TL;DR
Anemia is a common blood disorder characterized by a deficiency of red blood cells or hemoglobin, leading to reduced oxygen-carrying capacity. It’s a significant global health concern, particularly prevalent in developing countries, affecting productivity and economic development.
- Hemoglobin Physiology ▾: Hemoglobin is crucial for oxygen transport. Its structure, composed of four polypeptide chains (two alpha, two beta) each binding a heme molecule with an iron atom, allows for reversible oxygen binding and release, adapting to the body’s needs.
- Physiological Adaptations to Anemia ▾: The body compensates for reduced oxygen delivery through mechanisms like increased cardiac output and respiratory rate, enhanced tissue oxygen extraction, and alterations in red blood cell properties (shape, size, oxygen affinity).
- Anemia Symptoms and Factors Affecting Them ▾: Symptoms vary based on the rate of anemia development (acute vs. chronic) and individual characteristics (age, gender, health status). General symptoms include fatigue, weakness, pale skin, and shortness of breath. Specific disorders can present with additional signs like splenomegaly, jaundice, neurological symptoms (in B12 deficiency), or skin ulcers (in sickle cell anemia).
- Classification of Anemia ▾: Anemia is classified morphologically based on red blood cell size (normocytic, microcytic, macrocytic) and severity based on hemoglobin levels.
- Red Cell Balance and Production Assessment ▾: The balance between red blood cell production (erythropoiesis in bone marrow) and destruction determines anemia. Reticulocyte count, a measure of immature red blood cells, is used to assess the bone marrow’s red blood cell production capacity.
- Major Causes of Anemia ▾: Anemia can stem from four main categories
- Blood Loss: Acute (e.g., trauma) or chronic (e.g., gastrointestinal bleeding).
- Nutritional Deficiency: Most commonly iron, vitamin B12, or folate deficiency.
- Bone Marrow Failure: Syndromes where the bone marrow fails to produce blood cells (e.g., aplastic anemia, myelodysplastic syndromes).
- Hemolytic Anemia: Increased destruction of red blood cells due to intrinsic abnormalities, autoimmune responses, or infections.
- Diagnosis and Treatment ▾: Diagnosis typically begins with a Complete Blood Count (CBC) and reticulocyte count, followed by further specific tests to determine the underlying cause. Treatment strategies are tailored to the cause and may involve addressing the underlying condition, nutritional supplementation, or blood transfusions.
*Click ▾ for more information
Introduction
Anemia is a common blood disorder characterized by a deficiency of red blood cells or hemoglobin, leading to a reduced oxygen-carrying capacity of the blood. This condition can manifest in various forms and can have significant health consequences.
Definition
Anemia is defined as a reduction in the hemoglobin level below the normal range for age and gender or a reduction in the hemoglobin concentration of the blood according to gender and age.
Hemoglobin in the red blood cells is responsible for carrying oxygen from the lungs to the body’s tissues. When hemoglobin levels are reduced, the body’s tissues receive insufficient oxygen, leading to a range of anemia symptoms and potential complications.
Prevalence
Anemia is a global health concern, affecting an estimated 2.6 billion people worldwide. The prevalence of anemia is particularly high in developing countries, where factors such as malnutrition, infectious diseases, and inadequate healthcare contribute to its widespread distribution.
In Africa, it affects an estimated 40% of the population, with women and children being disproportionately affected. The World Health Organization (WHO) estimates that 27% of pregnant women and 40% of children under five in Africa are anemic.
In Southeast Asia, it affects approximately 32% of the population. Pregnant women and children under five are again the most vulnerable groups, with 30% of pregnant women and 40% of children under five in the region experiencing anemia.
These examples highlight the disproportionate burden of this disorder on developing countries. The prevalence of this disorder in these regions is significantly higher than in developed countries, where it affects an estimated 5% of the population.
The factors contributing to it’s high prevalence in developing countries are complex and interrelated. Malnutrition, particularly iron deficiency is often exacerbated by poor dietary intake, inadequate access to clean water and sanitation, and recurrent infections.
