TL;DR
Iron deficiency anemia is caused by defective heme or hemoglobin synthesis due to the lack of iron.
Iron deficiency symptoms & signs ▾
| General | Specific |
| Fatigue Weakness Pallor Headaches Dizziness or lightheadedness Shortness of breath Chest pain Palpitations Irritability Cold hands and feet Poor appetite in children and infants | Koilonychia Angular cheilosis Pica Sore tongues and papillary atrophy Paterson-Brown-Kelly or Plummer-Vinson syndrome |
Predisposing risk factors ▾
- Age
- Infants
- Children
- Elderly
- Dietary
- Vegetarians without adequate iron intakes
- Drug History
- Aspirin
- NSAIDs
- Gastrointestinal Tract
- Bleeding
- Gastric or bowel surgery
- Physiological
- Pregnancy
- Frequent blood donors
- Breastfeeding
- Renal
- Hematuria
- Reproduction
- Menorrhagia
- Sex
- Women especially women in childbearing age
Causes ▾
- Dietary
- Vegetarians
- Milk-fed infants
- Elderly
- Gastrointestinal Bleeding
- Esophagitis
- Esophageal varices
- Hiatus hernia (ulcerated)
- Peptic ulcer
- Inflammatory bowel disease
- Malignancy of stomach and colorectal
- Renal / bladder tumors
- Pulmonary hemosiderosis
- Hemorrhoids
- NSAIDs
- Gastrointestinal malabsorption
- Atrophic gastritis
- Helicobacter pylori gastritis
- Celiac disease
- Partial gastrectomy
- Physiological
- Growth spurts (prematurity, adolescents)
- Pregnancy
- Lactation
- Reproduction
- Menorrhagia
- Postmenopausal bleeding
- Social
- Hookworm infections (most common)
- Poverty
Laboratory Investigations ▾
- Complete blood count: ↓ hemoglobin, ↓ mean cell volume and ↓ mean cell hemoglobin. Reticulocytes can be normal or increased depending on the severity of the anemia. White blood cell and platelet counts are generally within the normal range.
- Peripheral blood smear: Hypochromic, microcytic red cells, anisopoikilocytosis with numerous pencil-shaped cells.
- Bone marrow smear: Perls’ Prussian blue stain will be negative if iron stores are absent.
- ↓ serum ferritin level (Reference range is 15 – 300 µg/dL).
- ↓ serum iron and transferrin saturation, and ↑ total iron binding capacity (TIBC).
- ↑ soluble transferrin receptor.
Iron deficiency treatment and management ▾
- Treat the underlying cause and replenish the iron stores.
- Dietary and lifestyle changes.
- Iron therapy through oral ferrous iron salts and parenteral iron for those unable to absorb the oral iron.
- Transfusion of packed RBCs for those with significant acute bleeding or in danger of hypoxia or coronary insufficiency.
*Click ▾ for more information
What is iron deficiency anemia (IDA)?
Iron deficiency anemia is a type of hypochromic microcytic anemia, a red blood cell disorder, caused by defective heme or hemoglobin synthesis due to the lack of iron in our body.
Hemoglobin, the oxygen-carrying protein in red blood cells, is the primary molecule affected by iron deficiency. Iron is an essential component of heme, the non-protein portion of hemoglobin that binds oxygen. When iron levels are insufficient, heme synthesis is disrupted, leading to reduced hemoglobin production.
The decrease in hemoglobin production results in smaller and less hemoglobinized red blood cells, known as microcytic hypochromic erythrocytes. These cells have a diminished capacity to carry oxygen, leading to tissue hypoxia, a state of inadequate oxygen supply to body tissues.
Anemia is a public health problem that affects almost two billion people globally. Anemia is defined as when a person’s hemoglobin level falls below the reference range or a reduction in the hemoglobin concentration of the blood according to gender and age. Iron deficiency anemia (IDA) contributes to approximately 50% of the cause; the most common nutritional disorder in the world.
How is iron regulated in our body?
