TL;DR
G6PD deficiency is an inherited hemolytic anemia due to red cell enzyme defect. It is an X-linked recessive disorder, thus, full expression is only seen in males.
- G6PD deficiency symptoms & signs ▾: It is usually asymptomatic. Acute intravascular hemolytic anemia and hemoglobinuria can be seen during crises.
- Causes of G6PD deficiency crises ▾:
- Fava beans
- Oxidative drugs (antimalarial drugs)
- Antihelminths (mothballs)
- Aspirin in high doses
- Infections
- Sulphonamides
- G6PD deficiency tests ▾:
- Supravital staining – presence of Heinz bodies
- Fluorescent spot test screening – reduced fluorescence
- DNA analysis – positive for mutation
- Full blood count: ↓ hemoglobin, reticulocytosis.
- Peripheral blood smear: Moderate anisopoikilocytosis, polychromatic cells, microspherocytes and bite cells in red blood cell morphology.
- ↑ serum bilirubin.
- ↑ lactate dehydrogenase.
- ↓ serum haptoglobin.
- G6PD deficiency treatment and management ▾:
- Stop ingestion of oxidative drugs or fava beans.
- Transfuse packed red cells if necessary.
- Treat underlying infection if present.
- Splenectomy may ameliorate hemolytic anemia in rare chronic hereditary nonspherocytic hemolytic anemia.
*Click ▾ for more information
What is G6PD deficiency?
G6PD deficiency is an inherited hemolytic anemia due to a red cell enzyme defect. For the RBC to be fully functional as an oxygen carrier, there are 3 important factors involved: the condition of the red blood cell membrane, the metabolic pathways involved in maintaining cell survival as well as the condition of the hemoglobin.
There are 4 major metabolic pathways in ensuring the survivability of red blood cells and they are interconnected. The key actions of these pathways include energy supply to the system, oxidant reduction of the cell, maintaining hemoglobin in a reduced state and regulation of oxygen release. These pathways use enzymes to convert specific molecules into their intended metabolites for the optimal functioning of the red blood cells.
Glucose-6-phosphate dehydrogenase (G6PD) is involved in the hexose monophosphate pathway in red blood cell metabolism and functions to convert hydrogen peroxide to water in the erythrocyte.
Inheritance and Epidemiology
