TL;DR
Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic malignancies due to impaired maturation of myeloid progenitors leading to cytopenias and dysplasia of myeloid-lineage cells. Peak incidence > 70 years old.
Signs and symptoms ▾
- Fatigue, shortness of breath
- Neutropenia
- Thrombocytopenia
Causes ▾
- Sporadic
- DNA-damaging agents e.g. radiation, chemotherapy, toxins
Laboratory Investigations ▾
- PBF: Dysplasia of myeloid lineage including erythroblasts with misshapen nucleus, ringed sideroblasts, bilobed neutrophils or abnormal cytoplasmic granules or multinucleated or small megakaryocytes
Treatment and management ▾
- Allogeneic stem cell transplantation
- Lenalidomide for MDS with anemia and 5qdel only
- Many patients progress to AML
*Click ▾ for more information
Introduction
Myelodysplastic syndromes (MDS) encompass a diverse group of hematopoietic neoplasms caused by acquired mutations that disrupt the normal maturation of myeloid progenitors, leading to cytopenias in the peripheral blood. In myelodysplastic syndrome (MDS), hematopoiesis is ineffective and the cells produced are often malformed or dysplastic. Myelodysplastic syndrome (MDS) is primarily confined to the elderly, with a peak incidence in individuals more than 70 years of age.
Myelodysplastic Syndrome (MDS) Symptoms
Most patients with myelodysplastic syndrome (MDS) present with vague symptoms related to the slow onset of anemia, neutropenia, or thrombocytopenia as the disease progression is not acute. Anemia can manifest as fatigue, shortness of breath, pale skin, and dizziness. Additionally, patients may experience easy bruising and excessive bleeding due to a lack of functional platelets. In severe cases, recurrent infections may occur as a consequence of neutropenia.
Some other myelodysplastic syndrome (MDS) individuals may experience vague, non-specific symptoms such as weight loss, fatigue, and fever. These seemingly trivial symptoms, when considered in conjunction with other factors like age and family history, can raise suspicion of underlying myelodysplastic syndrome (MDS).
Causes Related to Myelodysplastic Syndrome (MDS)
Myelodysplastic syndrome (MDS) arises sporadically, but some cases are related to exposure to DNA-damaging agents. While the exact mechanisms are still under investigation, several key contributors have emerged as leading suspects.
1. Acquired genetic mutations: The most significant culprit in myelodysplastic syndrome (MDS) is the presence of acquired genetic mutations within blood stem cells. These mutations disrupt the normal process of blood cell development, leading to abnormal and dysfunctional cells. Age is a major risk factor for acquiring these mutations, as the accumulation of genetic errors over time increases the likelihood of developing myelodysplastic syndrome (MDS).
2. Exposure to certain toxins and chemicals: Certain environmental exposures have been linked to an increased risk of developing myelodysplastic syndrome (MDS). These include:
- Benzene: a chemical found in gasoline and tobacco smoke
- Pesticides and herbicides: commonly used in agriculture and gardening
- Radiation: exposure to high doses of radiation, such as during cancer treatment
3. Previous cancer treatment: Individuals who have undergone chemotherapy or radiation therapy for other cancers are at a higher risk of developing myelodysplastic syndrome (MDS) as a secondary complication. This risk is related to the damage these treatments can inflict on bone marrow and blood stem cells.
4. Certain inherited conditions: While uncommon, some genetic disorders can increase the risk of developing myelodysplastic syndrome (MDS). These include Fanconi anemia, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome.
5. Smoking: Tobacco smoking is a well-established risk factor for various cancers, and it is also associated with an increased risk of myelodysplastic syndrome (MDS). This is likely due to the presence of harmful chemicals in tobacco smoke that can damage DNA and contribute to the development of cancer and other blood disorders.
6. Unknown factors: In many cases, the specific cause of myelodysplastic syndrome (MDS) remains elusive. Several other factors, such as dietary deficiencies, autoimmune disorders, and viral infections, are being investigated for their potential role in the development of this disease.
WHO Classifications of Myelodysplastic Syndrome (MDS) Based on Morphology
The World Health Organization (WHO) classification system for myelodysplastic syndromes (MDS) utilizes morphology, meaning the microscopic appearance of blood and bone marrow cells, as a key diagnostic and prognostic tool. This approach provides a framework for understanding the diverse presentations of MDS and guiding therapeutic decisions.
MDS with Single Lineage Dysplasia (MDS-SLD)
- Characterized by dysplasia affecting only one cell line: erythroid, myeloid, or megakaryocytic.