Infectious diseases such as malaria, hookworm, and schistosomiasis also contribute significantly to anemia in developing countries. These diseases can cause direct destruction of red blood cells or lead to chronic blood loss, further depleting the body’s iron stores.
Inadequate healthcare services and lack of access to treatment and prevention programs further perpetuate the high prevalence of anemia in these regions. Early diagnosis and treatment are crucial for preventing complications and improving quality of life, but these services are often limited in developing countries.
Significance of anemia as a global health concern
Anemia poses a significant threat to individual productivity and economic development at a national level. Its widespread prevalence, particularly in developing countries, hinders individuals’ ability to contribute effectively to the workforce and undermines overall economic growth.
It’s impact on productivity stems from its physiological consequences. Reduced oxygen-carrying capacity leads to symptoms for example fatigue, weakness, and impaired cognitive function, all of which diminish an individual’s ability to engage in physical labor, perform mental tasks, and maintain focus. This can lead to decreased work output, increased absenteeism, and higher rates of workplace accidents.
The economic ramifications of anemia extend beyond individual productivity losses. Anemia’s prevalence affects a nation’s overall labor force, reducing its capacity to contribute to economic growth. A weakened workforce can hinder industrial production, agricultural output, and service sector performance, thus slowing down economic development.
Moreover, it’s negative impact on maternal and child health has further economic implications. Pregnant women with anemia are more likely to experience complications during pregnancy and childbirth, increasing healthcare costs and potentially reducing the number of healthy children entering the workforce in the future.
Addressing this requires a multifaceted approach that encompasses preventive measures, early diagnosis, and effective treatment. Nutritional interventions, such as iron supplementation and fortification of staple foods, can significantly reduce the prevalence of iron deficiency anemia, a major cause of anemia worldwide. Additionally, improving access to healthcare services and promoting early detection and treatment of can help individuals return to productive work and contribute to economic growth.
Combating this disorder is not only a humanitarian imperative but also an economic necessity. Investing in prevention and control programs can yield significant returns in terms of improved individual health, increased productivity, and enhanced economic development. Prioritizing anemia reduction strategies is a crucial step towards achieving sustainable development goals and creating a more prosperous future for all.
Erythropoiesis
Red blood cell (RBC) production, a critical process known as erythropoiesis, occurs primarily in the red bone marrow, the specialized tissue responsible for blood cell formation. This intricate process involves a series of coordinated events, tightly regulated by various factors, to ensure the continuous supply of red blood cells and maintain adequate hemoglobin levels.
The journey of RBC production begins with hematopoietic stem cells, residing in the niches of the red bone marrow. These stem cells possess the remarkable ability to self-renew and differentiate into various blood cell lineages, including red blood cells. Under the influence of specific growth factors, hematopoietic stem cells give rise to myeloid progenitor cells, a committed group of cells destined to become either red blood cells, granulocytes, monocytes, or macrophages.