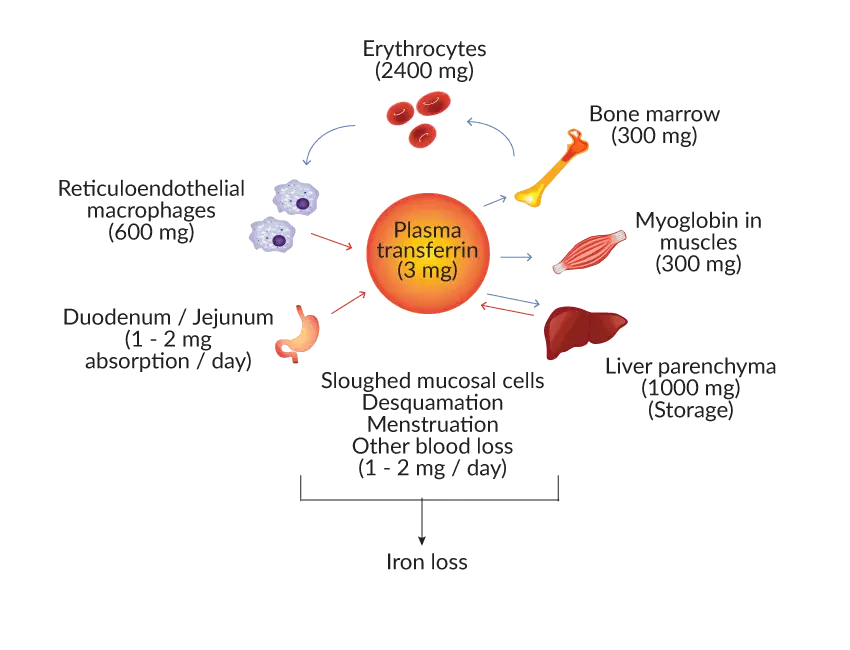
Iron, a vital micronutrient, plays a pivotal role in various physiological processes, including oxygen transport, energy production, and DNA synthesis. Maintaining iron homeostasis, the balance between iron absorption, utilization, and storage, is essential for optimal health. This intricate process involves a complex interplay of specialized proteins, regulatory mechanisms, and target tissues.
Iron Absorption
Organic dietary iron is partly absorbed as heme and partly broken down in the gut to inorganic iron. Absorption occurs through the duodenum. Inorganic iron absorption is favored by factors such as acid and reducing agents like ferrireductase that keep iron in the gut lumen and in the Fe2+ rather than the Fe3+ state.
The protein DMT-1 is involved in transfers of iron from the lumen of the gut across the enterocyte microvilli. The amount of iron absorbed is partly regulated according to the body’s needs by changing the levels of DMT-1 according to the iron status of the duodenal villous crypt enterocyte. Heme is absorbed through a specific receptor HCP-1, exposed on the apical membrane of the duodenal enterocyte. Heme is then digested to release iron.
Iron Transport
Ferroportin at the basolateral surface controls the exit of iron from the cell into portal plasma. Hepcidin is also a major regulator by affecting ferroportin concentration. Low hepcidin levels in iron deficiency increase ferroportin levels and allow more iron to enter portal plasma. Another enzyme, hephaestin, converts Fe2+ to Fe3+ at the basal surface prior to binding to transferrin.
Iron Utilization
From our daily meal, the duodenum absorbs about 1 – 2 mg of dietary iron. It will then be carried by transferrin to be distributed into various compartments, mainly as functional iron, in which the iron is used to synthesize hemoglobin in the bone marrow. Additionally, iron is essential for various enzymatic processes, including DNA synthesis, energy production, and cellular respiration.
At the end of their life, red cells are broken down in the macrophages of the reticuloendothelial system (RES) and the iron is released from the hemoglobin, enters the plasma and is transported around the body in transferrins. Iron is also present in the muscle as myoglobin and in most cells of the body in iron-containing enzymes.
Iron Storage
Excess iron, not immediately required for utilization, is stored in specialized proteins, ferritin and hemosiderin. Ferritin, the primary iron storage protein, is found in the liver, spleen, and bone marrow, and it reversibly binds iron for long-term storage. Hemosiderin, an insoluble form of iron storage, is derived from ferritin degradation and is primarily found in macrophages.
