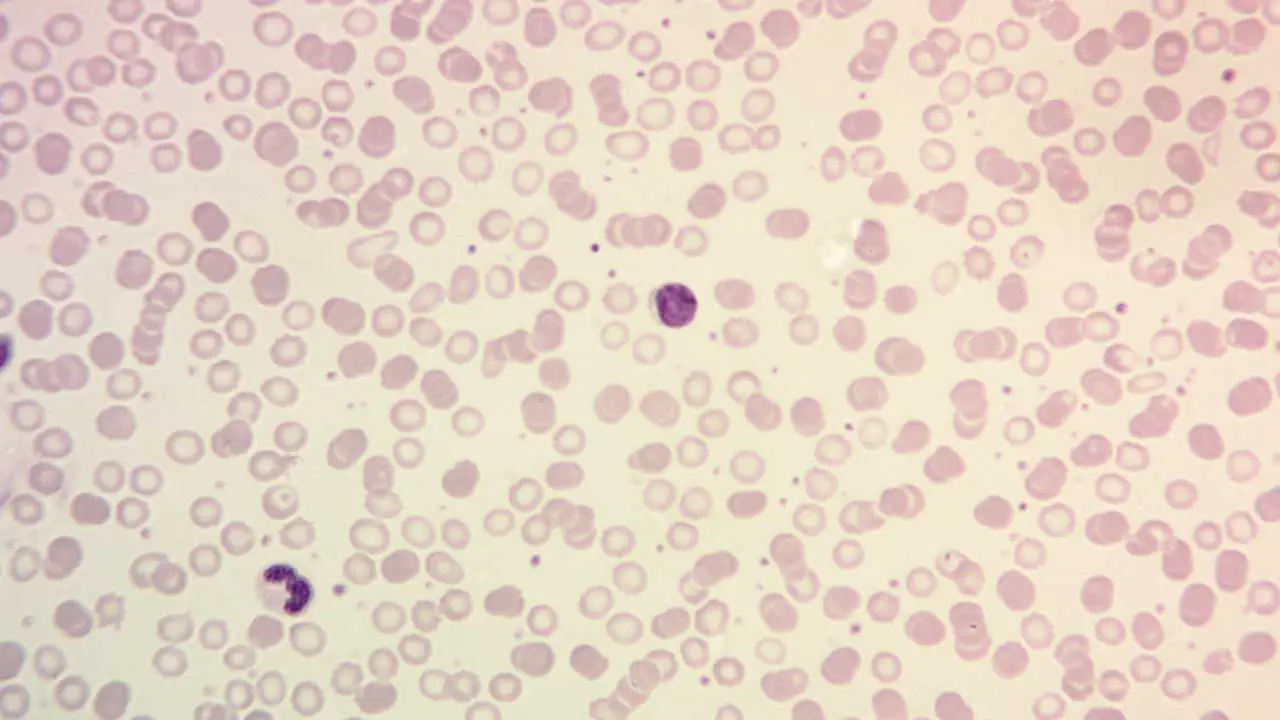
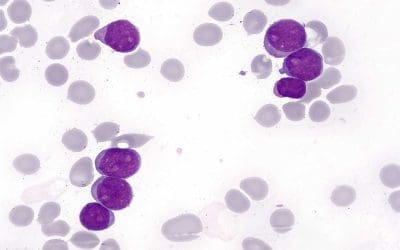
What is hematopathology?
Hemato-what? Ah, hematopathology! This exciting field bridges the gap between these two specialties. It combines the expertise of both hematology and pathology, focusing specifically on diseases and disorders affecting:
- Blood cells: Their production, morphology, and function.
- Bone marrow: The factory where blood cells are born.
- Lymphoid organs: Lymph nodes, spleen, and thymus, essential for immune function.
Hematology: Hematology delves into the composition, function, and disorders of blood cells, including red blood cells, white blood cells, and platelets. Hematologists analyze blood tests, diagnose blood-related diseases like anemia or leukemia, and manage their treatment.
Pathology: Pathology analyzes body samples (biopsies) to diagnose diseases, identify their origin and progression, and help guide treatment decisions. Pathologists play a crucial role in understanding and managing various medical conditions.
What does a hematopathologist do?
