TL;DR
Definition ▾
Sickle cell anemia is a hereditary chronic hemolytic anemia caused by the production of Hb S (a qualitative hemoglobin defect due to a β-globin gene codon 6 mutation [glutamic acid → valine])
Pathophysiology ▾
Sickle cell anemia is a genetic disorder characterized by the abnormal production of hemoglobin S. This abnormal hemoglobin distorts red blood cells into a crescent or sickle shape when they lose oxygen. These sickle cells are less flexible and more prone to clumping together, blocking small blood vessels. This blockage, known as vaso-occlusion, leads to severe pain crises, tissue damage, and a cascade of complications, including anemia, organ damage, and stroke. Additionally, the sickle cells have a shorter lifespan, leading to chronic anemia and increased risk of infection.
Signs and symptoms ▾
- Symptoms appear after 6 months of age when hemoglobin switching occurs
- Acute problems in infants and children i.e. severe infections, acute chest syndrome, splenic sequestration and stroke
- Generalized impairment of growth and development in children with chronic hypoxia
- Chronic organ damage in adults
Sickle cell crisis ▾
- Sickling crises (vaso-occlusive / painful / infarctive)
- Hemolytic crises (marked increase in hemolysis)
- Aplastic crises (associated with parvovirus B19 infection)
- Sequestration crises (sudden trapping of blood in the spleen or liver leading to rapid enlargement of the organ and drop in hematocrit resulting in hypovolemic shock)
Confirmatory / Diagnostic tests ▾
- Sickling test
- Hemoglobin electrophoresis
- High performance liquid chromatography (HPLC)
- DNA mutation test (PCR)
- Peripheral blood smear (screening)
Laboratory diagnosis of sickle cell anemia
- Full blood count: Moderate anemia, increased reticulocyte count
- Peripheral blood smear: Moderate anisopoikilocytosis, polychromatic cells, sickled erythrocytes
- ↑ Unconjugated bilirubin
- ↑ Urine urobilinogen
- ↓ Serum haptoglobin
- Pigment bilirubin gallstones
- Cholecystitis
Sickle cell anemia treatment and management ▾
- Prophylactic penicillin
- Pneumococcal and meningococcal vaccine
- Avoid dehydration, hypoxia and circulatory stasis
- Active treatment for bacterial infections
- Oral / IV fluids, analgesics, oxygen, exchange transfusion and ventilatory support during painful crises
- Transfusion when necessary
- Bone marrow transplantation
- Regular cerebral blood flow surveillance and prophylactic transfusions
- Hydroxyurea for adults
- Splenectomy in splenic sequestration crises
- Gene therapy
*Click ▾ for more information
What is sickle cell anemia?
Sickle cell anemia is a hereditary chronic hemolytic anemia caused by the production of Hb S as a result of a mutation in codon 6 of the beta globin gene. This mutation causes the hemoglobin to sickle when the red cell is deoxygenated.
What is the function of the red blood cell?
The main function of the red blood cell is to carry oxygen from the lungs to the tissues and then transport carbon dioxide back from the tissues to be expelled through the lungs. For the red blood cell to function, it depends on the red blood cell metabolism pathways, the membrane structure as well as the hemoglobin content. As the main function of the red blood cell is as an oxygen carrier, a mature erythrocyte is essentially a bag full of hemoglobins.
In a healthy adult, there are approximately 270 million hemoglobins in 1 red blood cell. The hemoglobin is a tetrameric structure of 2 alpha-like and 2 beta-like globin chains interconnected by specific points and each folded globin chain carries a heme peptide with an iron atom in the center. Each iron atom can carry 1 molecule of oxygen.
The alpha globin gene cluster is found on chromosome 16 while the beta globin gene cluster is found on chromosome 11. The genes in the cluster are arranged according to the order of development and at different stages of development, different hemoglobin subtypes are formed to have different oxygen affinities. For example, fetal hemoglobin or Hb F have a higher oxygen affinity compared to Hb A to allow for oxygen exchange between the mother and the developing fetus. However, Hb F switches to Hb A (at around 6 months after birth) which is more efficient in oxygen delivery once the baby is born.
What are hemoglobin disorders?
Disorders of the hemoglobin or hemoglobinopathies are mutations affecting the globin genes and are usually inherited. They can be loosely classified into structural hemoglobinopathies which is a qualitative disorder of the hemoglobin for example Hb S. In structural hemoglobinopathies, the hemoglobins formed are not able to carry oxygen as efficiently as the normal hemoglobins. The other group of hemoglobinopathy is thalassemia which is a quantitative reduction or absence of one or more of the globin chain production.