Iron Loss
Apart from the iron cycle, approximately 1-2 mg iron is lost through sloughed mucosal cells, desquamation, menstruation and other causes of blood loss. Iron homeostasis is well-regulated to protect cells from iron-mediated oxidative stress. Unfortunately, there is no physiological pathway for iron excretion from the human body. Therefore, to avoid detrimental effects, iron homeostasis involves the interaction of different types of iron-binding proteins like iron storage protein, iron transport proteins and function proteins, so that iron is stored and transported in a safe state.
Iron Cycle
Iron deficiency symptoms
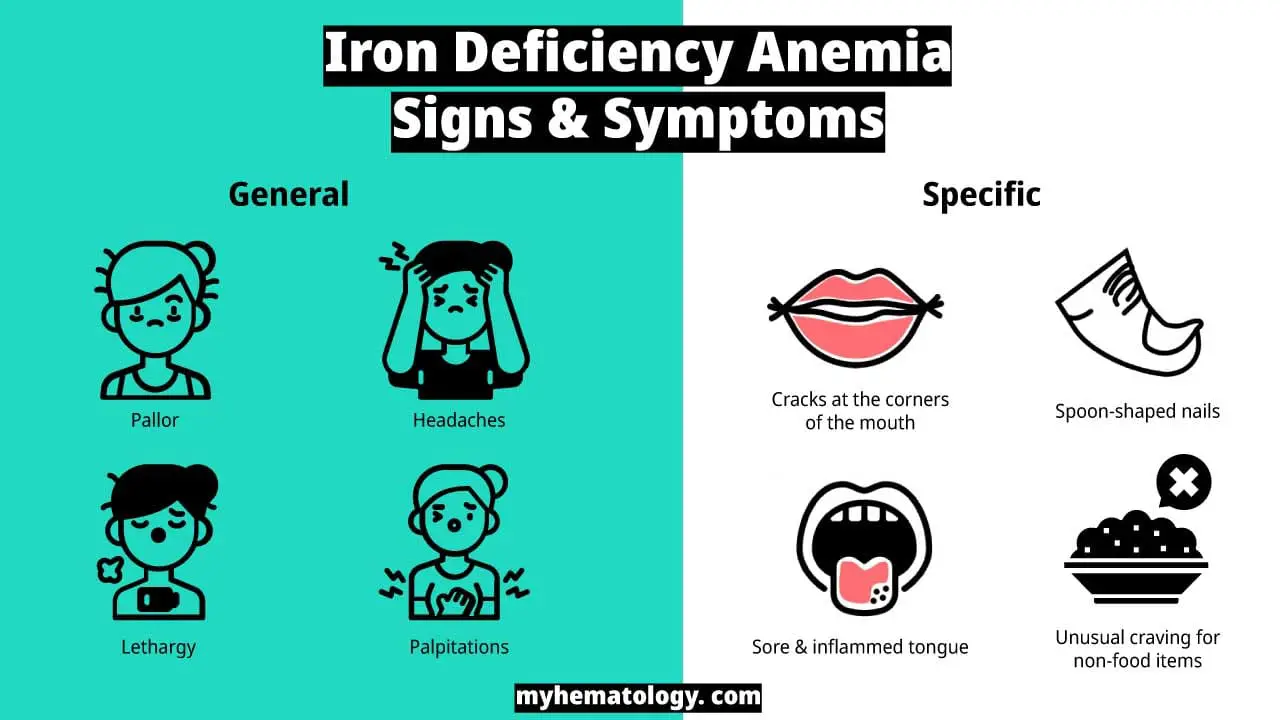
Iron deficiency anemia (IDA) symptoms range from mild to severe. While general symptoms of anemia, such as fatigue, weakness, pale skin, shortness of breath, and dizziness, are common, iron deficiency can also present with more specific signs and symptoms.