- Subtypes include:
- RA (refractory anemia): affecting erythroid lineage.
- RC (refractory cytopenia): affecting either the myeloid or megakaryocytic lineage.
- 5q- syndrome: characterized by isolated del(5q) cytogenetic abnormality and dysplastic erythropoiesis.
MDS with Multilineage Dysplasia (MDS-MLD)
- Features dysplasia in two or more cell lines.
- Requires at least 10% dysplastic cells in at least two lineages to meet diagnostic criteria.
MDS with Ring Sideroblasts (MDS-RS)
- Defined by the presence of ring sideroblasts, which are red blood cell precursors with iron deposits surrounding the nucleus.
- This subtype is further categorized based on the percentage of ring sideroblasts:
- MDS-RS with single lineage dysplasia (RS-SLD): <50% ring sideroblasts and dysplasia in only one lineage.
- MDS-RS with multilineage dysplasia (RS-MLD): <50% ring sideroblasts and dysplasia in two or more lineages.
- MDS-RS with ring sideroblasts and thrombocytosis (RS-T): >50% ring sideroblasts and thrombocytosis.
MDS with Excess Blasts (MDS-EB)
- Defined by the presence of 5-9% blasts in the bone marrow.
- Subtypes include:
- MDS-EB1: 5-9% blasts and dysplasia in one or more cell lines.
- MDS-EB2: 5-9% blasts with multilineage dysplasia and Auer rods (abnormal cytoplasmic granules) in blasts.
MDS with Myelofibrosis (MDS/MF)
- Occurs when MDS co-exists with myelofibrosis, a condition characterized by excessive bone marrow fibrosis.
Unclassifiable MDS (MDS-U)
- Cases that do not meet the criteria for any other MDS subtype.
Benefits of WHO Classification
- Provides a standardized approach to diagnosis and prognostication.
- Guides therapeutic decisions.
- Improves communication and collaboration among healthcare professionals.
Limitations of WHO Classification
- Relies primarily on morphology, which can be subjective and prone to observer bias.
- May not fully capture the molecular heterogeneity of MDS.
Myelodysplastic Syndrome (MDS) Defining Abnormalities
Myelodysplastic syndrome (MDS)-defining abnormalities are specific genetic mutations or chromosomal changes that play a significant role in the development and progression of myelodysplastic syndromes (MDS). These abnormalities help differentiate myelodysplastic syndrome (MDS) from other blood disorders and influence prognosis and treatment decisions.
Here are some of the most common MDS-defining abnormalities:
Cytogenetic abnormalities
- Deletion 5q: This is the most common and well-characterized myelodysplastic syndrome (MDS)-defining abnormality. It is associated with a relatively favorable prognosis and can be treated with less intensive therapies.
- Deletion 20q: This abnormality is also associated with a relatively good prognosis and can be responsive to immune-modulating agents.
- Trisomy 8: This abnormality is associated with an intermediate prognosis and may require more aggressive treatment.
- Monosomy 7/Deletion 7q: This abnormality is associated with a poor prognosis and often requires stem cell transplantation.
- Complex karyotype: This refers to the presence of multiple chromosomal abnormalities and is associated with a poor prognosis.
Gene mutations
- SF3B1 mutations: These mutations are the most common genetic mutations in myelodysplastic syndrome (MDS) and are associated with a favorable prognosis. They may be responsive to specific targeted therapies.
- TP53 mutations: These mutations are associated with a poor prognosis and often indicate a higher risk of progression to acute myeloid leukemia (AML).
- TET2 mutations: These mutations are relatively common in myelodysplastic syndrome (MDS) and are associated with a variable prognosis.
- ASXL1 mutations: These mutations are associated with a poor prognosis and may be indicative of a higher risk of AML.
It’s important to note that the presence of an myelodysplastic syndrome (MDS)-defining abnormality does not necessarily mean that a person will develop myelodysplastic syndrome (MDS). However, these abnormalities are significant risk factors for the disease and should be considered in the context of other clinical features when making a diagnosis.
The identification of myelodysplastic syndrome (MDS)-defining abnormalities plays a crucial role in the management of myelodysplastic syndrome (MDS) by:
- Providing prognostic information: Helping to predict the course of the disease and guide treatment decisions.
- Identifying patients eligible for targeted therapies: Some targeted therapies are only effective for patients with specific genetic mutations.
- Informing clinical trial enrolment: Patients with certain abnormalities may be eligible to participate in clinical trials testing new therapies.