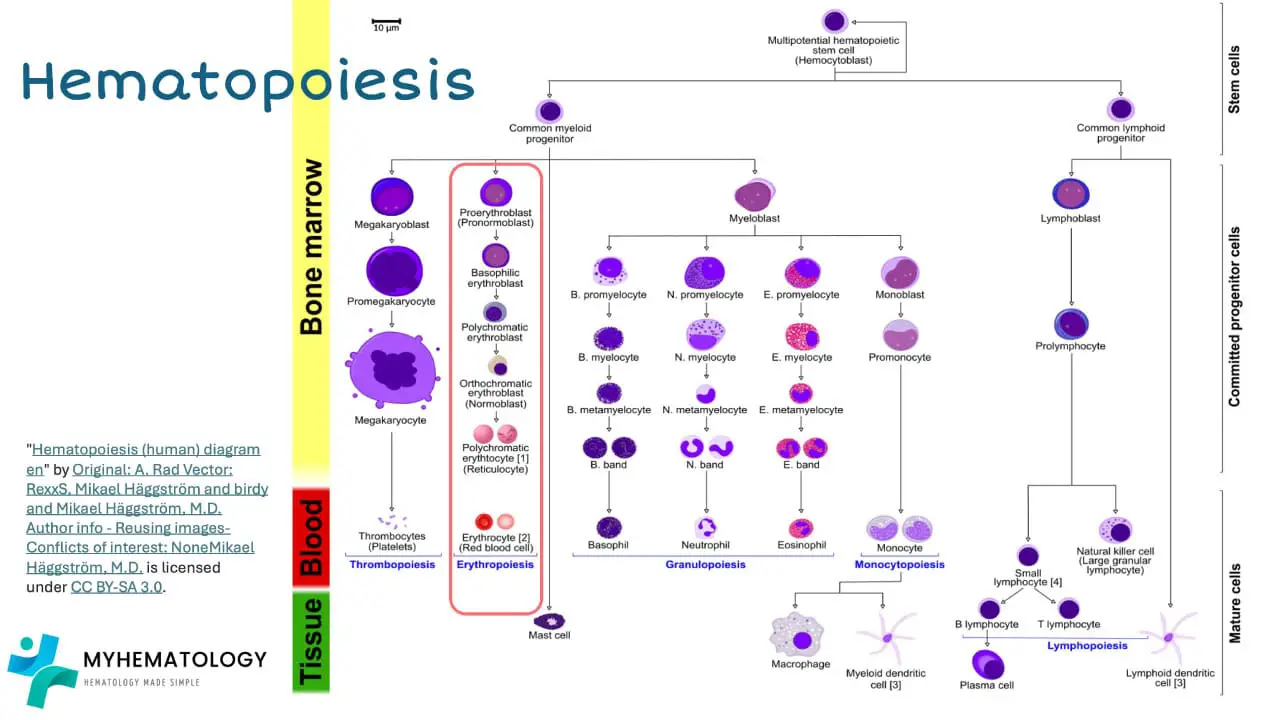
Myeloid progenitor cells further differentiate into erythroid progenitor cells, which are dedicated precursors to red blood cells. These erythroid progenitor cells undergo a series of rapid divisions, accompanied by the synthesis of globin chains, the protein subunits of hemoglobin. Simultaneously, heme, the non-protein component of hemoglobin containing the oxygen-binding iron atom, is also synthesized.
As the erythroid progenitor cells mature, they expel their nuclei and transform into reticulocytes, immature red blood cells. Reticulocytes retain some residual RNA and organelles, which are gradually removed as they mature into fully functional red blood cells. These mature red blood cells, laden with hemoglobin, are released into the bloodstream, ready to embark on their oxygen-carrying mission.
The regulation of erythropoiesis is a complex interplay of various factors, including the body’s oxygen demand, the availability of essential nutrients, and hormonal signaling. Erythropoietin (EPO), a hormone produced by the kidneys in response to low oxygen levels, plays a pivotal role in stimulating erythropoiesis. EPO binds to specific receptors on erythroid progenitor cells, triggering a cascade of signaling events that promote their proliferation, differentiation, and hemoglobin synthesis.
Physiology of Hemoglobin
Hemoglobin, the oxygen-carrying protein within red blood cells, plays a vital role in transporting oxygen from the lungs to the body’s tissues. Its unique structure and intricate mechanisms enable it to efficiently capture, transport, and release oxygen, ensuring the continuous supply of this essential molecule to all cells.