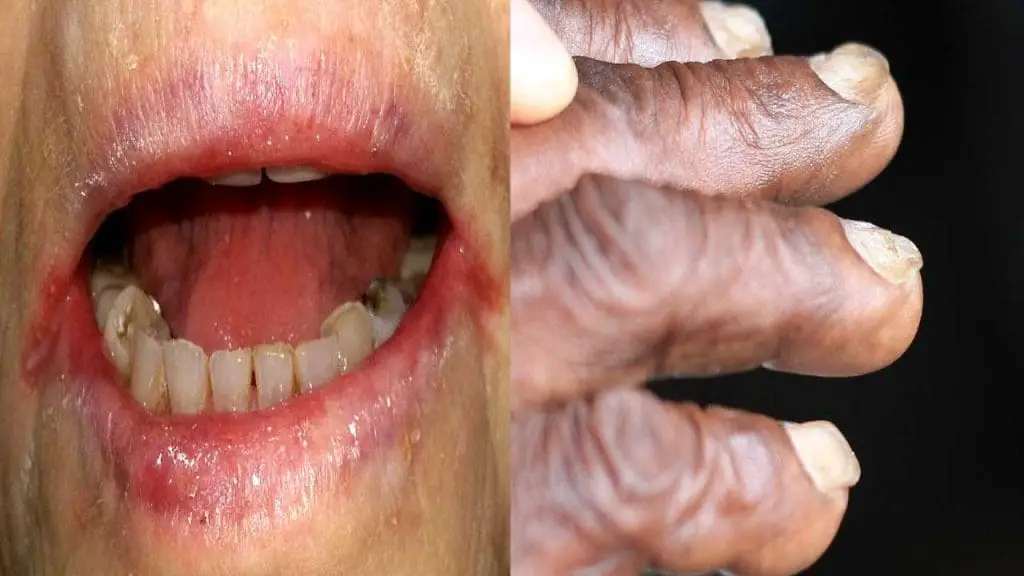
More specific iron deficiency signs include glossitis (an inflammation of the tongue) that can cause a smooth, red, and sore tongue. Angular cheilitis, characterized by cracks at the corners of the mouth, can be painful and persistent. Koilonychia, the development of spoon-shaped nails, is a rare but distinctive sign of iron deficiency anemia (IDA). Pica, an unusual craving for non-food items such as ice, dirt, or paper, can be observed in some individuals with iron deficiency anemia (IDA).
Am I at risk of iron deficiency?
High-risk groups that are most vulnerable are
- Vegetarians in terms of dietary habits or a poor diet in many poor economic countries
- Certain age groups like infants, adolescents, elderly people
- Chronic blood loss through gastrointestinal bleeding for example peptic ulcer or gluten-induced enteropathy
- Partial or total gastrectomy and atrophic gastritis can cause malabsorption
- Usage of certain drugs in causing chronic blood loss like aspirin or non-steroidal anti-inflammatory drugs
- Women of reproductive age, pregnant and breastfeeding women, postmenopausal women, in terms of physiological demand
- Chronic blood loss through menorrhagia
- Gastrointestinal bleeding due to hookworm or parasitic infestation.
What are the stages of iron deficiency?
Iron deficiency anemia (IDA) develops progressively, with distinct stages characterized by varying levels of iron depletion and impact on red blood cell production.
Stage 1: Iron Depletion
- Iron stores: Depleted bone marrow iron stores (ferritin < 15 ng/mL)
- Red blood cells: Normal red blood cell production and indices (hemoglobin, MCV, MCH)
- Iron deficiency symptoms: Often asymptomatic, but fatigue may be present.
- Diagnostic tests: Low ferritin, normal transferrin saturation (TSAT), and increased transferrin receptor (TfR).
Stage 2: Iron-Deficient Erythropoiesis
- Iron stores: Further depletion of bone marrow iron stores, but still detectable.
- Red blood cells: Normal red blood cell count, but decreased hemoglobin production (microcytic carrier state). Hemoglobin may remain within normal range initially.
- Iron deficiency symptoms: Fatigue, pallor, and exertional dyspnea become more prominent.
- Diagnostic tests: Low ferritin, decreased TSAT (< 20%), and elevated TfR. Bone marrow aspirate may show microcytic precursors.