Pathogenesis of sickle cell anemia
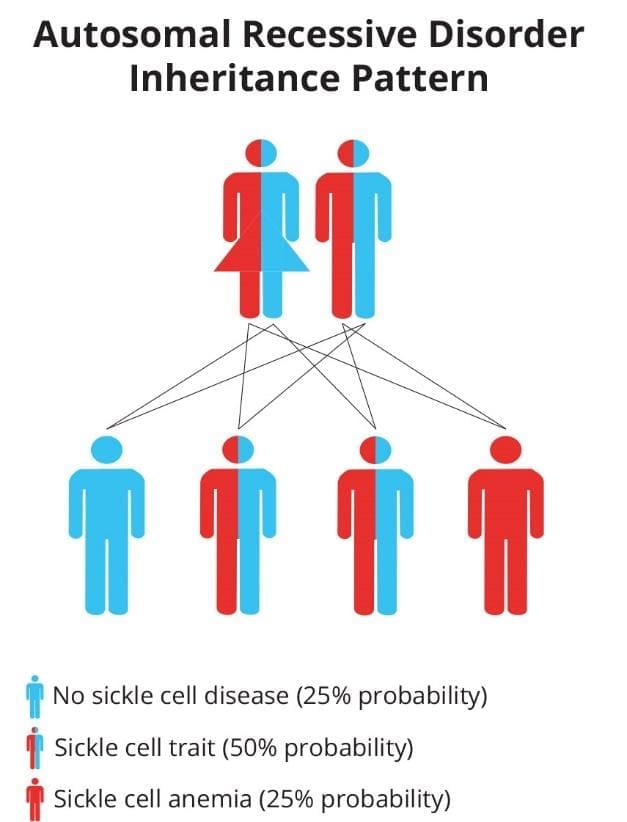
Genetically speaking, hemoglobinopathies are mainly autosomal recessive disorders where you will need two mutant alleles to be clinically symptomatic. Sickle cell trait or carriers are generally asymptomatic.
Sickle cell disease is common among the Africans and also the Indians. The sickle red cells confer some protective mechanism against malaria and has allowed this faulty gene to persist historically. Even just one copy of the sickle gene (sickle cell trait) is enough to offer some resistance towards malaria.
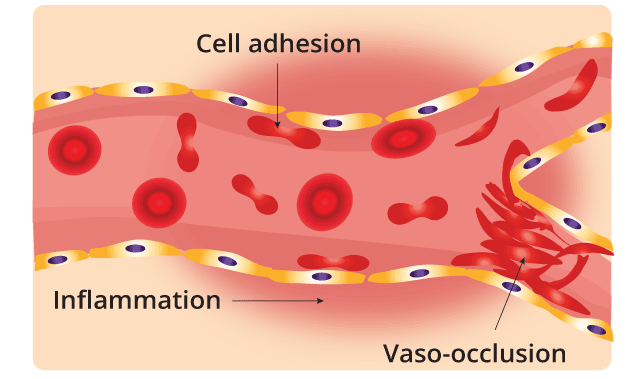
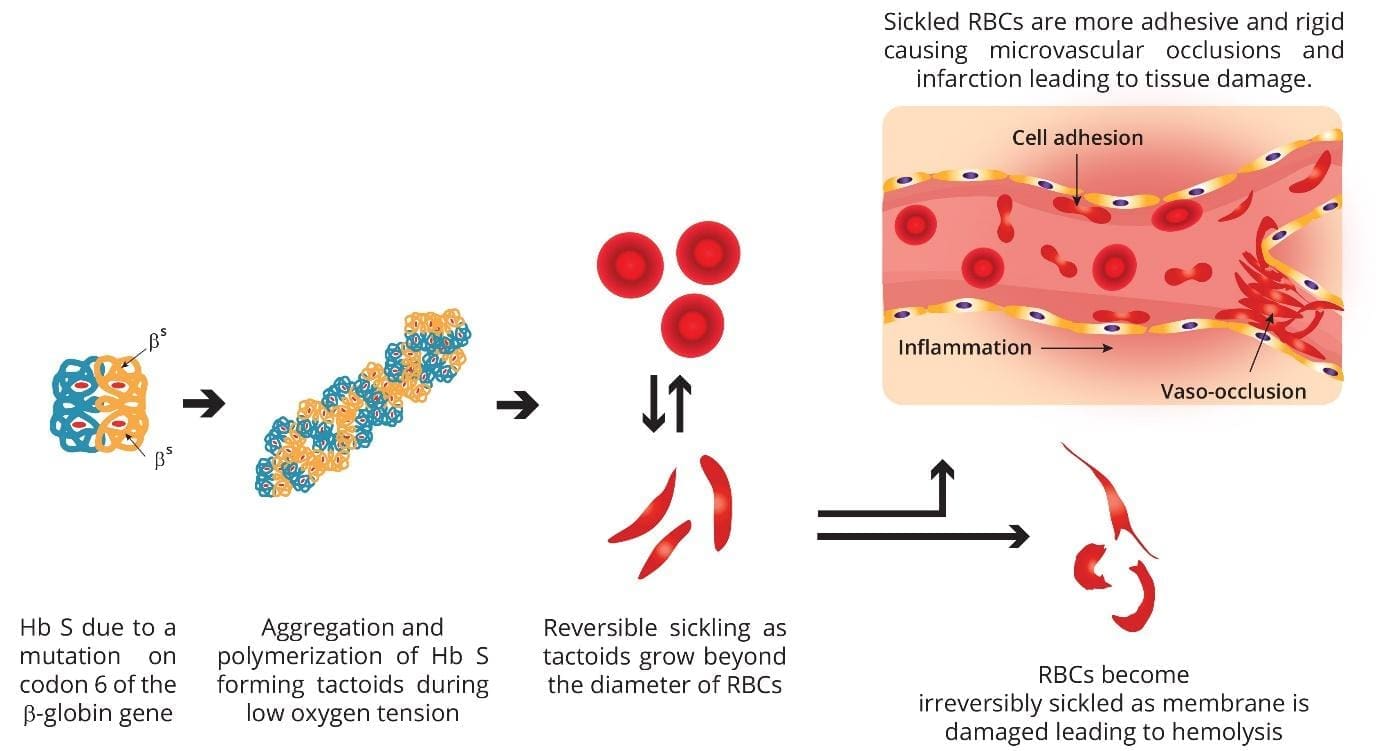
Sickle cell anemia is caused by a mutation in codon 6 of the beta globin gene, where the wild type glutamic acid has been changed to valine. Hemoglobin S (Hb S) is insoluble and forms crystals when exposed to low oxygen tension. Deoxygenated sickle hemoglobins tend to clump and polymerize into long fibers called tactoids. Initially, when the sickle hemoglobins clump together to form tactoids during the deoxygenation phase, the red blood cells are still reversibly sickled. But as more and more Hb S forms the long fibers, the red cells become irreversibly sickled and are unable to revert back to its biconcave shape. These red cell membranes are sticky and cause microvascular occlusions. They also cause vascular inflammation when they adhere to the vascular walls and in the spleen, there is a lot of hemolysis, congestion and infarction as these cells accumulate in the reticuloendothelial system to be destroyed.