Classification Summary
| Classification | Dysplastic lineages | Cytopenias | Bone marrow (BM) and peripheral blood (PB) blasts | Cytogenetics |
| MDS with single lineage dysplasia | 1 | 1 or 2 | BM <5%, PB <1%, no Auer rods | Any, unless meets criteria for MDS with isolated (del)5q |
| MDS with multilineage dysplasia | 2 or 3 | 1 – 3 | BM <5%, PB <1%, no Auer rods | Any, unless meets criteria for MDS with isolated (del)5q |
| MDS with isolated del(5q) | 1 – 3 | 1 -2 | BM <5%, PB <1%, no Auer rods | (del)5q alone or with 1 additional abnormality except -7 or del(7q) |
| MDS with ring sideroblasts (MDS-RS) | ||||
| MDS-RS with single lineage dysplasia | 1 | 1 or 2 | BM <5%, PB <1%, no Auer rods | Any, unless meets criteria for MDS with isolated (del)5q |
| MDS-RS with multilineage dysplasia | 2 or 3 | 1 – 3 | BM <5%, PB <1%, no Auer rods | Any, unless meets criteria for MDS with isolated (del)5q |
| MDS with excess blasts (MDS-EB) | ||||
| MDS-EB-1 | 0 – 3 | 1 – 3 | BM 5-9% or PB 2-4%, no Auer rods | Any |
| MDS-EB-2 | 0 – 3 | 1 – 3 | BM 10-19% or PB 5-19%, Auer rods | Any |
| MDS, unclassifiable (MDS-U) | ||||
| MDS-U with 1% blood blasts | 1 – 3 | 1 – 3 | BM <5%, PB <1%, no Auer rods | Any |
| MDS-U with single lineage dysplasia and pancytopenia | 1 | 3 | BM <5%, PB <1%, no Auer rods | Any |
| MDS-U based on defining cytogenetic abnormality | 0 | 1 – 3 | BM <5%, PB <1%, no Auer rods | MDS-defining abnormality* |
| Refractory cytopenia of child | 1 – 3 | 1 – 3 | BM <5%, PB <2% | Any |
*MDS-defining abnormality:
- Loss of chromosome 7 or del(7q)
- del(5q)
- Isochromosome 17q or t(17p)
- Loss of chromosome 13 or del(13q)
- del(11q)