Stage 3: Iron Deficiency Anemia
- Iron stores: Severely depleted bone marrow iron stores (ferritin often < 10 ng/mL).
- Red blood cells: Decreased red blood cell count (anemia), microcytosis (MCV < 80 fL), and hypochromia (MCH < 27 pg).
- Iron deficiency symptoms: Severe fatigue, weakness, pallor, dyspnea at rest, cognitive impairment, and pica.
- Diagnostic tests: Confirmed anemia with microcytosis and hypochromia, low ferritin, decreased TSAT, and elevated TfR. Bone marrow aspirate confirms iron deficiency.
General laboratory tests for iron deficiency anemia (IDA)
Laboratory investigations include red cell indices from the complete blood count showing low hemoglobin, mean cell volume (MVC) and mean cell hemoglobin (MCH) values.
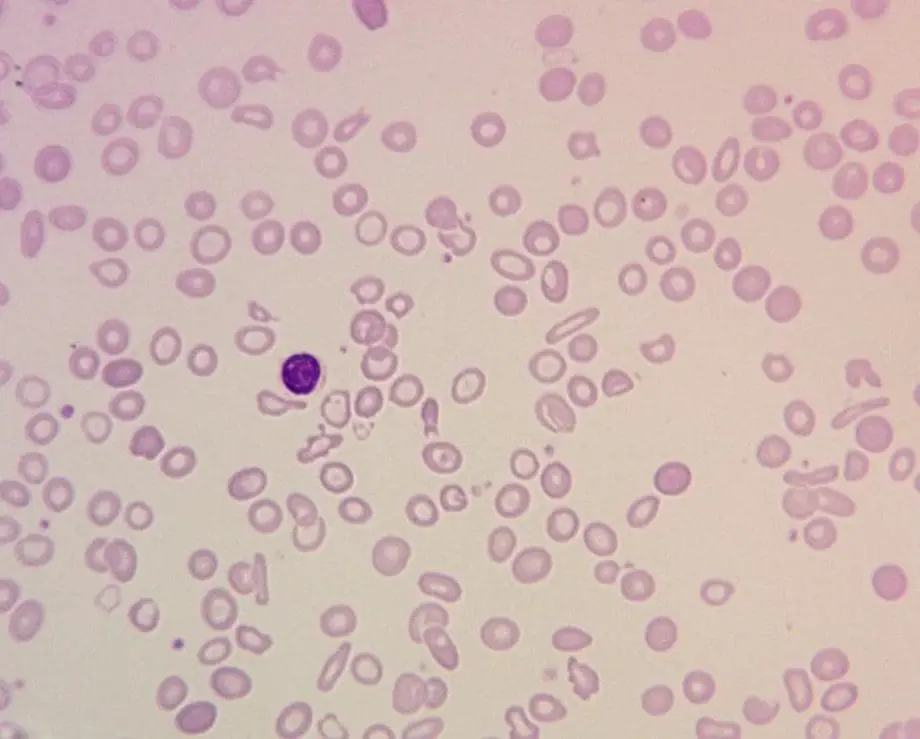
Peripheral blood film reveals microcytic hypochromic anemia with pencil shaped cells and sometimes target cells. The distortion of the red cell morphology is highly dependent on the severity of anemia in the patient. These are mainly for the indications of hypochromic microcytic anemia. To get proper confirmation, further tests need to be carried out.
In certain regions of the world, it is important to be able to differentiate between iron deficiency and thalassemia as both of them have quite similar clinical pictures i.e. the pallor, lethargy, weakness and even initial laboratory investigations have similar findings as both disorders present with hypochromic, microcytic red cells.
This disorder is rarely diagnosed using bone marrow aspiration as the method is rather invasive. However, the intensive grading method of assessing marrow iron provides a precise iron status classification especially using Perls’ Prussian blue stain to stain presence of iron.