Structure and function of hemoglobin
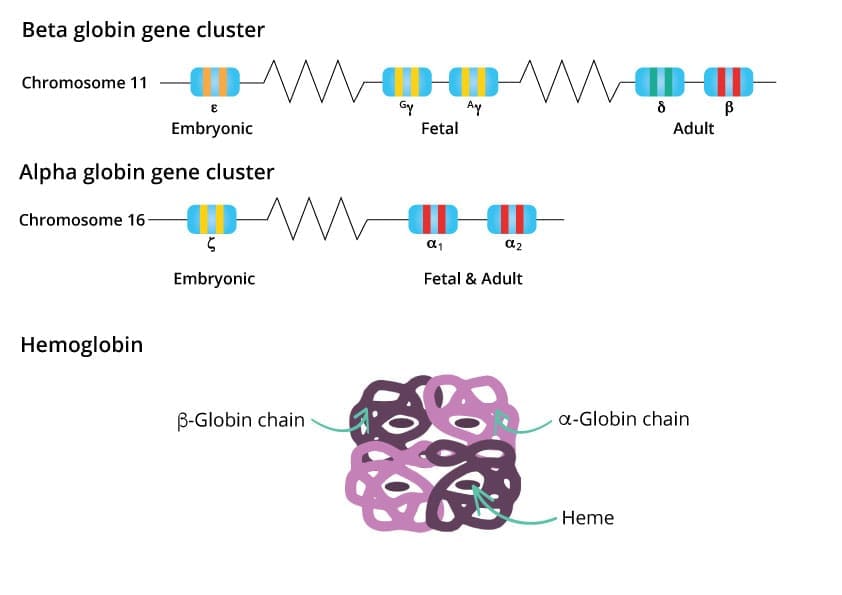
Hemoglobin is a complex molecular protein tetramer that provides an efficient transport of oxygen from the lungs to the body’s tissues. Its intricate structure and dynamic function are essential for maintaining life.
Hemoglobin is a tetramer, meaning it is composed of four polypeptide chains, two alpha chains and two beta chains. Each globin chain, the structural unit of hemoglobin, is folded into a complex three-dimensional structure that provides a binding site for a heme molecule. The heme molecule is a prosthetic group, a non-protein component that plays a crucial role in hemoglobin’s function.
At the heart of the heme molecule lies an iron atom (Fe2+), the oxygen-binding site. This iron atom coordinates with four nitrogen atoms from the porphyrin ring, a flat, ring-shaped molecule that forms the core of the heme group. The remaining two coordination sites on the iron atom are available for oxygen binding.
The specific sites where the heme molecules are anchored to the globin chains are critical for hemoglobin’s function. These binding sites are precisely positioned within hydrophobic pockets of the globin chains, creating a stable environment for the heme molecule and facilitating its interaction with oxygen molecules.
The interaction between hemoglobin and oxygen is reversible, allowing hemoglobin to pick up oxygen in the lungs and release it in the tissues. This reversible binding is facilitated by the conformational changes that hemoglobin undergoes as it transitions between its deoxygenated (deoxyhemoglobin) and oxygenated (oxyhemoglobin) states.
In the deoxygenated state, the iron atom in the heme group is slightly out of plane, creating a distorted structure. This distortion reduces the affinity of hemoglobin for oxygen. Upon oxygen binding, the iron atom pulls back into the plane, causing a conformational change in the heme group and the surrounding globin chains. This conformational change increases the affinity of hemoglobin for oxygen, allowing it to bind additional oxygen molecules.
Normal hemoglobin levels and variations between genders
Normal hemoglobin levels vary slightly between genders, reflecting physiological differences and hormonal influences.
In adult men, the average hemoglobin level ranges from 14 to 17 grams per deciliter (g/dL), while in adult women, the range is from 12 to 15 g/dL. This difference in hemoglobin levels is primarily attributed to the influence of testosterone, the primary male sex hormone.
Testosterone has a direct stimulatory effect on erythropoiesis, the process of red blood cell production. This stimulatory effect is mediated by several mechanisms, including increased production of erythropoietin (EPO), the hormone that regulates erythropoiesis. EPO stimulates the proliferation and differentiation of erythroid progenitor cells, leading to increased red blood cell production and, consequently, higher hemoglobin levels.
