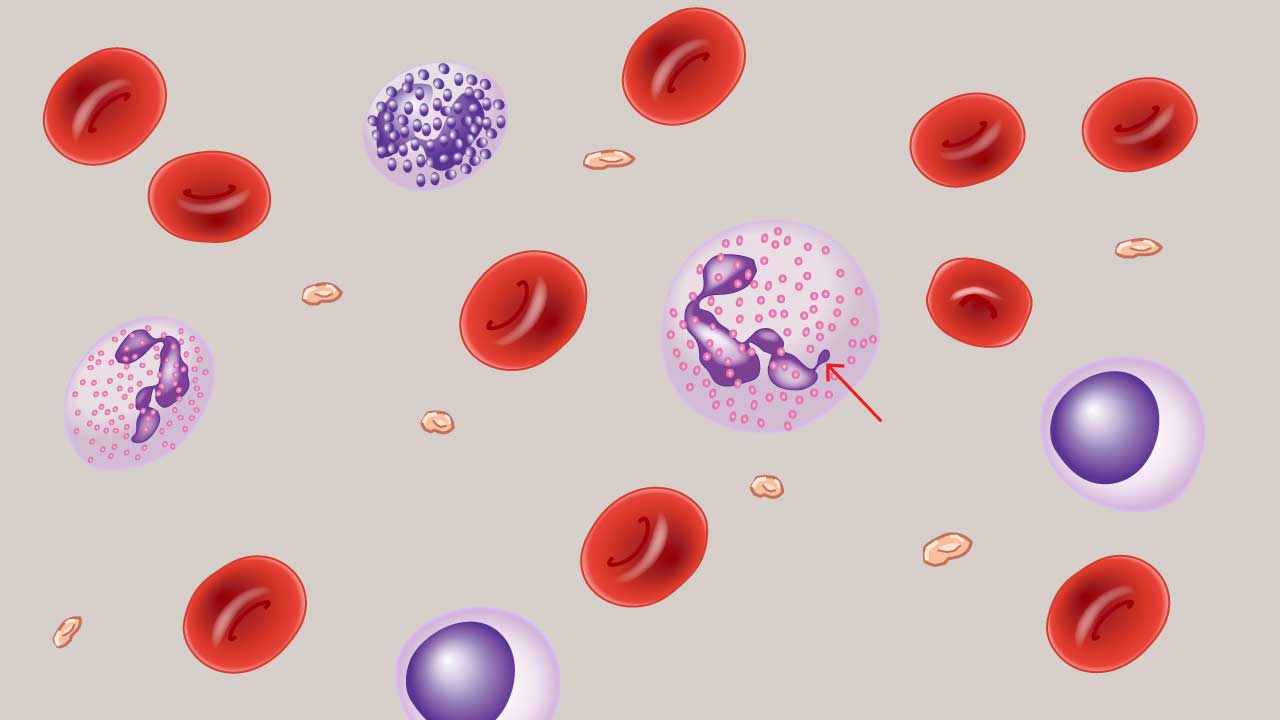
Causes of Drumsticks in Neutrophils
While drumsticks are primarily associated with sex chromatin in females, there are a few other considerations.
- Males: The presence of drumsticks in males is very rare (less than 0.1%) and might be associated with Klinefelter syndrome (XXY karyotype) or other chromosomal abnormalities.
- Non-Specific Nuclear Appendages: Occasionally, neutrophils can exhibit other nuclear appendages besides drumsticks as abnormal white blood cell morphology. These appendages can be:
- Sessile nodules: Small, round protuberances attached to the nucleus.
- Racquet forms: Resemble a drumstick but with a wider, flattened head. These non-specific appendages are usually smaller and lack the distinct drumstick shape. However, in rare cases, they might require further evaluation to rule out underlying conditions.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Blumenreich MS. The White Blood Cell and Differential Count. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 153.