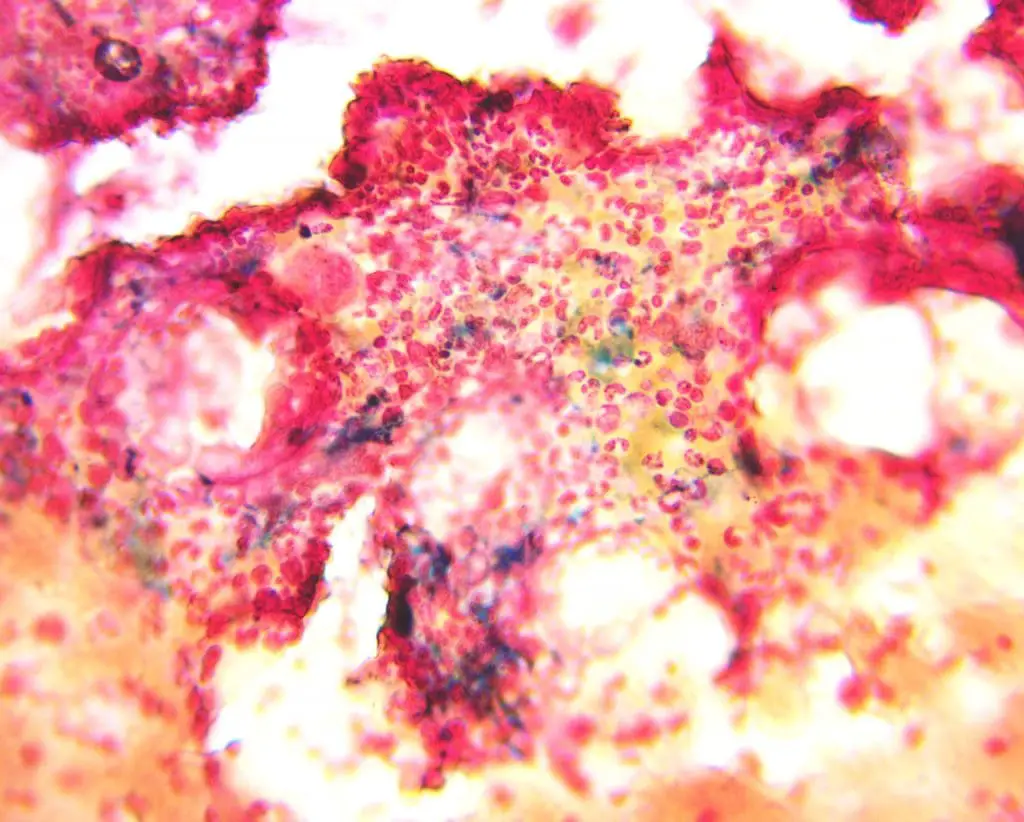
Specific biochemical tests for iron deficiency anemia (IDA)
Specific biochemical tests include low serum ferritin level, low serum iron, increased total iron binding capacity (TIBC) and increased soluble transferrin receptors.
Biochemical parameters for assessing iron status are derived from immunoturbidimetric assay by using a biochemical autoanalyzer and/or enzyme-linked immunosorbent assay (ELISA). These biochemical indicators can be categorized into two main iron distribution compartments: transport iron and storage iron.
Transport iron involves transferrin and transferrin receptors. Iron bound to transferrin is indicated as serum iron whereas transferrin binding capacity to iron is indicated as total iron binding capacity. TIBC measures the total amount of iron the blood can bind to a protein called transferrin. When there is iron deficiency, the body’s iron stores (ferritin) are depleted. This makes less iron available to bind to transferrin. However, the body needs to maintain the capacity to transport iron, even when there’s not much of it. So, as a response, the body produces more transferrin, increasing the TIBC.
For storage iron, serum ferritin is the widely accepted parameter that reflects the size of the iron stores similar to bone marrow iron. Ferritin plays an important role in iron detoxification and iron storage, however, it is an acute phase protein, rising with any inflammatory state. Therefore, it is evaluated together with inflammatory markers such as C-reactive protein.
Although biochemical parameters can detect the earliest onset of iron deficiency, its diagnostic shortcomings due to inappropriate distorted results in the presence of anemia of chronic diseases such infection, inflammation and cancer, have reduced the potential utility as an effective marker.