A hematopathologist wears many hats, playing a crucial role in diagnosing and managing various blood-related disorders. Here’s a glimpse into their diverse responsibilities.
Diagnostic Roles
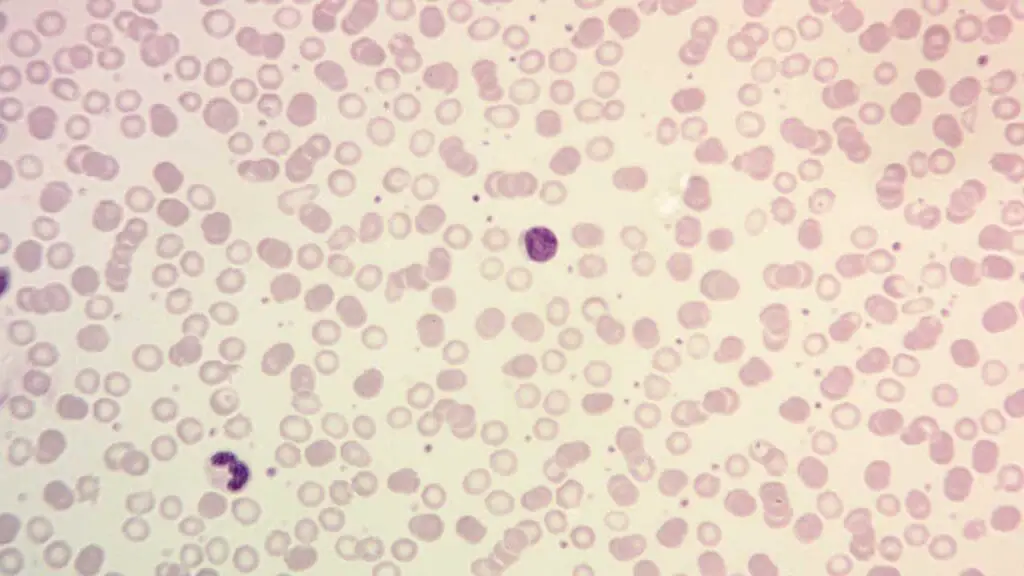
- Examining samples: They analyze blood smears, bone marrow biopsies, and tissue samples from lymph nodes and other lymphoid tissues under a microscope.
- Identifying abnormalities: Their keen eye detects subtle changes in cell morphology, number, and distribution, pointing towards potential diseases.
- Employing advanced tools: Flow cytometry, immunohistochemistry, and molecular diagnostics assist in pinpointing specific cell types and genetic mutations.
- Delivering diagnoses: They interpret all findings and provide accurate diagnoses of various blood disorders like leukemias, lymphomas, anemias, and hemoglobinopathies.
Clinical Collaborator
- Consulting with clinicians: They work closely with doctors, sharing their findings and insights to guide patient management and treatment decisions.
- Providing prognostic information: They assess the stage and aggressiveness of the disease, offering valuable insights into the likely course of illness and potential outcomes.
- Supporting clinical trials: Their expertise helps evaluate new medications and therapies for blood disorders, advancing research and patient care.
Research Activities
- Contributing to ongoing research: They actively participate in research projects to better understand the causes, progression, and treatment of blood diseases.
- Developing new techniques: They explore novel diagnostic methods and therapeutic strategies to improve patient outcomes.
- Staying at the forefront: They continuously update their knowledge through conferences, publications, and collaborations, ensuring they provide the most advanced care.
Remember, this is just a snapshot! Hematopathologists wear many other hats depending on their specific subspecialty and work setting. Some may focus primarily on diagnosing specific diseases, while others might delve deeper into research or education.