Pathophysiology
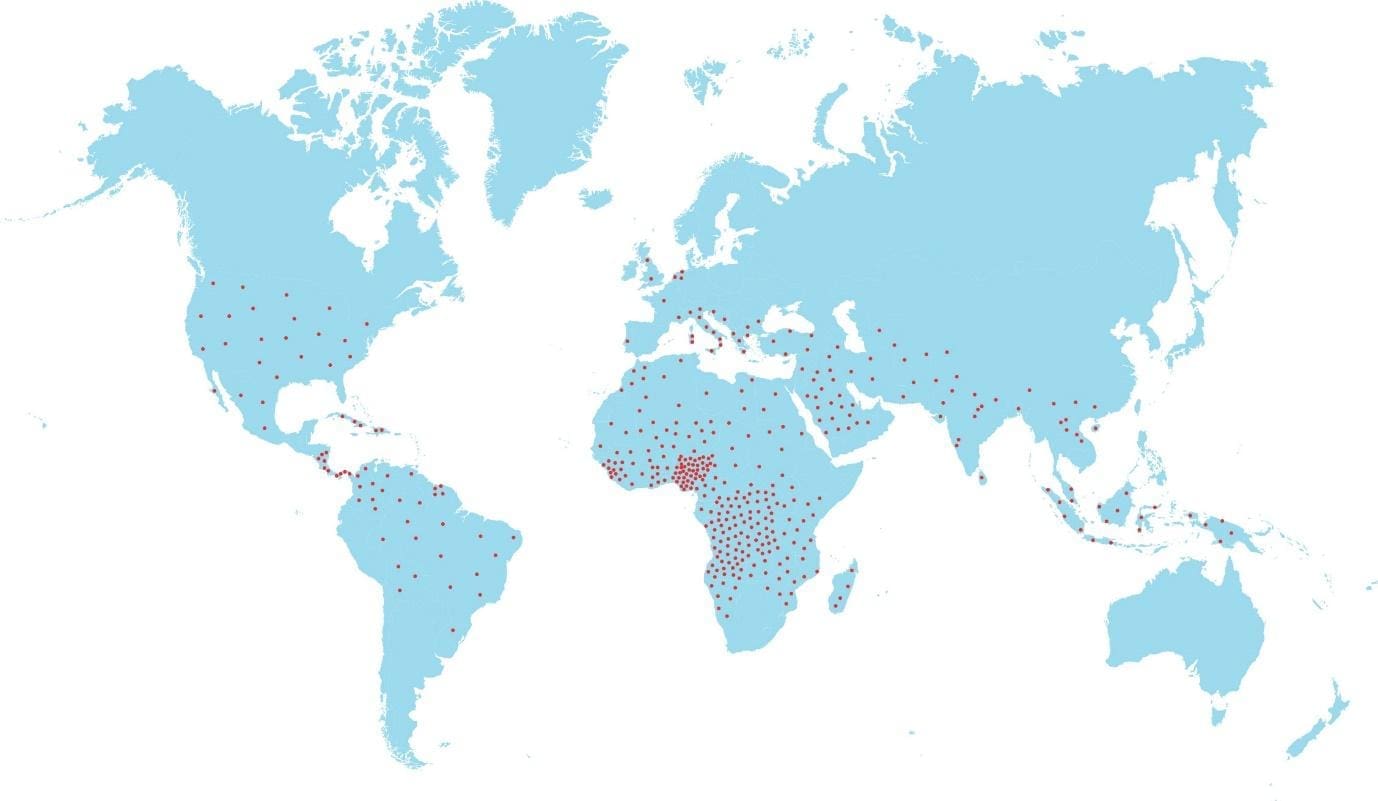
Epidemiology
Most frequent in Africa, Middle East and India.
Inheritance pattern
Autosomal recessive disorder.