The G6PD gene is found on the X chromosome. Specifically, it is located on the long arm (q) of the X chromosome, at band Xq28. Thus, G6PD deficiency follows an X-linked recessive inheritance pattern.
Because males have only one X chromosome (XY), they are typically more affected. If they inherit the altered gene on their single X chromosome, they will manifest the condition.
Females, having two X chromosomes (XX), are often carriers if they inherit one altered gene, as their other healthy X chromosome can compensate; however, due to a process called X-inactivation, some female carriers can experience varying degrees of G6PD deficiency if the X chromosome carrying the normal gene is primarily inactivated in their cells.

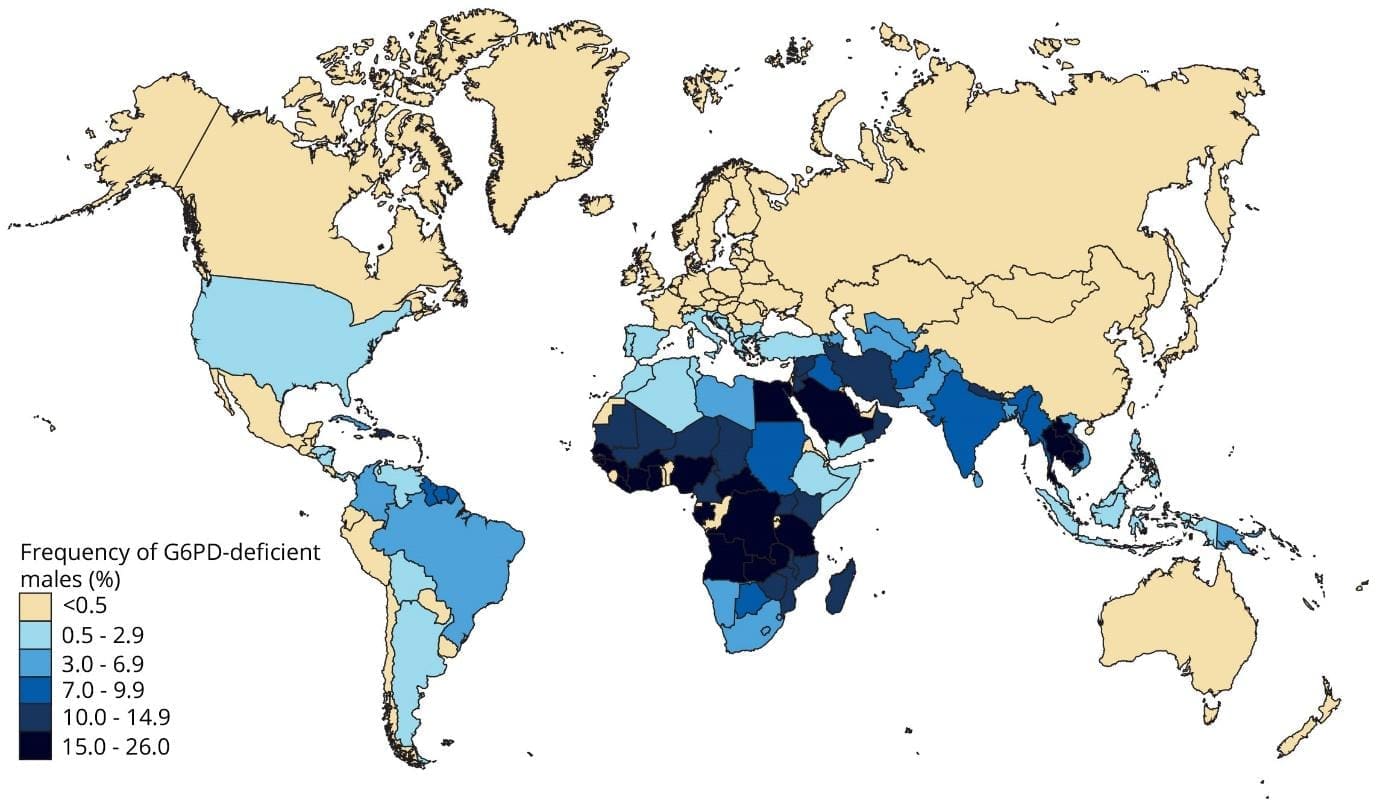
This disorder is one of the most common anemia disorders worldwide. The degree of deficiency varies and is often mild in black Africans, more severe in Orientals and most severe in Mediterraneans.
Approximately 5% of the global population is affected by this disorder.
Why is G6PD important?
Glucose-6-phosphate dehydrogenase (G6PD) deficiency affects the red blood cell metabolism pathway particularly the hexose monophosphate shunt.
Glucose-6-phosphate dehydrogenase (G6PD) is the catalyst in the rate-limiting first step of the pentose phosphate pathway, which uses glucose-6-phosphate to convert nicotinamide adenine dinucleotide phosphate (NADP) into its reduced form, NADPH.
In red blood cells, NADPH is critical in preventing damage to cellular structures caused by oxygen-free radicals (e.g., hydrogen peroxide). It does this by serving as a substrate to the enzyme glutathione reductase.
Reduced glutathione can be used to convert hydrogen peroxide to water and prevent damage to cellular structures, particularly the cell wall of RBCs since they have limited capacity for repair once mature.
Pathophysiology of G6PD deficiency
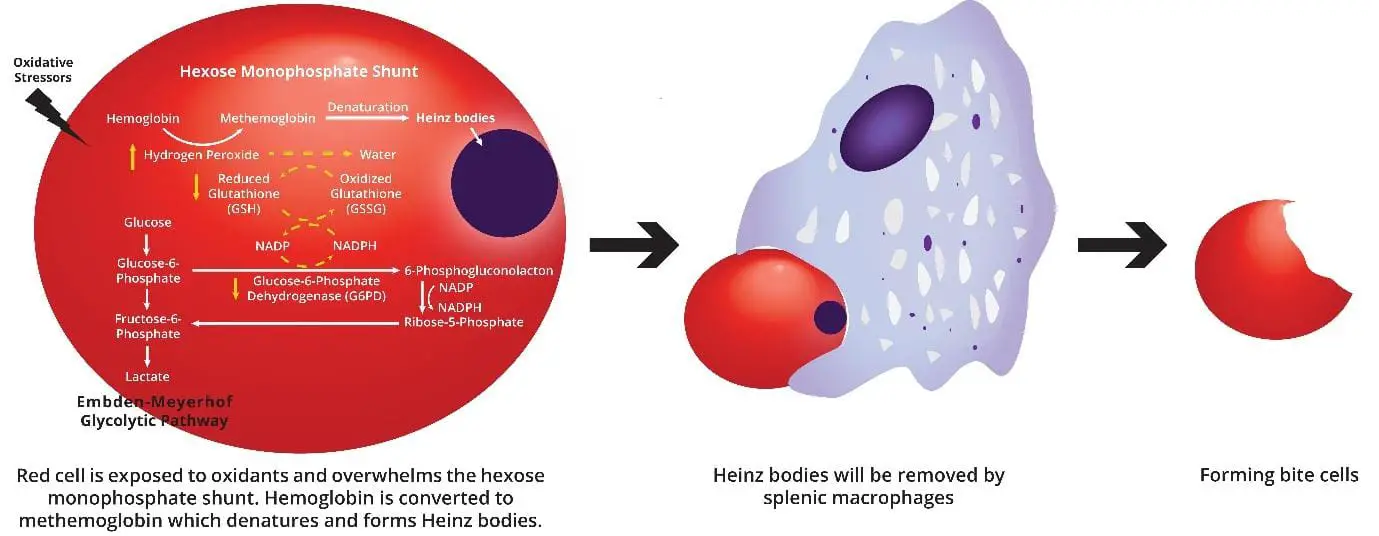
When the red cell is challenged by an oxidant stress, for example ingestion of moth balls by a glucose-6-phosphate dehydrogenase (G6PD) deficient individual, both lipids and red cell membrane proteins of the individual become oxidized as there is not enough reduced glutathione produced to convert the increased level of oxidants to water. Hemoglobin denatures and precipitates intracellular to become Heinz bodies.