Subspecialties within hematopathology
Delving deeper into the fascinating world of hematopathology, we encounter various subspecialties, each offering a unique focus and expertise. These subspecialties allow hematopathologists to refine their skills and knowledge, becoming true masters in specific areas of blood disorders. Here’s a glimpse into some prominent subspecialties:
Lymphoma Pathology
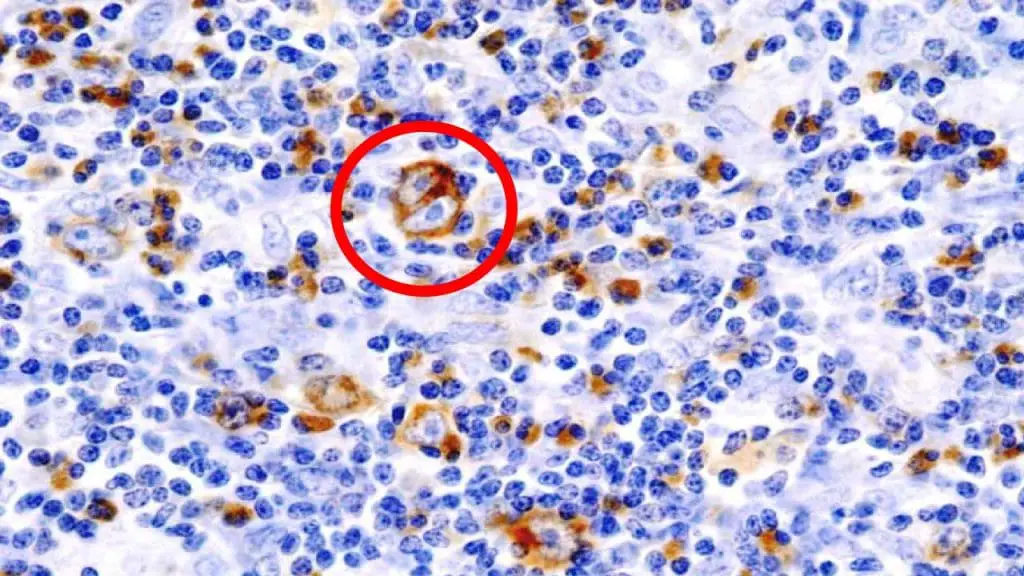
- Focus: Diagnosing and classifying lymphomas, cancers of the lymphatic system.
- Expertise: Identifying different lymphoma subtypes based on their morphology, immunophenotype, and genetic features.
- Importance: Accurate diagnosis is crucial for guiding treatment decisions and predicting patient outcomes.
Leukemia Pathology
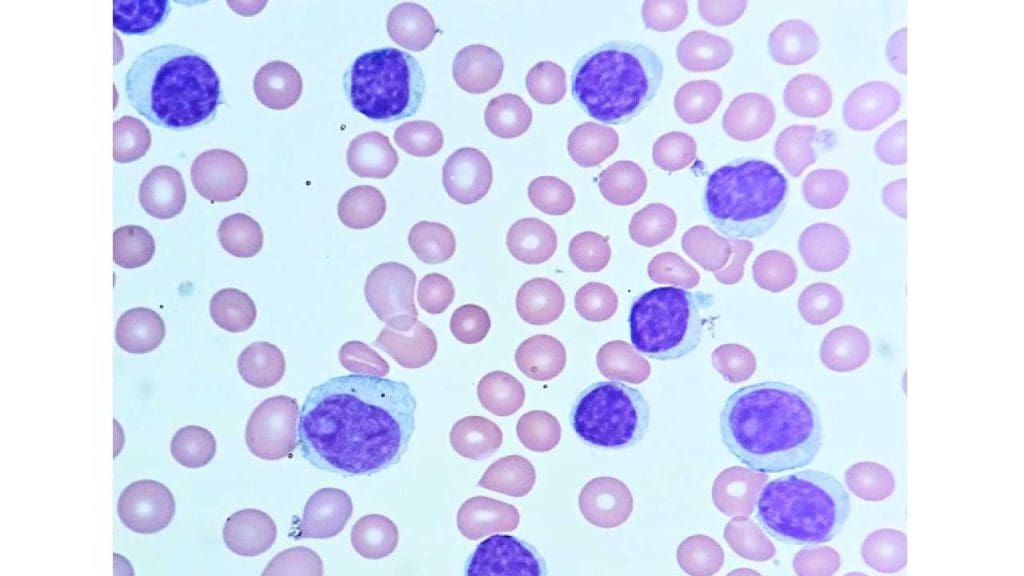
- Focus: Diagnosing and classifying leukemias, cancers of the white blood cells.
- Expertise: Distinguishing between different leukemia types based on cell morphology, immunophenotype, and cytogenetic abnormalities.
- Importance: Timely and accurate diagnosis is essential for initiating appropriate treatment, often involving chemotherapy or stem cell transplantation.