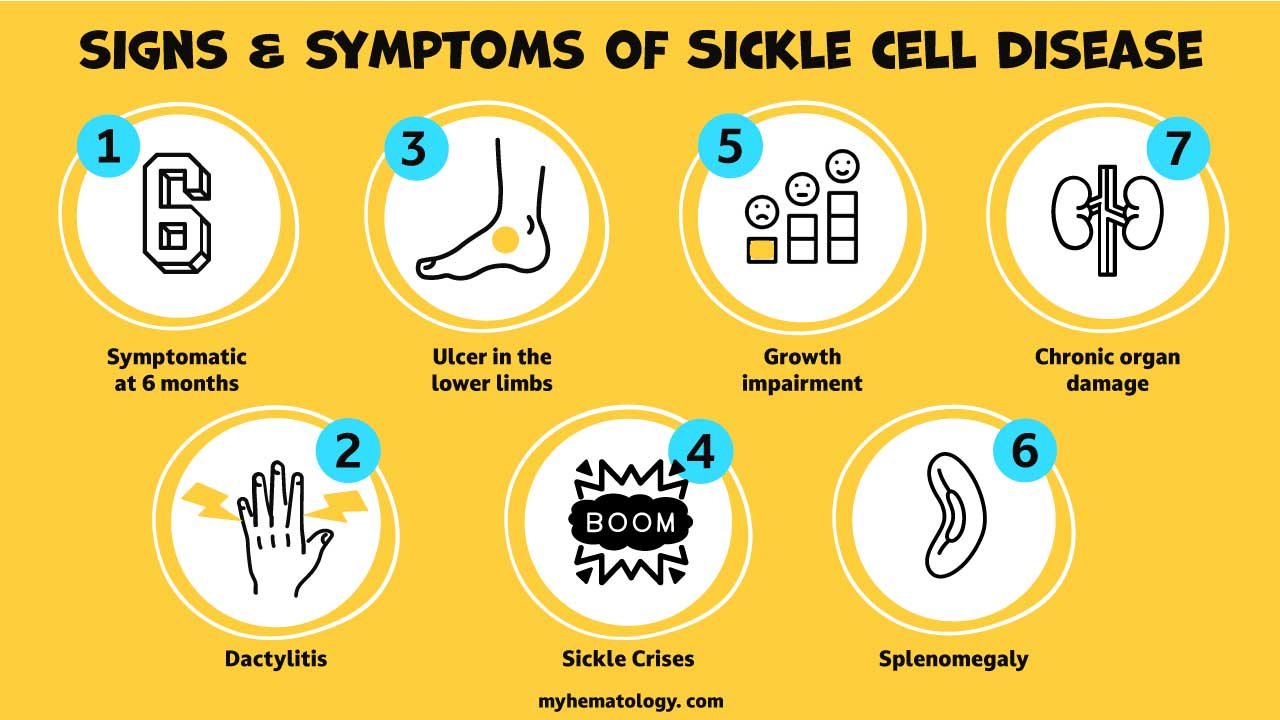
What are the signs and symptoms of sickle cell anemia?
Signs and symptoms of sickle cell anemia include
- Manifestations of sickle cell anemia symptoms 6 months after birth with hemoglobin switching predominantly from Hb F to Hb A, the adult hemoglobin.
- Acute problems in children for example painful swollen fingers or dactylitis or ulcers of the lower legs and splenomegaly but is often reduced in size later as a result of infarcts or autosplenectomy.
- The clinical expression of HbSS is very variable, some patients having an almost normal life, free of crises but others develop severe crises even as infants and may die in early childhood or as young adults.
- Chronic damage to the organs for example the kidneys or the liver and
- Growth impairment.
What are the complications of sickle cell anemia?
The hallmark of sickle cell anemia is severe hemolytic anemia punctuated by sickle cell crisis.
Sickle cell crisis may be vaso-occlusive, visceral, aplastic or hemolytic. Painful vaso-occlusive sickle cell crises are most frequent and are precipitated by infection, acidosis, dehydration or deoxygenation for example, altitude, surgeries, deliveries, circulation stasis, exposure to cold or violent exercise. Infarcts can occur in any organs including bones, lungs and spleen. The most serious vaso-occlusive sickle cell crisis is of the brain causing a stroke or the spinal cord.
Visceral sequestration crises are caused by sickling within organs and pooling of blood, often with a severe exacerbation of anemia. Severe complications include acute sickle chest syndrome which is the most common cause of death after puberty.
Splenic sequestration is usually seen in infants and presents with splenomegaly, falling hemoglobin and abdominal pain. Attacks tend to be recurrent and splenectomy is often needed.
Aplastic crises occur as a result of infection with parvovirus or from folate deficiency and are characterised by a sudden fall in hemoglobin which usually requires transfusion.