- del(12p) or t(12p)
- del(9q)
- idic(X)(q13)
- t(11;16)(q23.3;p13.3)
- t(3;21)(q26.2;q22.1)
- t(1;3)(p36.3;q21.2)
- t(2;11)(p21;q23.3)
- inv(3)(q21.3;q26.2)/ t(3;3)(21.3;q26.2)
- t(6;9)(p23;q34.1)
Laboratory Investigations Related to MDS
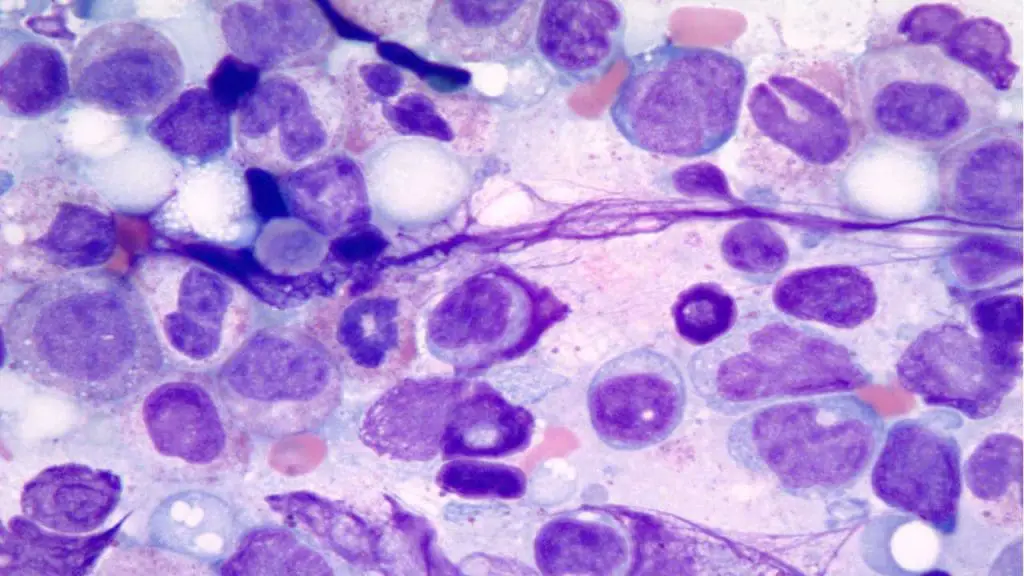
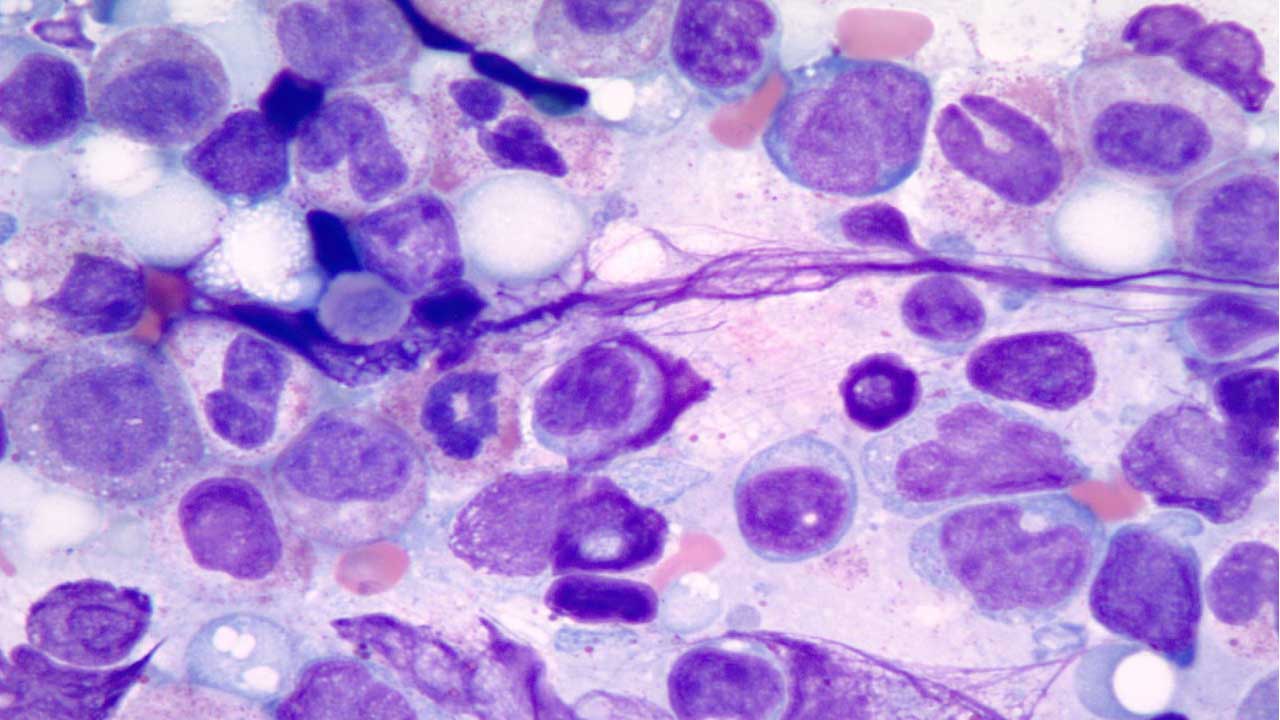
The diagnostic hallmark of this disorder is dysplasia which can affect all three lineages or just one or two.
1. Complete Blood Count (CBC): Presence of anemia, neutropenia or thrombocytopenia. It also helps in assessing the severity of cytopenias, which plays a crucial role in determining the appropriate course of treatment.
2. Peripheral Blood Smear: Dysplastic changes in red blood cells (macrocytosis, poikilocytosis), white blood cells (hypogranularity, vacuoles), and platelets (hypogranularity, giant platelets) can be observed, providing valuable clues to the diagnosis of myelodysplastic syndrome (MDS).
3. Bone Marrow Aspiration and Biopsy: Characteristic dysplastic findings include erythroid elements with misshapen nuclei or ring sideroblasts, neutrophils with only 2 nuclear lobes or abnormal cytoplasmic granulation, and multinucleated or small megakaryocytes with simple nuclei.
4. Cytogenetic Analysis: Specific chromosomal abnormalities, such as del(5q) or trisomy 8, can provide prognostic information and guide treatment decisions.
5. Flow Cytometry and Immunophenotyping: By employing flow cytometry and immunophenotyping techniques, researchers can identify, quantify, and characterize distinct populations of hematopoietic cells at various stages of development within the bone marrow and peripheral blood.