Myeloid Neoplasia
- Focus: Diagnosing and managing diseases affecting myeloid cells, which give rise to red blood cells, white blood cells, and platelets.
- Examples: Myeloproliferative disorders (increased cell production), myelodysplastic syndromes (abnormal cell development), and myelodysplastic/myeloproliferative neoplasms (overlapping features).
- Importance: Early diagnosis and appropriate treatment can improve patient outcomes and quality of life.
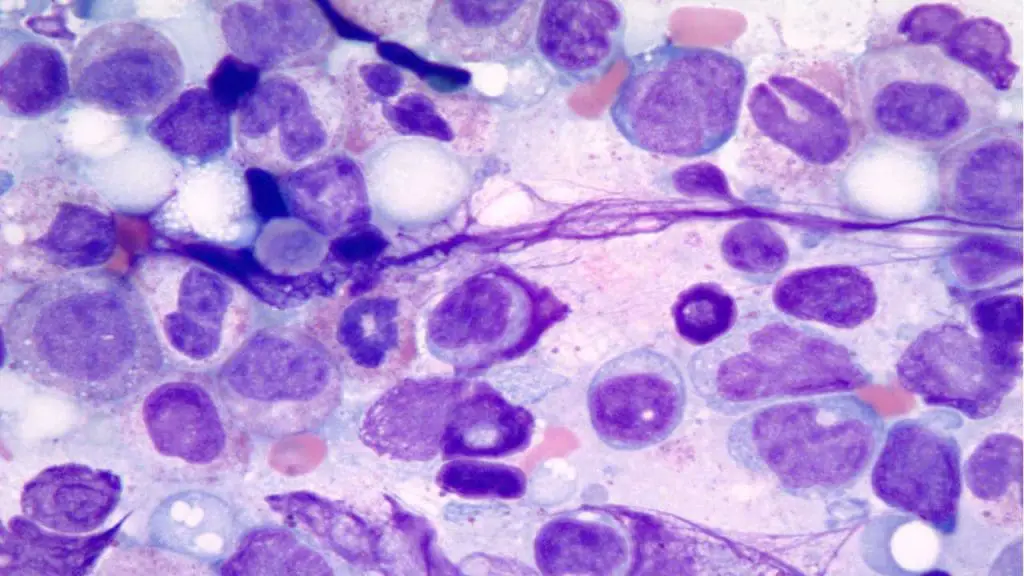
Hemoglobinopathy Diagnostics
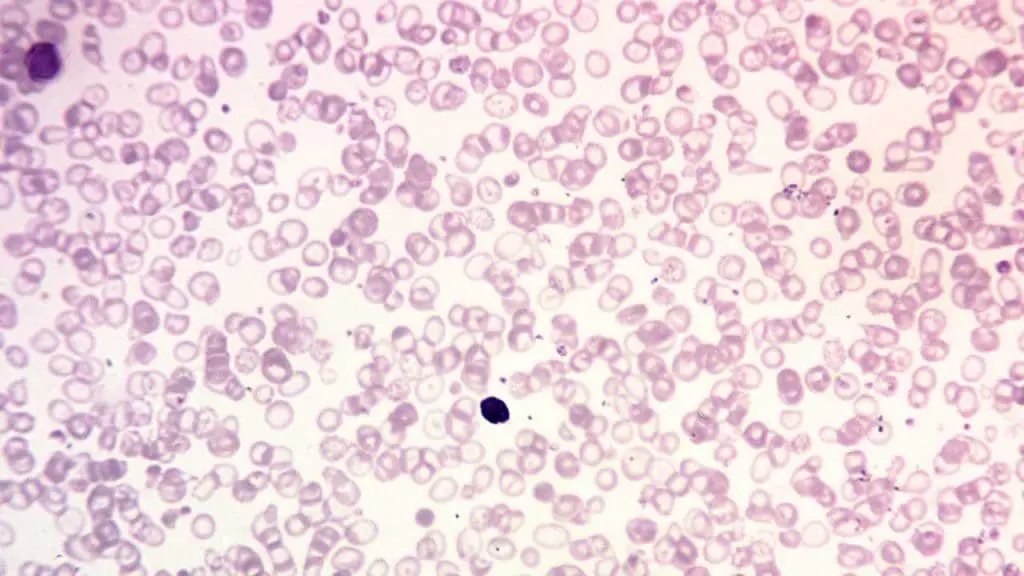
- Focus: Diagnosing and managing inherited disorders of hemoglobin, the protein responsible for oxygen transport in red blood cells.