Hemolytic crises are characterised by an increased rate of hemolysis with a fall in hemoglobin but a rise in reticulocytes and usually accompany a painful crisis.
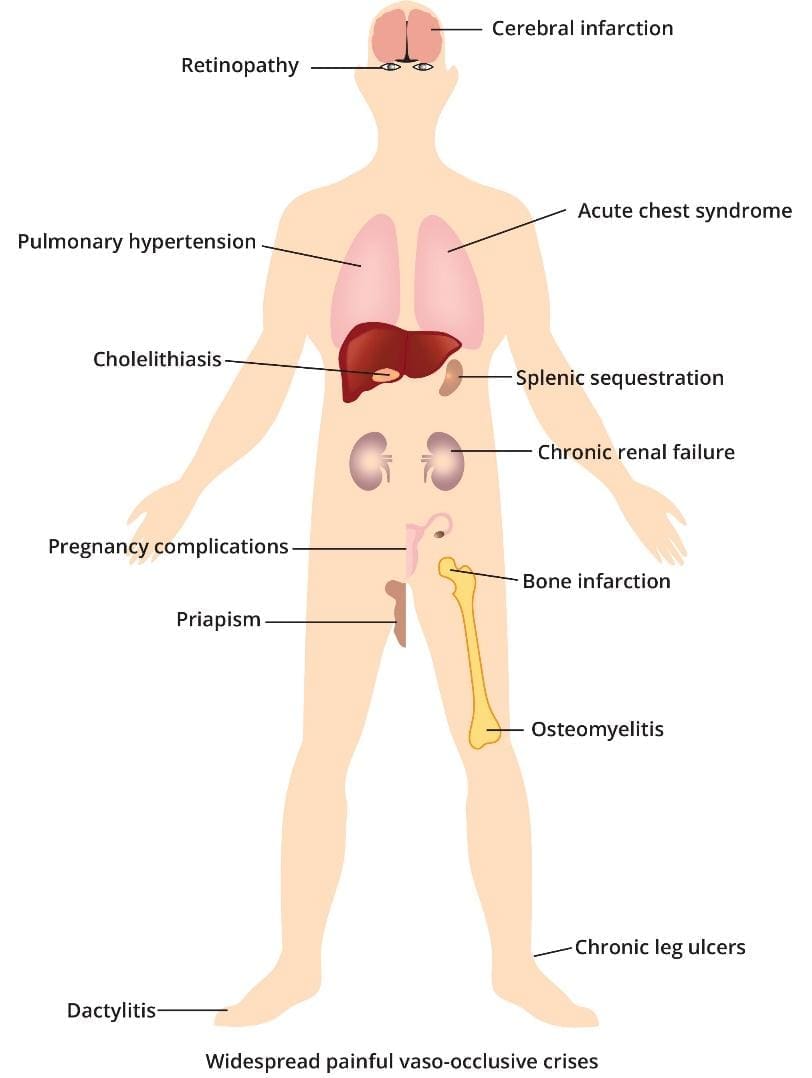
Complications of sickle cell anemia include acute chest pains when certain areas of the chest have their blood supply cut off, infections as the spleen is overwhelmed with destroying the abnormal red cells and also autosplenectomy when the spleen dies due to lack of blood supply. Stroke when certain parts of the brain do not receive enough blood and the same goes for organ failures like kidney failure, ulcers, eye damage and many more. All depends on where the microvessels get blocked or occluded. Other complications include brain infarcts, retinopathy, pulmonary hypertension, acute chest syndromes, cholelithiasis, autosplenectomy, chronic renal failure, obstetric complications, priapism, bone infarcts, osteomyelitis, chronic leg ulcers, dactylitis and generally widespread painful vaso-occlusive crises.
How do I test for sickle cell anemia?
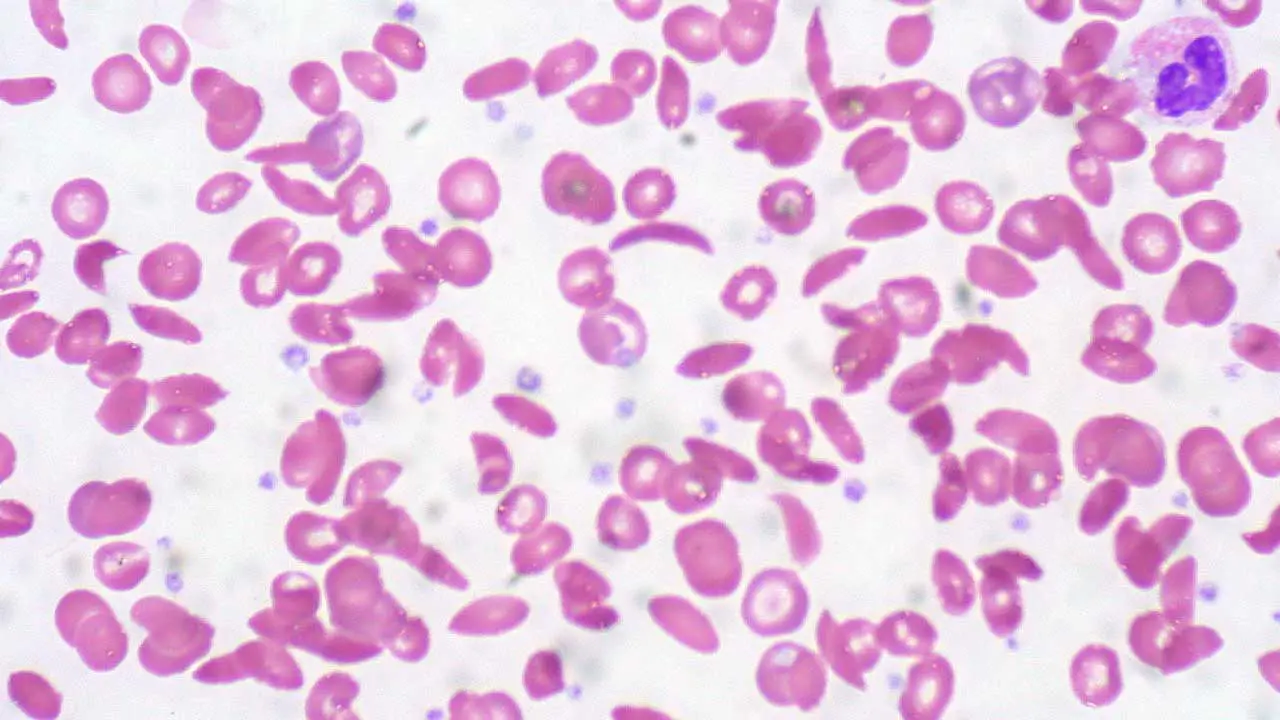
There will be a positive sickle solubility test. Elevated bilirubin and raised serum lactate dehydrogenase when there is high red cell destruction in a crisis. PCR will show a positive sickle mutation and infarcts like the brain infarcts can be detected using the Doppler ultrasonography. Hemoglobin electrophoresis and high performance liquid chromatography will reveal presence of hemoglobin S and also an elevated Hemoglobin F.
Newborn Screening
This is a routine screening for all newborns in many countries. It involves a simple heel prick blood test and often utilizes a combination of methods
- Hemoglobin Electrophoresis: This separates different hemoglobin types based on electrical charge. A normal result shows predominantly HbA (adult hemoglobin) with minimal or no HbS (sickle hemoglobin) in sickle cell trait, while sickle cell anemia will show a significant amount of HbS and also HbF.