The presence of Heinz bodies causes the red cell to be trapped in the spleen and sometimes splenic macrophages surgically excise the portion of the red cell that contains a Heinz body. In these circumstances, the red cell may escape with a gap, appearing as bite cells. These rigid and fragmented cells may also lead to intravascular hemolysis.
Causes of acute hemolysis in G6PD deficiency
Acute hemolysis in individuals with G6PD deficiency is primarily triggered by oxidative stress, which their red blood cells cannot adequately manage due to the enzyme deficiency.
- Other Oxidative Stressors: Less common triggers can include severe emotional stress, strenuous exercise, rapid correction of hyperglycemia in diabetics, and exposure to certain chemicals.
- Infections: Bacterial or viral infections (e.g., hepatitis, pneumonia, typhoid fever) can increase oxidative stress in the body, leading to the breakdown of red blood cells.
- Certain Medications: Various drugs that produce oxidative stress are known triggers.
- Antimalarials: Especially primaquine, but also some others.
- Sulfonamides: A class of antibiotics.
- Nitrofurans: Such as nitrofurantoin (an antibiotic).
- Aspirin and other NSAIDs: Especially in large doses.
- Dapsone
- Naphthalene: Found in mothballs.
- Some other less common drugs like phenazopyridine, nalidixic acid, and methylene blue (which paradoxically can also cause methemoglobinemia, a dangerous condition in G6PD deficient patients).
- Foods: The most well-known dietary trigger is fava beans (broad beans), which can cause a severe reaction called favism. Other foods like some berries (e.g., blueberries) and tonic water have also been implicated, though less commonly.
G6PD deficiency symptoms
G6PD deficiency often remains asymptomatic for a person’s entire life unless they are exposed to specific triggers that induce oxidative stress.
When red blood cells, which are already vulnerable due to insufficient G6PD enzyme, encounter these stressors, they break down prematurely in a process called hemolysis. This leads to a condition known as hemolytic anemia, and the signs and symptoms are a direct result of this rapid red blood cell destruction and the body’s attempt to cope.
The signs and symptoms of G6PD deficiency typically appear acutely following exposure to a trigger (like certain medications, fava beans, or infections) and can vary in severity.
Common Signs and Symptoms of Acute Hemolysis (Hemolytic Crisis):
- Pallor (Pale Skin): Due to the reduction in the number of red blood cells carrying oxygen, the skin, and sometimes the mucous membranes (like lips or tongue), may appear unusually pale.
- Jaundice (Yellowing of Skin and Eyes): When red blood cells break down, they release bilirubin. The liver processes bilirubin, but if red cells are destroyed too quickly, bilirubin can build up in the blood, leading to a yellowish discoloration of the skin and the whites of the eyes (sclera).
- Dark Urine: The rapid destruction of red blood cells can release hemoglobin into the urine, making it appear dark, tea-colored, or reddish-brown. This is known as hemoglobinuria.
- Fatigue and Weakness: A decreased red blood cell count means less oxygen is delivered to tissues, leading to tiredness, lethargy, and a general feeling of weakness.
- Shortness of Breath (Dyspnea) and Rapid Heart Rate (Tachycardia): The body tries to compensate for the reduced oxygen-carrying capacity by increasing heart rate and breathing rate to deliver more oxygen.
- Dizziness or Lightheadedness: Insufficient oxygen to the brain can cause these sensations.
- Back or Abdominal Pain: This can occur due to the rapid breakdown of red blood cells and the body’s reaction to the hemolytic process, potentially affecting organs like the kidneys or spleen.
- Fever: Infections are common triggers, and the inflammatory response can also cause a fever during a hemolytic crisis.
- Enlarged Spleen (Splenomegaly): The spleen works overtime to filter out damaged red blood cells, which can cause it to swell.
Specific Considerations in Newborns
G6PD deficiency is a significant cause of neonatal jaundice, which can be severe and prolonged. If left untreated, very high bilirubin levels in newborns can lead to a serious condition called kernicterus, causing irreversible brain damage. Signs in newborns may include:
- Pronounced jaundice (yellowing appearing quickly or being very deep)
- Lethargy and excessive sleepiness
- Poor feeding
- High-pitched crying
- Changes in muscle tone (either too floppy or too rigid)
- Arching of the back (opisthotonus)
- Seizures (in severe cases of kernicterus)
Long-term Symptoms/Complications
While most individuals are asymptomatic between acute episodes, severe or repeated hemolytic crises can potentially lead to long-term issues like:
- Kidney problems: Severe acute hemolysis can sometimes lead to acute kidney injury.