- Examples: Sickle cell disease, thalassemia, and other hemoglobinopathies.
- Importance: Accurate diagnosis and management can prevent complications and improve the lives of patients with these lifelong conditions.
Flow Cytometry
- Focus: Utilizing flow cytometry, a laser-based technology to analyze and characterize individual cells based on their size, granularity, and specific proteins they express.
- Applications: Diagnosing and monitoring various blood disorders, including leukemias, lymphomas, and minimal residual disease.
- Importance: Provides rapid and accurate information about cell populations, aiding in diagnosis, treatment selection, and monitoring response to therapy.
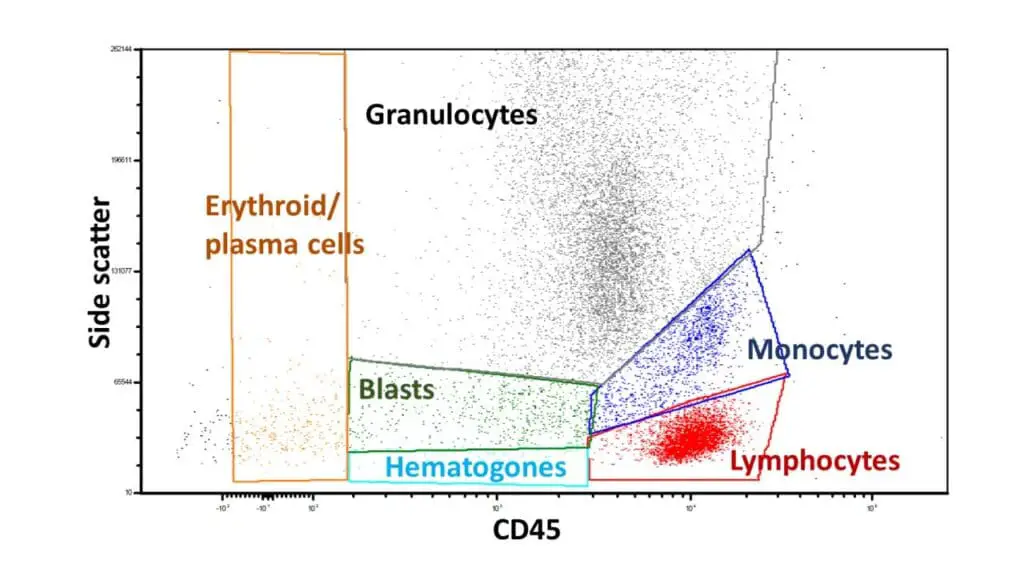
These are just a few examples, and the field of hematopathology continues to evolve, with new subspecialties emerging as technology and research advance.
Job scope and career paths in hematopathology
Being a hematopathologist offers a fulfilling and intellectually stimulating career path with diverse opportunities. Let’s explore the various aspects of their job scope and potential career trajectories.
Job Scope
- Diagnostic Duties: Analyzing blood smears, bone marrow biopsies, and tissue samples from lymph nodes and other organs to diagnose various blood disorders like leukemias, lymphomas, anemias, and hemoglobinopathies.
- Clinical Collaboration: Consulting with clinicians to guide patient management, treatment decisions, and provide prognostic information.
- Research Activities: Contributing to ongoing research, developing new diagnostic techniques and therapeutic strategies, and staying at the forefront of new developments.
- Education and Training: Some hematopathologists may also participate in teaching medical students, residents, and fellow pathologists.
Work Settings
- Hospitals and Medical Centers: This is the most common setting, where they provide diagnostic services to patients and collaborate with clinicians.
- Academic Institutions: They may conduct research, teach medical students and residents, and contribute to scientific publications.
- Reference Laboratories: These laboratories offer specialized diagnostic services to other healthcare providers and may require expertise in specific subspecialties.
- Private Practice: Some hematopathologists choose to establish their own practice, offering diagnostic services to patients and other healthcare providers.
Career Paths
- Clinical Practice: Focus primarily on patient diagnosis and care, potentially specializing in a specific subspecialty.