6. Molecular Testing: The presence of mutations in genes like SF3B1, TP53, and TET2 can influence prognosis and guide the selection of targeted therapies.
Hallmark Characteristics of Myelodysplastic Syndrome (MDS) in Laboratory Investigations:
- Cytopenias in one or more cell lines (anemia, neutropenia, thrombocytopenia)
- Dysplastic changes in blood and bone marrow cells
- Specific chromosomal abnormalities
- Gene mutations associated with MDS
Prognosis of Myelodysplastic Syndrome (MDS)
The International Prognostic Scoring System (IPSS) and its revised version, IPSS-R (Revised IPSS), are two important tools used to assess the prognosis and guide treatment decisions in patients with myelodysplastic syndromes (MDS). Both systems assign risk scores based on specific clinical and laboratory features, allowing for a more objective and standardized approach to risk stratification.
IPSS
Developed in 1997, the IPSS relies on three factors to predict overall survival:
- Cytopenia: Number of cytopenias (anemia, neutropenia, thrombocytopenia)
- Blast percentage: Percentage of immature blast cells in the bone marrow
- Cytogenetic risk category: Based on specific chromosomal abnormalities
Patients are assigned points based on these factors, and the total score determines their risk group:
- Very low risk: 0 points
- Low risk: 0.5-1 points
- Intermediate-1 risk: 1.5-2 points
- Intermediate-2 risk: 2.5-3 points
- High risk: ≥3.5 points
IPSS-R
Published in 2012, the IPSS-R incorporates additional factors to refine risk stratification:
- Sideroblasts: Percentage of ring sideroblasts in the bone marrow
- Serum lactate dehydrogenase (LDH) level: An indicator of disease activity
- Karyotype complexity: Presence of multiple chromosomal abnormalities
The IPSS-R assigns points based on these factors and classifies patients into five risk groups:
- Very low risk: ≤1.5 points
- Low risk: >1.5-3 points
- Intermediate risk: >3-4.5 points
- High risk: >4.5-6 points
- Very high risk: >6 points
Benefits of IPSS and IPSS-R
- Standardized prognostic assessment: Enhances comparison between patients and facilitates treatment decisions.
- Guidance for clinical trials: Helps stratify patients for eligibility and evaluate treatment efficacy.
- Improved patient counseling: Provides realistic expectations about disease course and prognosis.
Limitations of IPSS and IPSS-R
- Relies solely on clinical and cytogenetic data, potentially overlooking molecular factors.
- May not fully capture the complexities of individual cases.
The IPSS and IPSS-R systems provide valuable tools for risk stratification in MDS. Despite their limitations, they remain widely used in clinical practice and contribute significantly to improving the management of this complex group of disorders.

Myelodysplastic Syndrome (MDS) Treatment
The primary goal of myelodysplastic syndrome (MDS) treatment is to alleviate symptoms, improve quality of life, and prolong survival. Here’s an overview of the various myelodysplastic syndrome (MDS) treatment options:
Supportive care
- Blood transfusions: For managing anemia and thrombocytopenia.
- Growth factors: Stimulating the production of red blood cells and platelets.
- Antibiotics: To prevent infections in patients with neutropenia.
- Iron chelation therapy: For patients with chronic transfusion dependence.
Hypomethylating agents (HMAs)
- Medications like azacitidine and decitabine that work by reprogramming gene expression to promote the differentiation of immature blast cells into mature blood cells, induce programmed cell death in abnormal blood cells and trigger DNA damage pathways, leading to cell cycle arrest and apoptosis.
- Primarily used for lower-risk MDS and intermediate-risk MDS without del(5q).
- Can improve symptoms and prolong survival.
Chemotherapy
- Used for higher-risk myelodysplastic syndrome (MDS) and some patients with intermediate-risk myelodysplastic syndrome (MDS).
- Aims to destroy rapidly dividing cells, including abnormal blood cells.
- Aggressive chemotherapy is usually not effective, although it initially clears the marrow of tumor cells, the myelodysplastic syndrome (MDS) progenitors typically grow back quickly after chemotherapy is stopped and most patients never achieve a true remission.
Immunomodulatory drugs (IMiDs)
- Medications like lenalidomide that modulate the immune system and promote red blood cell production. One key mechanism of action involves hijacking the “garbage disposal” system of the cell, the ubiquitin-proteasome system. By binding to a key protein, lenalidomide directs the destruction of specific harmful proteins in MDS cells. This includes IKZF1/3, a protein that hinders blood cell development, and CK1α, which promotes cell growth.