- Chronic anemia: In very rare and severe cases, G6PD deficiency can lead to chronic anemia even without obvious triggers.
- Gallstones: Chronic red blood cell breakdown can increase bilirubin in bile, forming bilirubin gallstones.
What are the different variants of G6PD deficiency, and how do they influence severity?
G6PD deficiency, an X-linked genetic disorder, exhibits a spectrum of severity due to various mutations in the glucose-6-phosphate dehydrogenase (G6PD) gene. These mutations, known as variants, affect the enzyme’s activity, impacting red blood cell susceptibility to oxidative stress and influencing the severity of symptoms.
| Class | Severity | Enzyme Activity | Clinical Symptoms | Prevalence |
| I | Most severe, leading to chronic non-spehrocytic hemolytic anemia (CNSHA) | < 10% | Constant fatigue, jaundice, splenomegaly and possible gallstones | Relatively rare |
| II | Severe, causing acute hemolytic anemia triggered by specific exposures | 10 – 30% | Episodes of fatigue, dark urine, abdominal pain and jaundice after triggers | More common than Class I |
| III | Moderate, with potential for mild acute hemolytic anemia after triggers | 30 – 60% | May experience mild hemolytic episodes but often asymptomatic | More common than Classes I and II |
| IV | Mild, with very rare hemolysis even with triggers | 60 – 80% | Usually asymptomatic | Relatively common |
| V | Very mild, with no hemolysis even after triggers | > 80% | Usually asymptomatic | Relative Common |
- This classification is based on the World Health Organization (WHO) system, but other classification systems exist.
- The severity of symptoms can vary within each class due to individual genetic backgrounds, environmental factors, and nutritional status.
- Genetic testing can help identify the specific glucose-6-phosphate dehydrogenase (G6PD) variant and potential severity.
How do I test for G6PD deficiency?
Investigating G6PD deficiency involves a combination of clinical assessment and laboratory tests. The diagnostic approach can vary depending on whether the patient is experiencing an acute hemolytic episode or being screened, particularly in newborns or individuals from high-risk populations.
Initial Blood Tests (During a Hemolytic Crisis)
- Complete Blood Count (CBC): This will typically show anemia (low red blood cell count, hemoglobin, and hematocrit) during a hemolytic episode.
- Reticulocyte Count: An elevated reticulocyte count indicates that the bone marrow is trying to compensate for the rapid destruction of red blood cells by producing more immature red cells.
- Peripheral Blood Smear: Examination under a microscope can reveal characteristic abnormalities:
- Heinz bodies: These are precipitates of denatured hemoglobin that attach to the red blood cell membrane. They are a hallmark of oxidative damage and can be seen with special supravital stains (like crystal violet).
- Bite cells/Blister cells: These are red blood cells from which Heinz bodies have been “bitten off” by macrophages in the spleen, leaving a characteristic appearance.
- Bilirubin Levels: Elevated unconjugated (indirect) bilirubin is common due to the rapid breakdown of heme from destroyed red blood cells. This contributes to jaundice.
- Lactate Dehydrogenase (LDH): Elevated LDH levels are a general indicator of cell damage and are often increased during hemolysis.
- Haptoglobin: Haptoglobin is a protein that binds to free hemoglobin in the blood. During hemolysis, haptoglobin levels typically decrease as it is consumed by binding to the released hemoglobin.
- Urinalysis: May reveal hemoglobinuria (hemoglobin in the urine), making the urine appear dark or reddish-brown.
Specific G6PD Enzyme Activity Assays
These tests directly measure the amount of G6PD enzyme activity in red blood cells. It’s crucial to perform these tests when the patient is not in an acute hemolytic crisis, as recent transfusions or a high proportion of young red blood cells (reticulocytes, which have higher G6PD activity) can lead to falsely normal or high results, masking the deficiency.