- Research: Dedicate a larger portion of their time to research activities, collaborating with other scientists and contributing to advancements in the field.
- Academics: Combine clinical practice with teaching and research at a university or medical school.
- Industry: Collaborate with pharmaceutical or biotechnology companies on developing new diagnostic tools and therapies.
- Leadership and Administration: Hold leadership positions in hospitals, laboratories, or professional organizations.
Factors Influencing Career Path
- Personal Interests: Passion for research, clinical practice, education, or a specific subspecialty.
- Work-Life Balance: Preferences for research hours, patient interaction, and administrative duties.
- Geographical Location: Opportunities available in different regions and institutions.
Additional Information
- Salary: Hematopathologists typically earn competitive salaries, with factors like experience, subspecialty, and location impacting the specific range.
- Demand: The demand for hematopathologists is expected to grow due to an aging population and the increasing need for specialized blood disorder diagnosis.
- Certification: Board certification by the American Board of Pathology (ABP) is required for independent practice.
Remember: This is a general overview, and individual career paths will vary depending on personal goals, opportunities, and interests. Exploring different settings, networking with professionals, and attending conferences can help you navigate your career journey within this exciting field.
The rise of personalized medicine and its impact on hematopathology
Personalized medicine, tailoring treatment to an individual’s unique genetic and molecular makeup, is revolutionizing healthcare, and hematopathology is at the forefront of this exciting transformation. Here’s how:
Personalized Diagnosis
- Molecular profiling: Hematopathologists can now utilize advanced tools like next-generation sequencing to identify specific genetic mutations in a patient’s cancer cells. This allows for a more precise diagnosis, differentiating between subtypes of diseases with similar appearances under a microscope.
- Targeted therapies: Identifying specific mutations helps select therapies designed to attack those specific alterations, leading to more effective and less toxic treatment compared to “one-size-fits-all” approaches.
Precision Prognosis
- Predicting risk: Genetic and molecular markers can predict a patient’s risk of developing certain blood disorders or their disease progression, allowing for early intervention and preventive measures.
- Tailored monitoring: Personalized approaches can identify patients at risk for relapse, enabling more targeted monitoring and intervention strategies.
Treatment Optimization
- Drug response prediction: Genetic analysis can predict a patient’s response to specific therapies, avoiding ineffective treatments and potential side effects.
- Minimizing resistance: Personalized approaches can help identify patients at risk of developing resistance to specific therapies, allowing for timely adjustments in treatment plans.
Challenges and Opportunities
- Data integration: Integrating massive amounts of genetic and clinical data for comprehensive analysis and effective decision-making requires robust bioinformatics infrastructure and expertise.
- Ethical considerations: Access to and implications of genetic information present ethical and social challenges that need careful consideration and responsible management.
- Collaboration: Effective implementation of personalized medicine requires close collaboration between hematopathologists, oncologists, geneticists, and other healthcare professionals.
The Future of Hematopathology
- Expanding subspecialties: As personalized medicine advances, subspecialties in hematopathology focused on specific genetic alterations and targeted therapies are likely to emerge.
- Precision clinical trials: Hematopathologists will play a crucial role in designing and conducting clinical trials focused on specific patient populations with targeted therapies.
- Improved patient outcomes: With personalized approaches, hematopathology promises to contribute significantly to improved patient outcomes, longer survival rates, and better quality of life for individuals with blood disorders.
In conclusion, the rise of personalized medicine is transforming hematopathology, pushing the boundaries of diagnosis, prognosis, and treatment for blood disorders. While challenges exist, the future of this field is bright, offering exciting opportunities for innovation and delivering hope for patients.
Ethical considerations in diagnosing and managing blood disorders
As the field of hematopathology embraces personalized medicine and advanced technologies, navigating ethical considerations becomes increasingly crucial. Here are some key aspects to consider:
Informed Consent and Genetic Testing
- Patients have the right to understand the implications of genetic testing and give informed consent for its use in diagnosis and treatment decisions.
- Balancing detailed information with potential emotional strain on patients requires respectful communication and clear explanations of testing benefits and limitations.
- Access to genetic testing and its potential financial burden raise concerns about equitable healthcare and ensuring all patients have access to this information.