Therefore, combining several parameters, such as ferritin and serum transferrin together with at least one acute phase protein, such as CRP, might provide a more reliable overview than an individual test. Iron status quantification can be limited in some areas due to high cost and lack of access to these analyzers. Thus, more low-cost and efficient techniques are still needed for rapid detection of this disorder in rural areas.
Iron deficiency treatment
Iron deficiency treatment include treating the underlying cause with an addition of oral or parenteral iron to correct the anemia and replenish iron stores.
There should be changes in dietary and lifestyle to increase iron intake.
Parenteral iron as iron deficiency treatment is only given when there are high iron requirements as in gastrointestinal bleeding, severe menorrhagia, chronic hemodialysis with erythropoietin therapy and when oral iron is ineffective for example in atrophic gastritis or Crohn’s disease.
Transfusion of packed red cells in iron deficiency treatment is only indicated in emergency or urgent cases for example when a patient is in her third trimester of pregnancy.
Nutrition and IDA
Animal sources
- Red meat: Beef, lamb, and pork are excellent sources of heme iron, which is the most easily absorbed form of iron.
- Organ meats: Liver, kidney, and heart are incredibly rich in iron, but be mindful of their cholesterol content.
- Poultry: Chicken and turkey are good sources of iron, although not as rich as red meat.
- Fish and seafood: Tuna, sardines, salmon, and oysters are all good sources of iron, with some varieties also providing omega-3 fatty acids.
Plant-based sources
- Beans and lentils: These are affordable and versatile sources of iron and fiber.
- Tofu and tempeh: These soy-based products are good options for vegetarians and vegans, but their iron absorption is lower than heme iron.
- Dark leafy greens: Spinach, kale, and collard greens are packed with iron, vitamin C (which helps with iron absorption), and other nutrients.
- Nuts and seeds: Pumpkin seeds, cashews, sunflower seeds, and almonds are all good sources of iron, as well as healthy fats and protein.
- Dried fruits: Raisins, prunes, and apricots are concentrated sources of iron and other nutrients, but be mindful of their sugar content.
- Fortified foods: Cereals, breads, and plant-based milks are often fortified with iron, making them a convenient way to increase your intake.
Iron-rich drinks
- Iron-fortified orange juice: Vitamin C in orange juice helps with iron absorption from plant-based sources.
- Prune juice: This naturally sweet juice is a good source of iron and other nutrients, but limit your intake due to its sugar content.
Foods to avoid or limit
While no foods directly interfere with iron absorption, some can hinder it if consumed in large quantities. These include:
- Coffee and tea: These beverages contain tannins, which can bind to iron and reduce its absorption. Space out your coffee or tea intake from iron-rich meals by at least an hour.
- Calcium-rich foods: Dairy products, calcium-fortified foods, and antacids can interfere with iron absorption if consumed close together. Avoid taking iron supplements with milk or calcium-rich foods.
- Whole-grain wheat products: While whole grains are nutritious, the phytates they contain can slightly reduce iron absorption. Soak or sprout grains before consuming to improve their digestibility and nutrient bioavailability.
Frequently Asked Questions (FAQs)
What is the main cause of iron deficiency anemia (IDA)?
The main cause of iron deficiency anemia (IDA) depends on several factors, including age, gender, and underlying health conditions. However, there are two main categories:
1. Blood loss: This is the most common cause of iron deficiency anemia (IDA) in adults and men. It can occur through various mechanisms:
- Gastrointestinal bleeding: This can be caused by ulcers, hemorrhoids, cancer, or chronic inflammation, such as Crohn’s disease.
- Heavy menstrual bleeding: Women with heavy periods are at increased risk of iron deficiency anemia (IDA).
- Blood donation: Frequent blood donation can deplete iron stores, especially if not replenished properly.
2. Inadequate iron intake or absorption: This is more common in infants, children, and pregnant women:
- Diet low in iron-rich foods: Meat, poultry, fish, beans, and leafy green vegetables are excellent sources of iron. Limited access to these foods can contribute to iron deficiency anemia (IDA).
- Malabsorption: Certain medical conditions, such as celiac disease or Crohn’s disease, can interfere with iron absorption from the gut.