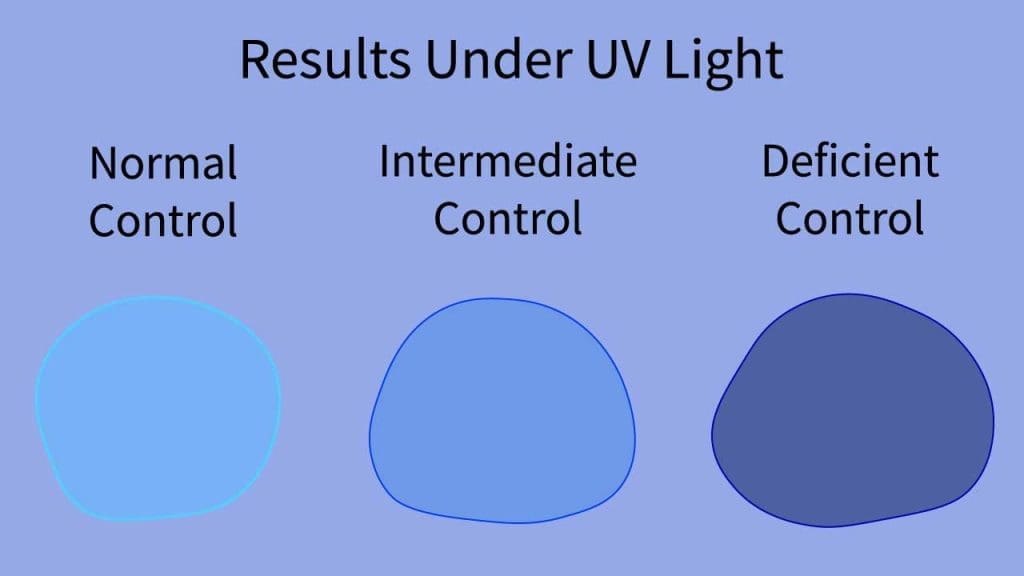
- Fluorescent Spot Test (FST): This is a widely used qualitative or semi-quantitative screening test. It’s simple and relatively inexpensive. The test relies on the principle that G6PD produces NADPH (nicotinamide adenine dinucleotide phosphate) as it metabolizes glucose-6-phosphate. NADPH fluoresces under UV light.
- Quantitative Spectrophotometric Assay (Gold Standard): This is the most accurate and definitive method for diagnosing G6PD deficiency. It precisely measures the enzyme’s activity by monitoring the rate of NADPH formation using a spectrophotometer.
- Point-of-Care (POC) Tests: Newer rapid diagnostic tests (RDTs), often lateral flow assays, are emerging for use in resource-limited settings. They provide quick qualitative (deficient/normal) or semi-quantitative results, useful for screening before administering drugs like primaquine for malaria.
Molecular Genetic Testing (DNA Analysis/Gene Sequencing)
While not typically the first-line diagnostic test, genetic testing can identify specific mutations in the G6PD gene. It’s particularly useful in cases with unclear phenotypic test results, for precise variant identification (especially for research or genetic counseling purposes), in neonates (where enzyme activity tests can be unreliable due to immature red cells or transfusions), or for carrier identification in females.
Screening Considerations
- Newborn Screening: In populations with a high prevalence of G6PD deficiency (like many Southeast Asian countries), routine newborn screening is common to identify affected infants early and prevent severe neonatal jaundice and kernicterus.
- Pre-drug Screening: Individuals from endemic areas, especially males, may be screened for G6PD deficiency before being prescribed certain medications known to cause hemolysis (e.g., primaquine for malaria treatment).
How is G6PD deficiency treated?
There is no cure for G6PD deficiency, as it is a genetic condition. Treatment primarily focuses on prevention of hemolytic crises and supportive care during acute episodes.
Prevention of Hemolytic Crises (Primary Management Strategy)
This is the most crucial aspect of managing G6PD deficiency and involves educating the individual and their family about triggers to avoid.
- Avoidance of Oxidative Drugs: Patients must be given a clear list of medications to avoid. This includes certain antimalarials (e.g., primaquine, chloroquine, mefloquine), sulfonamides (e.g., sulfamethoxazole, dapsone), nitrofurans (e.g., nitrofurantoin), and some other drugs like rasburicase and naphthalene (found in mothballs). Even large doses of Vitamin C and some over-the-counter pain relievers like aspirin can be problematic for some individuals.
- Avoidance of Certain Foods: The most notable food trigger is fava beans (broad beans), which can cause a severe reaction called favism. Other foods, though less common triggers, might also be advised to be avoided in some cases (e.g., blueberries, tonic water, some traditional Chinese herbs).
- Prompt Treatment of Infections: Infections are a very common trigger for hemolysis. Therefore, any infection should be treated promptly and effectively to minimize oxidative stress on red blood cells.
- Caution with Chemicals: Direct exposure to certain chemicals (e.g., naphthalene in mothballs, some hair dyes like henna in high concentrations, or industrial chemicals) should be avoided.
- Healthy Lifestyle: Maintaining overall health, managing stress, getting adequate rest, and avoiding excessive alcohol consumption and smoking can generally support the immune system and reduce overall oxidative stress.
Supportive Care During Acute Hemolytic Crisis
If a hemolytic crisis does occur, treatment is supportive and aims to manage the symptoms and complications.
- Removal of the Offending Agent: The immediate step is to identify and discontinue the drug, food, or chemical that triggered the hemolysis.
- Hydration: Intravenous fluids may be given to maintain hydration, especially if there’s significant hemoglobinuria, to help prevent kidney damage.