Data Privacy and Security
- Protecting patient genetic and medical data is paramount. Robust security measures and clear data governance policies are necessary to prevent unauthorized access or misuse.
- Balancing data sharing for research and collaboration with individual privacy raises complex ethical concerns, requiring careful consideration and patient consent.
Fairness and Equity in Treatment
- Personalized treatment based on genetic data could exacerbate existing healthcare disparities if access is limited by cost or other factors.
- Ensuring equitable access to personalized medicine for all patient populations regardless of ethnicity, socioeconomic status, or geographical location is crucial.
- Cultural and religious beliefs regarding genetic testing and targeted therapies must be carefully considered and respected.
Allocation of Resources and Cost-Effectiveness
- Advanced diagnostic tools and personalized therapies can be expensive, raising questions about resource allocation and ensuring their cost-effectiveness compared to alternative treatments.
- Balancing the potential benefits of personalized medicine with resource constraints and equitable access to other healthcare needs is vital.
Informed Decision-Making and Shared Responsibility
- Patients should be actively involved in treatment decisions based on a clear understanding of the risks and benefits of personalized approaches compared to traditional treatment options.
- Shared decision-making between patients, hematopathologists, and other healthcare professionals fosters trust and ensures alignment with patient preferences and values.
Ethical considerations are integral to responsible practice in hematopathology. By acknowledging these challenges and actively discussing them within the field and with patients, we can ensure that personalized medicine truly benefits all individuals and advances healthcare in a just and equitable manner.
Remember, this is not an exhaustive list, and ongoing research and discussions are shaping the ethical landscape of personalized medicine in hematopathology. By embracing ethical principles and open communication, we can ensure that this field advances responsibly and benefits all patients.
Emerging technologies and their potential to revolutionize the field
The field of hematopathology is constantly evolving, with exciting new technologies emerging that have the potential to revolutionize diagnosis, treatment, and research.
Artificial Intelligence (AI)
- Image analysis: AI algorithms can analyze microscopic images of blood smears and tissue samples with high accuracy, automating tasks currently performed by pathologists and potentially even improving diagnostic accuracy.
- Predictive modeling: AI can analyze vast datasets to predict patient outcomes, guide treatment decisions, and identify individuals at risk for developing blood disorders.
Liquid biopsies
- Non-invasive testing: Analyzing circulating tumor cells, DNA, or RNA in blood samples offers a minimally invasive alternative to traditional tissue biopsies, enabling earlier diagnosis and monitoring of disease progression.
- Personalized medicine: Analyzing genetic mutations in circulating tumor cells can guide targeted therapies tailored to individual patients.
Point-of-care diagnostics
- Rapid and decentralized testing: Portable and user-friendly diagnostic devices could enable rapid diagnosis of blood disorders in resource-limited settings or even at the point of care.
- Early intervention: Faster diagnoses can lead to earlier intervention and potentially improve patient outcomes.
Microfluidics
- Miniaturized analysis: Microfluidic devices can automate complex laboratory processes, allowing for faster and more efficient analysis of blood samples.
- Drug testing: Microfluidic chips can be used for personalized drug testing, predicting a patient’s response to specific therapies.
3D printing and tissue engineering
- Creating models: Printing of 3D models of patient tissues based on their specific genetic makeup can aid in personalized drug testing and surgical planning.
- Regenerative medicine: Tissue engineering holds potential for developing personalized therapies for blood disorders like sickle cell anemia.
Challenges and Opportunities
- Integration and validation: Integrating these technologies into clinical practice requires robust validation, standardization, and cost-effectiveness analysis.
- Data privacy and security: Ensuring patient data privacy and security is crucial when utilizing AI and other advanced technologies.
- Training and expertise: Healthcare professionals need training and support to effectively utilize these emerging technologies in daily practice.
Conclusion
Emerging technologies are poised to transform the field of hematopathology, leading to more accurate diagnosis, personalized treatment, and improved patient outcomes. By embracing these advancements responsibly and addressing the associated challenges, we can unlock the full potential of these technologies and revolutionize the care of patients with blood disorders.
Frequently Asked Questions (FAQs)
What is the difference between hematology and hematopathology?
While both hematology and hematopathology deal with blood and blood disorders, they differ in their specific focus and approach.
Hematology
- Focus: Primarily clinical, dealing with the diagnosis, management, and treatment of blood disorders.