- High-Performance Liquid Chromatography (HPLC): Similar to electrophoresis, this technique separates and quantifies hemoglobin types with high accuracy. The chromatogram will reveal presence of HbS and also an elevated HbF.
- Isoelectric Focusing: Another technique separating hemoglobin based on their electrical properties, sometimes used in conjunction with other methods.
Complete Blood Count (CBC)
This measures various blood cell parameters, including:
- Red Blood Cell (RBC) Count: May be lower than normal in SCD due to destruction of sickled cells.
- Hemoglobin Levels: May be lower than normal due to decreased RBC count.
- Mean Corpuscular Volume (MCV): RBC size indicator. Sickled cells sizes are variable, could be normal or slightly smaller.
- Reticulocyte Count: Measures immature red blood cells. May be elevated due to the body’s attempt to compensate for the destruction of sickled cells.
Hemoglobin Solubility Test (Dithionite Test)
A rapid screening test using sodium dithionite to remove oxygen from red blood cells.
- Positive result: Sickled-shaped erythrocytes as well as sickle-shaped erythrocytes with spikes.
- While a valuable initial screening tool, it’s not definitive and needs confirmation with other tests.
Molecular Hemoglobin Gene Analysis
Direct genetic testing can definitively identify the specific mutation responsible for HbS. This test can be performed prenatally or postnatally.
Doppler Ultrasonography for Brain Infarcts
Cerebral infarction, also known as a stroke, is a potential complication of SCD due to blocked blood vessels. Doppler ultrasonography can be used to evaluate blood flow in the brain arteries, but it’s not a first-line diagnostic tool for SCD itself.
- Role in SCD: Regular screening with Doppler ultrasound specifically for brain infarcts is not typically recommended for all SCD patients. However, it may be used in certain situations:
- If a patient experiences symptoms suggestive of a stroke (sudden weakness, numbness, speech difficulties, etc.).
- If a patient has a history of recurrent transfusions, which can increase the risk of stroke.
How is sickle cell anemia treated?
In spite of all the pain, the treatment of sickle cell anemia is largely symptomatic as it is not currently curable. Patients are given prophylactic oral penicillin to prevent infection, and told to always hydrate themselves and given painkillers, oxygen and antibiotics in painful crises.
Management of Sickle Cell Anemia Symptoms and Complications
- Pain Management: Painful episodes (crises) are a hallmark of sickle cell disease. Medications like opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and lidocaine can help manage pain during these episodes.
- Hydration: Drinking plenty of fluids helps prevent dehydration, a trigger for sickling crises.
- Folic Acid Supplementation: Folic acid helps the body produce healthy red blood cells, which is crucial in sickle cell disease.
- Hydroxyurea: This medication stimulates the production of fetal hemoglobin, which inhibits sickling. It can reduce the frequency of painful crises and the need for blood transfusions.
- Antibiotics: People with sickle cell disease are more susceptible to infections. Prophylactic antibiotics, especially in early childhood, can help prevent infections.
Blood Transfusions
- Only case by case basis depending on requirement.
- In severe cases or during crises, blood transfusions can be used to replace sickled red blood cells with healthy ones. This can improve oxygen delivery and alleviate symptoms.
- However, frequent transfusions can lead to iron overload, requiring additional chelation therapy to remove excess iron from the body.
Bone Marrow Transplant
- This is the only curative option available, but it’s a complex procedure with significant risks. It involves replacing the patient’s bone marrow with healthy bone marrow from a matched donor. This approach is typically reserved for severe cases when other treatments are not effective or when a suitable gene therapy option is unavailable.
Gene Therapy
- Casgevy™ is a recently approved gene therapy that offers a potential cure. It uses CRISPR-Cas9 technology to edit a patient’s own blood stem cells to reactivate the fetal hemoglobin production, thus reducing the occurrence of vaso-occlusive crises.
- Lyfgenia™, also recently approved, uses a viral envelope to deliver a healthy hemoglobin-producing gene.
- However, gene therapy comes with a heft price tag of USD $2 – 3 million each shot.
Frequently Asked Questions (FAQs)
Can sickle cell anemia be cured?
Currently, there is no cure for sickle cell anemia. However, there are effective treatment options that can significantly improve the lives of people with the disease. These treatments focus on managing symptoms, preventing complications, and potentially offering a chance for a cure in some cases.
Can sickle cell patient live long?
Sickle cell disease (SCD) can significantly impact life expectancy, but advancements in treatment have improved the outlook for patients.
Life Expectancy
- Traditionally, the average life expectancy for individuals with SCD was lower than the general population.
- Recent studies indicate some improvement, with the national median life expectancy in the US for publicly insured individuals with SCD at around 52.6 years (compared to the national average of 73.5 years for men and 79.3 years for women).
Factors Affecting Life Expectancy
- Severity of SCD: The specific type of SCD and the proportion of abnormal red blood cells can influence life expectancy. More severe forms with frequent complications generally have a lower life expectancy.
- Access to healthcare: Early diagnosis, comprehensive treatment, and good management of complications are crucial for maximizing life expectancy.
- Lifestyle factors: Maintaining a healthy lifestyle with proper hydration, a balanced diet, regular exercise (as tolerated), and avoiding smoking can significantly improve overall health and potentially lengthen lifespan.
What can trigger sickle cell crisis?