- Blood Transfusions: For severe anemia resulting from rapid red blood cell destruction, blood transfusions (packed red blood cells) are the primary treatment to restore oxygen-carrying capacity and alleviate symptoms like severe fatigue, shortness of breath, or dizziness.
- Management of Jaundice (especially in newborns):
- Phototherapy: For newborns with significant jaundice, phototherapy (exposure to special blue light) is used. This light helps convert unconjugated bilirubin in the skin into a form that can be excreted more easily.
- Exchange Transfusion: In severe cases of neonatal jaundice, particularly when there’s a risk of kernicterus (bilirubin-induced brain damage), an exchange transfusion may be performed. This procedure involves removing small amounts of the baby’s blood and replacing it with donor blood to quickly lower bilirubin levels and remove affected red blood cells.
- Monitoring: Close monitoring of blood counts (hemoglobin, hematocrit), bilirubin levels, and kidney function is crucial during an acute crisis.
Frequently Asked Questions (FAQs)
What is the difference between G6PD deficiency and hemolytic anemia?
Glucose-6-phosphate dehydrogenase (G6PD) deficiency and hemolytic anemia are not the same, although they are closely related.
G6PD deficiency
- Genetic disorder: This is a genetic condition where you have a reduced amount of the enzyme glucose-6-phosphate dehydrogenase (G6PD) in your red blood cells. The gene that produces glucose-6-phosphate dehydrogenase (G6PD) can be found on the X-chromosome.
- Underlying cause: This enzyme helps protect red blood cells from damage caused by oxidative stress. Without enough glucose-6-phosphate dehydrogenase (G6PD), red blood cells are more susceptible to damage and breakdown.
- Not always symptomatic: Many people with glucose-6-phosphate dehydrogenase (G6PD) deficiency may never experience any symptoms, especially if they avoid triggers like certain medications, fava beans, or infections.
- Symptom, not a disease: This is a condition where red blood cells are destroyed faster than they can be produced by the body. This can lead to various symptoms like fatigue, weakness, pale skin, jaundice, and dark urine.
- Can have various causes: G6PD deficiency is one of many causes of hemolytic anemia, but other causes include autoimmune diseases, infections, sickle cell disease, and certain medications.
- Always symptomatic: People with hemolytic anemia will always experience at least some symptoms, although the severity can vary depending on the cause and individual factors.
In simpler terms, glucose-6-phosphate dehydrogenase (G6PD) deficiency is a genetic predisposition, while hemolytic anemia is a symptom that can be caused by various factors, including G6PD deficiency.
What happens to a baby with G6PD deficiency?
Babies born with G6PD deficiency can experience a range of outcomes, depending on the severity of their variant and other factors.
Most Cases
- No symptoms: Many babies with G6PD deficiency, especially those with milder variants, have no noticeable symptoms and develop normally. Their G6PD levels may be lower than average, but their red blood cells still function adequately.
- Jaundice: This is a common occurrence in newborn babies with G6PD deficiency, particularly in the first few days of life. It happens when bilirubin, a byproduct of red blood cell breakdown, builds up in the blood faster than the liver can process it. Jaundice usually resolves itself within a week or two with increased feeding and phototherapy (light therapy).
- Mild anemia: Some babies may experience mild anemia, leading to symptoms like fatigue and pale skin. This usually improves with age and proper management.
Severe Cases
- Severe anemia: In babies with severe variants of G6PD deficiency, red blood cell breakdown can be significant, leading to severe anemia and that it is more likely to be severe enough to cause kernicterus. This can require blood transfusions and close medical monitoring.
- Complications: In rarer cases, severe hemolytic episodes can trigger other complications like liver problems, kidney problems, and heart failure.
Important Factors
- Variant type: The severity of symptoms and potential complications depend heavily on the specific glucose-6-phosphate dehydrogenase (G6PD) variant the baby has. Some variants are much more severe than others.
- Trigger exposure: Avoiding triggers like certain medications, fava beans, and infections is crucial for preventing hemolytic episodes.
- Early diagnosis and management: Early identification of G6PD deficiency allows for proactive measures to avoid triggers and address any problems promptly, improving the baby’s health outcomes.
How does G6PD deficiency affect other medical conditions or treatments?
G6PD deficiency can have various impacts on other medical conditions and treatments, mainly due to its influence on red blood cell health and susceptibility to oxidative stress.
Increased susceptibility to infections
- Damaged red blood cells are less efficient at carrying oxygen and fighting off infections, potentially increasing susceptibility to bacterial and viral infections.
- Certain antibiotics used to treat infections can trigger G6PD-related hemolysis, requiring careful medication selection and monitoring.
Complications during surgery
- Pre-operative evaluation for G6PD deficiency is crucial to prevent hemolysis during surgery due to potential blood loss and oxidative stress.
- Transfusion of glucose-6-phosphate dehydrogenase (G6PD)-normal red blood cells might be necessary in specific cases to manage hemolytic complications during surgery.