The higher testosterone levels in men compared to women contribute to their higher hemoglobin levels. However, it is important to note that hemoglobin levels can also be influenced by other factors, such as age, ethnicity, and overall health status.
Hormonal influences on hemoglobin levels extend beyond testosterone. In women, estrogen, the primary female sex hormone, has a mild suppressive effect on erythropoiesis. This suppressive effect is thought to be mediated by estrogen’s modulation of EPO production and its impact on iron metabolism.
While testosterone and estrogen play significant roles in regulating hemoglobin levels, other hormones also contribute to this process. Androgens, a group of hormones that includes testosterone, have a stimulatory effect on erythropoiesis, while thyroid hormones and corticosteroids can also influence red blood cell production and hemoglobin levels.
Factors affecting hemoglobin levels
Numerous factors can influence hemoglobin levels, causing them to deviate from the normal range. Some common causes of low hemoglobin levels, known as anemia, include:
- Iron deficiency: Iron is a crucial component of heme, and its deficiency is a leading cause of anemia worldwide. Poor dietary intake, blood loss, and certain medical conditions can all contribute to iron deficiency anemia (IDA).
- Vitamin B12 deficiency: Vitamin B12 is essential for DNA synthesis, and its deficiency can impair red blood cell production, leading to anemia.
- Folic acid deficiency: Folic acid is another essential nutrient for red blood cell production, and its deficiency can also cause anemia.
- Bone marrow failure: Conditions that affect the bone marrow’s ability to produce red blood cells, such as aplastic anemia or myelodysplastic syndrome.
- Hemolytic anemia: This group of anemias is characterized by the premature destruction of red blood cells, resulting in a decrease in hemoglobin levels.
Conversely, elevated hemoglobin levels, known as polycythemia, can arise from various causes, including:
- Chronic obstructive pulmonary disease (COPD): In COPD, the lungs become less efficient in exchanging oxygen, leading to compensatory increases in red blood cell production and hemoglobin levels.
- Congenital heart defects: Certain heart defects can cause the body to produce excessive red blood cells to compensate for reduced oxygen levels.
- High-altitude living: Individuals living at high altitudes have increased red blood cell production to adapt to the lower oxygen pressure.
Major Adaptations to Anemia
Anemia presents challenges to the body’s ability to transport oxygen effectively. To compensate for this reduced oxygen supply, the body undergoes a series of physiological adaptations, known as major adaptations to anemia, aimed at maintaining adequate oxygen delivery to the body’s tissues.
Physiological Mechanisms for Compensating Reduced Oxygen Delivery
Increased cardiac output and respiratory rate
The heart, acting as the body’s pump, plays a pivotal role in compensating for reduced oxygen delivery. When oxygen levels are low, the heart responds by increasing its output, the amount of blood it pumps per minute. This increase in cardiac output is achieved by two primary mechanisms:
- Increased heart rate: The heart beats more rapidly, allowing it to pump more blood with each stroke. This increase in heart rate is mediated by the autonomic nervous system, particularly the sympathetic nervous system, which triggers the release of hormones that stimulate the heart’s pacemaker cells.
- Increased stroke volume: The amount of blood pumped with each heartbeat increases, further contributing to the overall increase in cardiac output. This increase in stroke volume is achieved by several factors, including increased contractility of the heart muscle and reduced resistance to blood flow.
By increasing cardiac output, the heart delivers more oxygen-rich blood to the tissues, partially compensating for the reduced oxygen-carrying capacity caused by anemia. This adaptation is crucial for maintaining vital organ function and preventing tissue damage.
In addition to increased cardiac output, the body also responds to reduced oxygen delivery by increasing respiratory rate, the number of breaths taken per minute. This increase in respiratory rate enhances the intake of oxygen from the atmosphere and facilitates its transfer into the bloodstream.
The increased respiratory rate is triggered by chemoreceptors, specialized cells located in the carotid bodies and aortic arch that are sensitive to changes in blood oxygen levels. When oxygen levels fall, these chemoreceptors send signals to the brainstem, which in turn stimulates the respiratory center to increase breathing rate.