- Increased iron requirements: During pregnancy, the body needs more iron to support fetal development. Infants and young children are also rapidly growing and require adequate iron intake.
Therefore, the main cause of IDA can vary depending on individual circumstances.
What are the 4 common iron deficiency symptoms?
While iron deficiency anemia (IDA) can present with a variety of symptoms, here are 4 common iron defiency symptoms:
1. Fatigue and weakness: This is often the most noticeable iron deficiency symptom, and it can range from mild tiredness to feeling constantly exhausted. It occurs because the body’s tissues aren’t receiving enough oxygen due to the reduced number of healthy red blood cells.
2. Pale skin: Due to the lack of hemoglobin, which gives red blood cells their color and carries oxygen, the skin can appear pale or lackluster in iron deficiency. This may be most noticeable in the face, lips, and under the nails.
3. Shortness of breath: With fewer red blood cells transporting oxygen, the body has to work harder to deliver oxygen to the body’s tissues. This can lead to shortness of breath, especially during physical activity as a common iron deficiency symptom.
4. Dizziness or lightheadedness: As the body struggles to supply sufficient oxygen to the brain, a person may experience dizziness or lightheadedness, particularly when standing up quickly or changing positions in iron deficiency amemia (IDA).
It’s important to note that these are just a few common symptoms, and iron deficiency symptoms include:
- Headache
- Cold hands and feet
- Brittle nails
- Restless leg syndrome
- Difficulty concentrating
- Pica (craving non-food items)
How long does it take to correct iron deficiency?
The time it takes to correct iron deficiency anemia (IDA) depends on several factors, including:
Severity of the deficiency: More severe cases with depleted iron stores will take longer to correct than mild cases.
Treatment method: Oral iron supplements usually take longer than intravenous (IV) iron to replenish stores.
Individual absorption and response: Different people absorb iron differently, and some may respond faster to treatment than others.
Underlying cause: If the underlying cause of the iron deficiency anemia (IDA) like blood loss, isn’t addressed, it can hinder iron stores from replenishing fully.
Here’s a general timeframe:
- Oral iron supplements: It can take 3-6 months to see significant improvement in red blood cell counts and iron deficiency symptoms with oral iron supplements. However, iron stores may take longer to fully replenish, up to 12 months or more.
- IV iron: IV iron infusions can correct severe iron deficiency symptoms much faster, sometimes within weeks. This is because it bypasses the gut and delivers iron directly into the bloodstream.
Factors that can slow down progress:
- Skipping iron supplements or missing doses in iron deficiency treatment.
- Having underlying conditions that affect iron absorption, like celiac disease or Crohn’s disease.
- Continuing to lose blood (e.g., heavy menstrual bleeding, ulcers).
- Taking medications that interfere with iron absorption, like antacids or calcium supplements.
When is the best time to take iron for anemia?
In general, most guidelines recommend taking iron supplements on an empty stomach, as food can reduce absorption. However, if you experience side effects, it’s okay to take them with food. Avoid taking iron supplements with calcium-rich foods like milk, yogurt, or antacids, as these can also interfere with absorption. Take iron supplements with a glass of water and avoid coffee or tea, which can further reduce absorption.
Are there test kits available to test for iron at home?
Yes, there are at-home iron test kits available. These kits typically measure ferritin, a protein that stores iron in your body.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- World Health Organization. Iron deficiency anemia: Assessment, prevention, and control of a global public health problem. World Health Organization; 2008.
- Safiri, S., Kolahi, AA., Noori, M. et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. J Hematol Oncol 14, 185 (2021). https://doi.org/10.1186/s13045-021-01202-2.
- Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013 Jan 15;87(2):98-104. PMID: 23317073.
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Garrison C. The Iron Disorders Institute Guide to Anemia: Understanding the Causes, Symptoms, and Healing of Iron Deficiency and Other Anemias (Cumberland House). 2009.
- Johansson A. Iron Deficiency Anemia Cookbook: Essential Diet Guide, 50 + Iron Rich Recipes and a 2-Week Diet Plan to Help Low Iron Levels. 2021.