Impact on certain medical conditions
- Sickle cell anemia: Individuals with both G6PD deficiency and sickle cell anemia might experience more severe hemolysis due to the additive effect on red blood cell fragility.
- Thalassemia: While less common, co-occurrence with G6PD deficiency might further complicate red blood cell health management.
Challenges with certain treatments
- Chemotherapy: Certain chemotherapy drugs can trigger hemolysis in G6PD-deficient individuals, requiring dose adjustments or alternative medications.
- Radiation therapy: Radiation therapy, particularly in areas rich in red blood cell production, can increase oxidative stress and potentially trigger hemolysis.
Overall, G6PD deficiency doesn’t necessarily preclude other medical conditions or treatments, but it requires careful consideration and management by healthcare professionals.
Can G6PD deficiency be misdiagnosed as other conditions? If so, how can we avoid this?
Yes, G6PD deficiency can sometimes be misdiagnosed as other conditions, especially in its milder forms. This can delay proper diagnosis and management, potentially leading to unnecessary treatment for the wrong condition.
Reasons for Misdiagnosis
- Non-specific symptoms: Early signs like fatigue, pale skin, and jaundice can be common to various other conditions like anemia, iron deficiency, or infections. Without a high index of suspicion, G6PD deficiency might be overlooked.
- Mild cases with infrequent or subtle symptoms: Individuals with milder variants might not experience frequent or obvious hemolytic episodes, making diagnosis less apparent.
- Lack of awareness among healthcare professionals: G6PD deficiency is less common in some regions, and healthcare professionals might not readily consider it as a diagnostic possibility.
- Limited or unreliable testing: Inaccessible or inaccurate diagnostic tests, especially in resource-limited settings, can contribute to misdiagnosis.
Preventing Misdiagnosis
- Raising awareness: Educating healthcare professionals about G6PD deficiency, its signs and symptoms, and high-risk populations is crucial.
- Comprehensive clinical history: Detailed medical history, including family history and potential triggers like fava beans or medications, can provide valuable clues.
- Accurate and accessible testing: Utilizing reliable tests like the quantitative G6PD enzyme assay is essential for confirmation or ruling out G6PD deficiency.
- Maintaining a high index of suspicion: In areas with high G6PD variant prevalence or for individuals with suggestive symptoms, a high degree of suspicion can promote timely diagnosis.
What are the differential diagnoses for G6PD deficiency?
Diagnosing G6PD deficiency isn’t always straightforward, especially in milder cases or when symptoms overlap with other conditions. Here’s a list of potential differential diagnoses, conditions that might initially seem like G6PD deficiency but require further investigation:
Non-spherocytic hemolytic anemias
- Thalassemia: Genetic disorder affecting hemoglobin production, leading to anemia and similar symptoms like fatigue and pale skin.
- Autoimmune hemolytic anemia: Immune system attacks red blood cells, causing destruction and symptoms similar to G6PD deficiency.
- Enzymopathies: Deficiencies in other red blood cell enzymes like pyruvate kinase can also cause hemolysis and mimic G6PD deficiency symptoms.
- Microangiopathic hemolytic anemia: Certain conditions like HELLP syndrome or thrombotic thrombocytopenic purpura can damage red blood cells in small blood vessels, leading to hemolysis.
Other conditions with some overlapping symptoms
- Iron deficiency anemia: This can cause fatigue, pale skin, and weakness, mimicking G6PD deficiency but responds differently to treatment.
- Vitamin B12 or folate deficiency: These deficiencies can also cause anemia and pale skin, requiring specific vitamin supplementation.
- Chronic infection: Some chronic infections can lead to mild anemia and fatigue, potentially appearing similar to G6PD deficiency.
- Liver disease: Liver problems can interfere with red blood cell production and cause jaundice, a symptom sometimes seen in G6PD deficiency.
Distinguishing factors
- Detailed medical history: Exploring family history, potential triggers, and past episodes can differentiate G6PD deficiency from other conditions.
- Physical examination: Checking for specific signs like splenomegaly (enlarged spleen), common in chronic hemolysis, can provide clues.
- Laboratory tests: G6PD enzyme assay is the definitive test for diagnosis, but other blood tests like complete blood count, reticulocyte count, and bilirubin levels can help distinguish between different causes of anemia and jaundice.
What are the limitations of current G6PD test methods?
While current glucose-6-phosphate dehydrogenase (G6PD) test methods have significantly improved diagnosis and management of the condition, they still have some limitations:
Accuracy
- Qualitative tests: These tests, like the Fluorescent Spot Test (FST), provide a yes/no answer for deficiency but don’t measure enzyme activity levels. This can be misleading, especially for heterozygous females who might have borderline enzyme activity but still be at risk of hemolysis under certain triggers.
- Quantitative tests: These tests, like the glucose-6-phosphate dehydrogenase (G6PD) enzyme assay, measure enzyme activity but may not accurately reflect individual sensitivity to triggers or the full spectrum of glucose-6-phosphate dehydrogenase (G6PD) variants.