By taking more breaths per minute, the lungs allow more oxygen to diffuse into the blood, compensating for the reduced oxygen-carrying capacity associated with anemia. This adaptation ensures that oxygen-rich blood is available for delivery to the body’s tissues.
Enhanced tissue oxygen extraction
Under normal conditions, cells extract approximately 25% of the oxygen carried by hemoglobin. However, when oxygen delivery is reduced, cells can adapt to extract a greater proportion of oxygen, ensuring that they receive adequate oxygen for their metabolic needs.
This enhanced tissue oxygen extraction is facilitated by several mechanisms:
- Increased blood flow to active tissues: During periods of increased activity, blood flow to the affected tissues increases to provide more oxygen. This increase in blood flow is mediated by local vasodilation, which allows more blood to reach the active tissues.
- Increased capillary density: In response to chronic oxygen deprivation, tissues can increase their capillary density, creating a more extensive network of capillaries that facilitates the exchange of oxygen between the blood and the tissues.
- Metabolic adaptations: Cells can adapt their metabolism to utilize oxygen more efficiently under conditions of reduced oxygen availability. This may involve switching to anaerobic metabolism, which produces less energy but can sustain cellular function in the absence of adequate oxygen.
- Increased glycolysis: Glycolysis, a metabolic pathway that produces energy from glucose in the absence of oxygen, can be upregulated in response to reduced oxygen delivery. This increased glycolysis provides an alternative source of energy for cellular function.
Alterations in red blood cell properties
Alterations in RBC Shape and Size
In response to reduced oxygen delivery, RBCs can undergo shape and size changes to enhance their ability to deliver oxygen to the tissues.
- Increased RBC deformability: RBCs become more deformable, allowing them to squeeze through narrow capillaries and reach even the most peripheral tissues. This increased deformability is achieved by changes in the RBC membrane composition and cytoskeletal structure.
- Increased mean corpuscular volume (MCV): RBCs may increase in size, becoming macrocytic, which can increase their oxygen-carrying capacity. This increase in MCV is associated with increased hemoglobin synthesis and RBC production.
Alterations in RBC Oxygen Affinity
RBCs can also adapt their oxygen affinity to optimize oxygen delivery under conditions of reduced oxygen availability.
- Increased 2,3-DPG production: 2,3-diphosphoglycerate (2,3-DPG) binds to hemoglobin, reducing its affinity for oxygen. This increased 2,3-DPG production facilitates oxygen release from hemoglobin in the tissues, ensuring adequate oxygen delivery.
- Alterations in allosteric properties of hemoglobin: The allosteric properties of hemoglobin, which govern its oxygen-binding affinity, can be altered in response to changes in oxygen levels and other factors. This modulation of hemoglobin’s oxygen affinity can further optimize oxygen delivery to the tissues.
Factors Affecting Anemic Symptoms
Anemia manifests in a range of clinical features that vary in severity and depend on the underlying cause, the duration of anemia, and the individual’s overall health status. Understanding the factors that affect anemia symptoms is crucial for accurate diagnosis, effective treatment, and preventing complications.
Rate of Development
Acute vs. Chronic Anemia
Acute
Acute anemia develops rapidly over a short period, typically within a few days or weeks. The sudden decrease in red blood cells or hemoglobin can lead to a constellation of symptoms, including:
- Severe fatigue and weakness: The reduced oxygen-carrying capacity of the blood causes marked tiredness and a lack of energy even during minimal activity.
- Dyspnea (shortness of breath): The body struggles to maintain adequate oxygen supply to the tissues, leading to breathlessness, especially upon exertion.
- Tachycardia (rapid heart rate): The heart compensates for the reduced oxygen delivery by increasing its rate to pump more blood per minute.
- Pallor (pale skin): The deficiency of oxygen-rich blood circulating in the capillaries causes the skin and mucous membranes to appear pale.