- Activities: Performing blood tests, analyzing results, diagnosing conditions like anemia, leukemia, and clotting disorders, recommending and administering treatment plans, and monitoring patient progress.
- Scope: Broader, encompassing the entire blood system, including red blood cells, white blood cells, platelets, and bone marrow.
- Expertise: In understanding blood cell function, physiology, and diseases, using clinical skills and knowledge to manage patient care.
Hematopathology
- Focus: Primarily diagnostic, using laboratory techniques and microscopic examination of blood and tissues to identify and categorize blood disorders.
- Activities: Analyzing blood smears, bone marrow biopsies, lymph node samples, and other tissues, diagnosing specific diseases based on morphological and molecular features, collaborating with clinicians to guide treatment decisions.
- Scope: More specialized, focusing on the microscopic and molecular analysis of blood cells and tissues to aid in diagnosing blood disorders.
- Expertise: In microscopic examination, immunohistochemistry, flow cytometry, and molecular diagnostics to characterize and classify blood disorders.
Is hematopathology anatomic or clinical pathology?
Hematopathology is considered a subspecialty of both anatomic and clinical pathology. While this might seem confusing, it’s because it uniquely bridges the gap between these two major branches of pathology:
Anatomic Pathology
- Focuses on analyzing tissues and organs removed from the body (biopsies, autopsies) to diagnose diseases.
- Techniques include gross examination, microscopic evaluation, and various staining methods.
- Subspecialties include surgical pathology, neuropathology, and dermatopathology.
Clinical Pathology
- Focuses on analyzing bodily fluids and samples like blood, urine, and cerebrospinal fluid.
- Techniques include various laboratory tests, chemical analysis, and microscopy.
- Subspecialties include clinical chemistry, microbiology, and hematology.
Hematopathology
- Borrows aspects from both disciplines:
- Analyzes tissues and organs like lymph nodes and bone marrow samples, similar to anatomic pathology.
- Focuses on blood cells and their disorders, closely linked to clinical hematology.
- Utilizes a blend of techniques from both, including microscopy, immunohistochemistry, and flow cytometry.
Therefore, while hematopathologists have expertise in diagnosing diseases through tissue analysis, their specific focus on blood cells and disorders aligns them closely with clinical hematology. This unique combination contributes to their vital role in diagnosing and managing a wide range of blood-related conditions.
What is the difference between hematopathology and cytology?
While both hematopathology and cytology deal with examining cells under a microscope to diagnose diseases, they have some key differences.
Focus
- Hematopathology: Primarily focuses on blood cells and tissues directly related to the blood system, such as bone marrow, lymph nodes, and spleen. Its main concern is diagnosing disorders of blood cell production and function, like leukemias, lymphomas, and anemias.
- Cytology: Examines cells from various body sites, including organs, fluids like urine and cerebrospinal fluid, and even brushings or scrapings from skin or other surfaces. Its scope is broader, covering a wide range of conditions like cancer (not just blood cancers), infections, and inflammatory diseases.
Sample types
- Hematopathology: Analyzes blood smears, bone marrow biopsies, tissue samples from lymph nodes and other lymphoid organs.
- Cytology: Examines Pap smears, fine-needle aspirations (FNAs) of organs, body fluids, and even brushings from various body sites.
Diagnostic approach
- Hematopathology: Often uses specialized techniques like flow cytometry and immunohistochemistry to analyze specific cell markers and assess their function.
- Cytology: Primarily relies on morphological features of cells observed under a microscope to identify abnormalities and suggest potential diagnoses.
Treatment involvement
- Hematopathology: Plays a crucial role in diagnosing and classifying blood disorders, which helps guide treatment decisions made by clinicians.
- Cytology: Primarily provides diagnostic information to guide further investigations or treatment decisions made by other specialists.
Example
- If you have an enlarged lymph node, a hematopathologist would analyze a biopsy from that node to diagnose whether it’s a lymphoma or another blood-related issue.
- If you have an abnormal Pap smear, a cytologist would examine the cells to see if they show precancerous changes, but wouldn’t necessarily diagnose the specific type of cancer.
Essentially
- Think of hematopathology as specialists for blood cells and related tissues.
- Think of cytology as generalists for examining cells from various body sites.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.