Accessibility
- Cost: The glucose-6-phosphate dehydrogenase (G6PD) enzyme assay can be expensive, limiting access in resource-limited settings where the deficiency is most prevalent.
- Equipment and expertise: Performing the glucose-6-phosphate dehydrogenase (G6PD) enzyme assay requires specialized equipment and trained personnel, further limiting accessibility in certain regions.
Individual variability
- Enzyme activity levels: Even within the same glucose-6-phosphate dehydrogenase (G6PD) variant, individual enzyme activity can vary, making it challenging to predict individual risk of hemolysis based solely on enzyme levels.
- Trigger sensitivity: Individual susceptibility to triggers can vary, and current tests might not fully capture this variability.
Newborn screening
- False positives: Newborn screening programs using qualitative tests can have high false-positive rates, potentially leading to unnecessary anxiety and follow-up tests.
- Limited variant detection: Newborn screening programs might not detect all G6D variants, potentially missing individuals at risk.
Ongoing research
- New technologies like point-of-care tests and genetic analysis are being explored to improve accuracy, accessibility, and individual-specific risk assessment.
- Efforts are underway to develop more affordable and user-friendly testing methods for resource-limited settings.
Conclusion
While current glucose-6-phosphate dehydrogenase (G6PD) test methods have limitations, they remain crucial for diagnosis and management. Ongoing research aims to address these limitations and ensure accurate, accessible, and personalized testing for all individuals potentially affected by G6PD deficiency.
Is cetirizine safe for G6PD deficiency?
The safety of cetirizine for individuals with G6PD deficiency is a complex issue with varying opinions and ongoing research.
Potential Concern
- Cetirizine belongs to the antihistamine class, some of which are known triggers for hemolytic episodes in G6PD-deficient individuals. Examples include:
- Trimeprazine: This antihistamine has been widely documented to cause hemolysis in G6PD-deficient individuals.
- Dapsone: While primarily used for leprosy, it also has antihistamine properties and can trigger hemolysis in G6PD patients.
Limited Evidence
- No clear consensus: Despite being an antihistamine, cetirizine is not explicitly listed as a contraindicated medication for G6PD deficiency in major drug reference databases like MIMS (Malaysia) or the G6PD Deficiency Association website.
- Limited studies: Research specifically analyzing the safety of cetirizine in G6PD patients is scarce.
Reasons for Potential Safety
- Different mechanism: Cetirizine, unlike trimeprazine and dapsone, exerts its antihistamine effect through a different mechanism that might not directly affect red blood cell membranes, potentially reducing the risk of hemolysis.
- Individual variability: G6PD deficiency severity and individual sensitivity to triggers vary greatly. Some individuals with mild G6PD deficiency might tolerate cetirizine without issues, while others might be more susceptible.
Recommendations
- Consult your doctor: Discussing your G6PD deficiency with your doctor before taking cetirizine is crucial. They can consider your individual risk factors, medical history, and potential alternatives to ensure your safety.
- Start with low doses: If your doctor deems cetirizine appropriate, consider starting with a low dose and monitoring for any adverse reactions.
- Be aware of symptoms: Pay close attention to any potential signs of hemolysis like fatigue, jaundice, dark urine, or abdominal pain. If you experience any of these symptoms, stop taking cetirizine and seek medical attention immediately.
- Alternative options: If you’re concerned about potential risks, discuss alternative antihistamines considered safe for G6PD individuals with your doctor.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Luzzatto, L., Mehta, A. and Vulliamy, T. (2001) Glucose 6-Phosphate Dehydrogenase Deficiency. In: Scriver, C.R., Beaudet, A.L., Sly, W.S. and Valle, D., Eds., The Metabolic and Molecular Bases of Inherited Disease, 8th Edition, McGraw-Hill, New York, 4517-4533.
- Herz F, Kaplan E, Scheye ES. Diagnosis of erythrocyte glucose-6-phosphate dehydrogenase deficiency in the negro male despite hemolytic crisis. Blood 1970;35:953-954.
- Gautam, Keyoor. (2016). Glusoce-6-phosphate dehydrogenase- History and diagnosis. Journal of Pathology of Nepal. 6. 1034. 10.3126/jpn.v6i12.16260.
- Richardson SR, O’Malley GF. Glucose-6-Phosphate Dehydrogenase Deficiency. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470315/
- Ainoon, O., Boo, N. Y., Yu, Y. H., Cheong, S. K., Hamidah, H. N., & Lim, J. H. (2004). Complete molecular characterisation of glucose-6-phosphate dehydrogenase (G6PD) deficiency in a group of Malaysian Chinese neonates. The Malaysian journal of pathology, 26(2), 89–98.
- Luzzatto, L., Ally, M., & Notaro, R. (2020). Glucose-6-phosphate dehydrogenase deficiency. Blood, 136(11), 1225–1240. https://doi.org/10.1182/blood.2019000944