- Lightheadedness and dizziness: The reduced oxygen supply to the brain can lead to dizziness, lightheadedness, and even fainting.
Chronic
Chronic anemia develops gradually over a longer period, often months or even years. The body may have some time to adapt to the reduced oxygen-carrying capacity, leading to less pronounced symptoms. However, as chronic anemia worsens, symptoms can become more noticeable and may include:
- Fatigue and weakness: Fatigue is a common symptom, but it may be less severe than in acute anemia.
- Dyspnea (shortness of breath): Exertion can become increasingly difficult due to the reduced oxygen supply to the muscles.
- Tachycardia (rapid heart rate): The heart compensates for the reduced oxygen delivery by increasing its rate.
- Pallor (pale skin): The skin and mucous membranes may appear pale, but it may be less noticeable than in acute anemia.
- Cognitive impairment: Chronic anemia can affect concentration, memory, and overall cognitive function.
Impact of rapid versus gradual onset on clinical presentation
The rate of anemia development significantly impacts the clinical presentation. Rapid-onset anemia’s sudden onset leads to more severe and immediate symptoms, while gradual-onset anemia’s slow development allows for some adaptation, resulting in less pronounced anemia symptoms initially. However, chronic anemia can progress over time, leading to anemia worsening symptoms and potential complications.
Understanding the distinction between rapid-onset and gradual-onset anemia is essential for clinicians to accurately assess the severity of the condition, identify the underlying cause, and provide appropriate treatment. Prompt diagnosis and management of rapid-onset anemia are crucial to prevent severe symptoms and complications. Chronic anemia requires ongoing monitoring and treatment to maintain adequate oxygen supply and prevent long-term health consequences.
Individual Patient Characteristics
The clinical presentation of anemia can be influenced by individual patient characteristics, including age, gender, and overall health status. These factors can affect the severity of symptoms, the likelihood of developing anemia, and the response to treatment.
Age
Age plays a significant role in the presentation of anemia. Older adults are more prone to developing anemia due to a combination of factors, including:
- Increased prevalence of underlying medical conditions: Chronic conditions such as heart disease, kidney disease, and cancer can contribute to anemia.
- Reduced absorption of nutrients: Older adults may have impaired absorption of iron, vitamin B12, and folic acid, which are essential for red blood cell production.
- Increased risk of blood loss: Older adults may experience more frequent blood loss due to gastrointestinal issues or medications.
The symptoms in older adults may be less noticeable or attributed to other aging-related changes, making it more challenging to diagnose.
Gender
Gender differences in anemia presentation are primarily attributed to physiological factors, particularly hormonal influences.
- Women: Women of reproductive age are more susceptible to IDA due to menstrual blood loss. Additionally, pregnancy can increase iron requirements, putting women at risk if iron intake is inadequate.
- Men: Men are more prone to hemolytic anemias, such as sickle cell anemia, which is an inherited blood disorder.
Overall Health Status
The overall health status of an individual significantly impacts the presentation of anemia. Individuals with underlying medical conditions, such as heart disease, lung disease, or malnutrition, may experience more severe anemia symptoms and complications.
- Heart disease: Anemia can worsen heart function in individuals with existing heart conditions, leading to increased fatigue, shortness of breath, and swelling of the legs.
- Lung disease: It can exacerbate the symptoms of lung disease, such as shortness of breath and difficulty breathing, causing further impairment of respiratory function.
- Malnutrition: Individuals with malnutrition may have deficiencies in essential nutrients, such as iron, vitamin B12, and folic acid, which can predispose them to anemia.