Sickle cell crisis occurs when the abnormal, sickle-shaped red blood cells clump together and block blood flow in small blood vessels. This blockage can cause pain, tissue damage, and a variety of complications. Several factors can trigger a sickle cell crisis, although the exact cause may not be identifiable in every instance. Here are some of the most common triggers:
- Infection: People with SCD are more susceptible to infections, and even minor infections can trigger a crisis.
- Dehydration: Not drinking enough fluids can thicken the blood and make it easier for sickle cells to clump.
- Pain: Pain itself can trigger a crisis, creating a vicious cycle.
- Stress: Physical or emotional stress can activate the body’s fight-or-flight response, leading to physiological changes that promote sickling.
- Sudden temperature changes: From hot to cold or vice versa can trigger a crisis.
- Low oxygen levels: This can occur at high altitudes or during strenuous exercise.
- Deoxygenation: Sickle cells are more prone to sickling when they’re not carrying oxygen. This can happen during sleep or anesthesia.
- Certain medications: Some medications can worsen sickling or interfere with other treatments. Discussing all medications with your doctor is crucial.
- Puberty and menstruation: Hormonal changes during these times might increase the risk of crises for some individuals.
It’s important to note that:
- Triggers can vary from person to person. Some people might have specific triggers they can identify, while others may experience crises without an obvious cause.
- Avoiding triggers whenever possible can help reduce the frequency and severity of crises.
Who is most at risk for sickle cell anemia?
People with a family history of sickle cell disease are most at risk for inheriting the condition.
- Genetic Inheritance: Sickle cell anemia is an autosomal recessive genetic disorder. This means a person needs to inherit two copies of the abnormal sickle cell gene, one from each parent. Inheriting only a single copy of the mutation leads to sickle cell trait.
- Ancestral Background: The sickle cell gene mutation is more common in people with ancestry from regions where malaria was historically prevalent. These regions include:
- Sub-Saharan Africa
- South Asia (India, Saudi Arabia)
- Mediterranean countries (Greece, Turkey, Italy)
- Hispanic populations in Central and South America
What is the difference between thalassemia and sickle cell anemia?
Both thalassemia and sickle cell anemia are inherited blood disorders that affect red blood cells, but they have distinct causes and effects.
| Feature | Thalassemia | Sickle Cell Anemia |
|---|---|---|
| Cause | Reduced hemoglobin production | Abnormal hemoglobin S (HbS) production |
| Genetic Defect | Mutation in alpha or beta globin genes | Mutation in beta globin gene |
| Red Blood Cells | Reduced number, may be slightly abnormal shape | Normal number, sickle-shaped when deoxygenated |
| Symptoms | Fatigue, weakness, pale skin, slow growth (severe) | Painful crises, tissue damage, increased infection risk |
| Complications | Enlarged spleen, bone deformities (less common) | Organ damage (lungs, spleen, liver, etc.) |
Is sickle cell painful?
Yes, sickle cell anemia is a very painful condition. Pain is one of the most common and distressing symptoms of sickle cell disease.
Here’s a breakdown of the pain in sickle cell anemia:
- Cause: The abnormal, sickle-shaped red blood cells can get stuck in small blood vessels, blocking blood flow and causing tissue oxygen deprivation. This lack of oxygen triggers pain signals.
- Painful Episodes (Crises): These are the hallmark of sickle cell disease and can occur unpredictably. They can last for hours, days, or even weeks.
- Location of Pain: Pain can occur anywhere in the body, but commonly affects the
- Chest
- Back
- Abdomen
- Arms and legs
- Joints
- Severity of Pain: Pain can vary from mild to severe and can be debilitating. Some people describe it as a constant dull ache, while others experience excruciating pain.
Additional factors contributing to pain
- Inflammation: The body’s response to the damaged and blocked blood vessels can further contribute to pain.
- Nerve damage: Chronic pain episodes can damage nerves, leading to increased pain sensitivity.
Why is iron not good for sickle cell patients?
Iron is not inherently bad for people with sickle cell disease, but excess iron due to transfusions and potential absorption issues can be detrimental. Excess iron acts like a toxin in the body, damaging organs like the liver, heart, and pancreas. This damage can lead to serious complications like liver fibrosis and cirrhosis, heart failure and diabetes. Careful management through chelation therapy, monitoring, and potentially dietary adjustments is crucial to prevent iron overload and its associated complications.
What are the first signs of sickle cell crisis?
The initial signs of a sickle cell crisis can vary depending on the individual and the type of crisis, but some common early indicators include:
- Pain: This is often the first and most prominent symptom. Pain can develop gradually or come on suddenly, and can occur anywhere in the body, although it frequently affects the:
- Chest (acute chest syndrome)
- Back
- Abdomen
- Arms and legs
- Joints
- Fatigue: Feeling unusually tired or sluggish can be an early sign, especially if accompanied by other symptoms.
- Shortness of breath: This can occur due to blocked blood flow to the lungs (acute chest syndrome) or simply due to fatigue.
- Fever: A low-grade fever can be an early indicator, but high fevers may also occur during a full-blown crisis.
- Headache: This is a common symptom, especially during a vaso-occlusive crisis (painful blockage of blood vessels).
What to do if you experience early signs of a crisis
- Rest: Take it easy and avoid strenuous activity.
- Hydration: Drink plenty of fluids to help thin your blood and improve circulation.
- Pain management: If you have pain medication prescribed by your doctor, take it as directed. However, don’t hesitate to seek medical attention, especially if the pain is severe or worsens.
- Warm or cold therapy: Applying a heating pad or cold compress to the affected area might provide some pain relief.