- Used for some patients with lower-risk myelodysplastic syndrome (MDS) with del(5q).
- Can improve anemia and reduce transfusion dependence.
Stem cell transplantation (SCT)
- The only potentially curative treatment option for myelodysplastic syndrome (MDS).
- Involves replacing the patient’s diseased bone marrow with healthy stem cells from a donor.
- Only suitable for younger patients with good performance status.
Choosing the right treatment approach depends on various factors, including
- Myelodysplastic syndrome (MDS) subtype and risk category
- Patient’s age and health status
- Availability of a suitable donor for SCT
- Patient’s preferences and goals of treatment
Despite these diverse therapies, the overall prognosis is poor. Many patients progress to AML with those with higher-risk disease doing so more rapidly and more frequently. When AML supervenes, it usually responds poorly to chemotherapy and is associated with median survival times of only a few months.
Frequently Asked Questions (FAQs)
Is myelodysplastic syndrome (MDS) cancer curable?
Myelodysplastic syndrome (MDS) is not typically curable. However, there are treatments available that can manage the symptoms, improve quality of life, and, in some cases, slow down the progression of the disease.
Stem cell transplant is often considered the most promising myelodysplastic syndrome (MDS) treatment, as it can potentially replace the damaged bone marrow with healthy cells. However, it is not suitable for everyone due to the risks involved.
What is the life expectancy of a person with myelodysplastic syndrome (MDS)?
The life expectancy of a person with myelodysplastic syndrome (MDS) varies greatly depending on several factors, including:
- Type of MDS: Some types of MDS are more aggressive than others.
- Stage of the disease: The earlier the MDS is diagnosed, the better the prognosis.
- Overall health: Other underlying health conditions can affect life expectancy.
- Treatment response: How well the individual responds to treatment can significantly impact their outlook.
It’s important to note that while myelodysplastic syndrome (MDS) can be a serious condition, there are advancements in treatment options that can improve quality of life and extend survival.
What organ does myelodysplastic syndrome (MDS) affect?
Myelodysplastic syndrome (MDS) primarily affects the bone marrow. The bone marrow is responsible for producing blood cells, including red blood cells, white blood cells, and platelets. In myelodysplastic syndrome (MDS), the bone marrow becomes damaged and is unable to produce enough healthy blood cells. This can lead to a variety of symptoms, including fatigue, anemia, bleeding, and infections.
What are signs that MDS is progressing?
Here are some signs that MDS may be progressing:
- Increasing anemia: This can lead to more severe fatigue, weakness, and shortness of breath.
- Frequent infections: A weakened immune system due to low white blood cell count can increase the risk of infections.
- Excessive bleeding: This can occur due to low platelet count and may manifest as easy bruising, nosebleeds, or bleeding gums.
- Bone pain: As MDS progresses, the damaged bone marrow can cause pain in the bones.
- Enlarged spleen or liver: These organs may become enlarged as the body tries to compensate for the damaged bone marrow.
Is myelodysplastic syndrome (MDS) an aggressive cancer?
Myelodysplastic syndrome (MDS) can be aggressive, but it’s not always. The aggressiveness of myelodysplastic syndrome (MDS) varies depending on the specific type and stage of the disease. Some forms of MDS progress slowly, while others can be more rapidly aggressive.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstöcker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012 Sep 20;120(12):2454-65. doi: 10.1182/blood-2012-03-420489. Epub 2012 Jun 27. PMID: 22740453; PMCID: PMC4425443.
- Khan C, Pathe N, Fazal S, Lister J, Rossetti JM. Azacitidine in the management of patients with myelodysplastic syndromes. Ther Adv Hematol. 2012 Dec;3(6):355-73. doi: 10.1177/2040620712464882. PMID: 23606938; PMCID: PMC3627328.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391-405. doi: 10.1182/blood-2016-03-643544. Epub 2016 Apr 11. PMID: 27069254.
- Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023 Aug;98(8):1307-1325. doi: 10.1002/ajh.26984. Epub 2023 Jun 8. PMID: 37288607.
- Hadley A. Myelodysplastic Syndrome (MDS): A guide for MDS (symptoms, prognosis and common treatment options) (Understanding Your immune system). (2020).
- Gotlib J, Fechter L. 100 Questions & Answers About Myelodysplastic Syndromes. (2015).
- Thomas JT. Myelodysplastic Syndrome: Fast Focus Study Guide. (2015).