- Early intervention: Seeking medical attention at the first sign of a crisis can help prevent it from worsening and potentially reduce the duration and severity of the episode.
Does sickle cell get worse with age?
Yes, sickle cell disease (SCD) can worsen with age, but the progression and severity can vary depending on several factors.
Increased Complications
- As people with SCD age, they are more likely to experience complications from the disease. These complications arise due to chronic damage caused by repeated episodes of blocked blood vessels and hemolysis (destruction of red blood cells). Some common complications include:
- Organ damage: Organs like the lungs, liver, heart, kidneys, and spleen can be progressively damaged by recurrent sickling crises.
- Chronic pain: Frequent episodes of pain can become more chronic and debilitating over time.
- Increased risk of infections: Damage to the spleen and compromised immune function can make individuals with SCD more susceptible to infections.
- Pulmonary complications: Acute chest syndrome (blocked blood flow to the lungs) can become more frequent and severe with age.
- Stroke: Sickling can block blood flow to the brain, increasing the risk of stroke.
Cumulative Effects
- The longer someone lives with SCD, the greater the cumulative effect of the disease on their body. Tissue damage from repeated blockages and hemolysis can progressively worsen over time.
Does sickle cell cause blood clots?
Yes, people with sickle cell disease (SCD) are at a greater risk for developing blood clots compared to the general population because of:
- Sickled Red Blood Cells: The abnormal, crescent-shaped red blood cells in SCD are less flexible and more prone to clumping together. These clumps can block blood flow in small blood vessels, creating an environment conducive to clot formation.
- Chronic Inflammation: SCD is associated with chronic inflammation throughout the body. This inflammatory state can further contribute to blood clotting.
- Damaged Blood Vessel Walls: Repeated episodes of blocked blood flow can damage the inner lining of blood vessels, making them more susceptible to clot formation.
Does sickle cell compromise the immune system?
People with SCD are more prone to infections due to the weakened immune system. This is especially true for certain types of infections, such as those caused by bacteria encapsulated by a polysaccharide coat (e.g., Streptococcus pneumoniae). However, the hyperinflammatory response can also contribute to complications like vasoocclusive crises (painful episodes due to blocked blood flow).
Are sickle cell patients immune to malaria?
No, sickle cell disease (SCD) does not provide immunity to malaria. In fact, the relationship between SCD and malaria is complex and depends on the type of SCD a person has:
- Sickle cell trait: Offers some protection against malaria, especially the severe form.
- Sickle cell disease: Does not provide immunity and may increase the risk of severe complications from malaria.
How does sickle cell lead to death?
Sickle cell disease (SCD) can lead to death through various complications arising from the abnormally shaped red blood cells. Here’s a breakdown of the main causes:
Organ Damage
- Sickled red blood cells are rigid and sticky, causing them to get lodged in small blood vessels. This blockage can lead to:
- Organ damage: Over time, chronic blockage of blood flow can damage organs like the lungs, heart, kidneys, liver, and spleen. This damage can eventually lead to organ failure, which can be life-threatening.
- Acute chest syndrome (ACS): This complication arises when sickled red blood cells block blood vessels in the lungs, causing difficulty breathing, chest pain, and fever.
Infections
- People with SCD are more susceptible to infections due to a compromised immune system. This can be caused by:
- Damaged spleen: The spleen plays a vital role in filtering out bacteria from the bloodstream. In SCD, chronic damage to the spleen weakens its ability to fight infections.
- Reduced red blood cell production: Sickling and destruction of red blood cells can limit the body’s ability to produce enough healthy red blood cells, which can also carry oxygen-carrying proteins crucial for immune cell function.
- Severe infections, particularly those affecting the lungs (pneumonia) or bloodstream (sepsis), can be life-threatening in individuals with SCD.
Other Complications
- Pulmonary embolism: A blood clot that breaks loose and travels to the lungs, blocking blood flow and potentially leading to death.
- Stroke: Blocked blood flow to the brain can cause a stroke, leading to permanent neurological damage or death.
- Splenic sequestration: A large number of sickled red blood cells get trapped in the spleen, causing it to enlarge and potentially rupture, which can be a medical emergency.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000 May 1;151(9):839-45. doi: 10.1093/oxfordjournals.aje.a010288. PMID: 10791557.
- Schnog JB, Duits AJ, Muskiet FA, ten Cate H, Rojer RA, Brandjes DP. Sickle cell disease; a general overview. Neth J Med. 2004 Nov;62(10):364-74. PMID: 15683091.
- Aliyu ZY, Tumblin AR, Kato GJ. Current therapy of sickle cell disease. Haematologica. 2006 Jan;91(1):7-10. PMID: 16434364; PMCID: PMC2204144.
- Cisneros GS, Thein SL. Recent Advances in the Treatment of Sickle Cell Disease. Front. Physiol., Sec. Red Blood Cell Physiology Volume 11 – 2020 | https://doi.org/10.3389/fphys.2020.00435
- Williams TN, Thein SL. Sickle Cell Anemia and Its Phenotypes. Annu Rev Genomics Hum Genet. 2018 Aug 31;19:113-147. doi: 10.1146/annurev-genom-083117-021320. Epub 2018 Apr 11. PMID: 29641911; PMCID: PMC7613509.
- Steinberg MH, Forget BG, Higgs DR, Weatherall DJ. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (Cambridge Medicine) 2nd Edition. 2009.
- Bain, B.J. (2017). A beginner’s guide to blood cells. Wiley Blackwell.
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- https://www.yalemedicine.org/news/gene-therapies-sickle-cell-disease