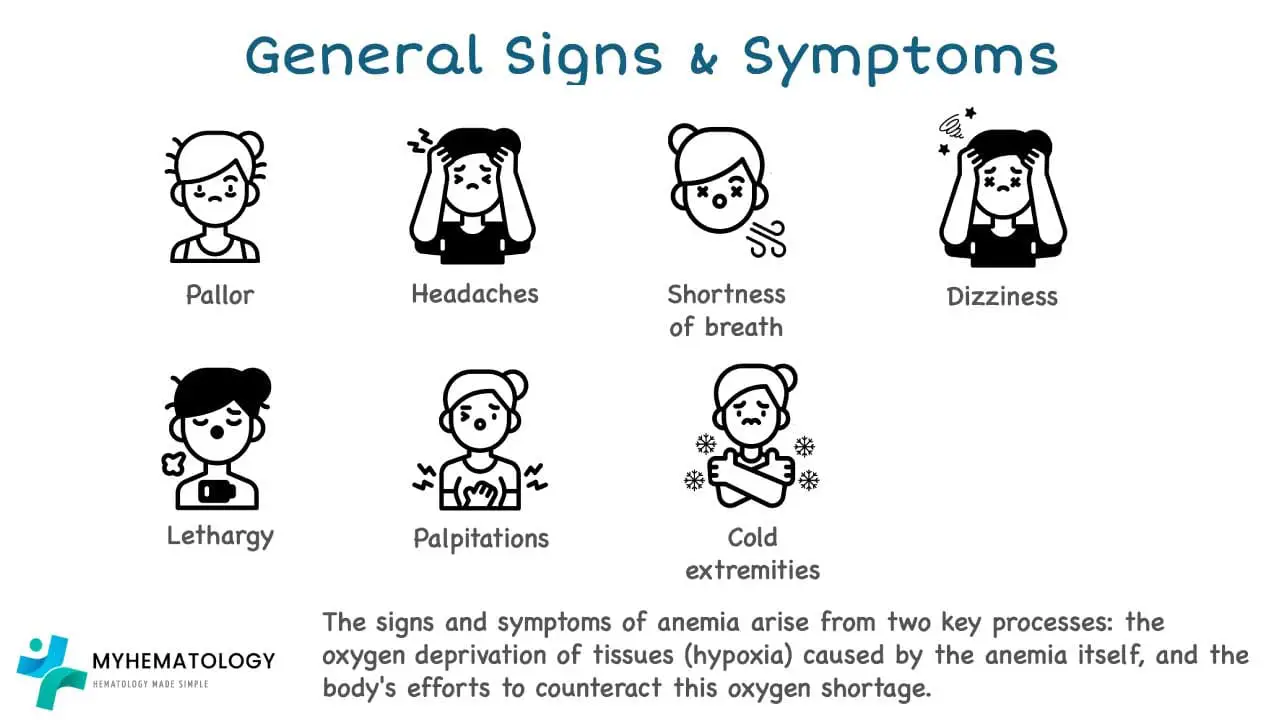
General Signs and Symptoms
Fatigue and Weakness
Fatigue and weakness are common symptoms, a condition characterized by a deficiency in red blood cells or hemoglobin. The reduced oxygen-carrying capacity of the blood in anemia leads to a generalized feeling of tiredness and lack of energy, which can significantly impact an individual’s daily activities and quality of life.
Fatigue
Fatigue is a hallmark symptom and is often described as an overwhelming tiredness that persists even after rest or sleep. The reduced oxygen supply to the body’s tissues interferes with cellular energy production, leading to a feeling of exhaustion and a decreased ability to perform physical or mental tasks.
Weakness
Weakness, the inability to exert muscle force, is another common symptom. The reduced oxygen delivery to the muscles in anemia impairs their ability to contract effectively, leading to muscle weakness and a decreased ability to perform physical activities.
Severity of Fatigue and Weakness
The severity of fatigue and weakness varies depending on the underlying cause and the degree of anemia. In mild anemia, fatigue and weakness may be subtle and only noticeable during exertion. However, as anemia worsens, these symptoms become more pronounced and can significantly impact an individual’s daily life.
Impact on Daily Activities
Fatigue and weakness can have a significant impact on an individual’s ability to perform daily activities. These symptoms can make it difficult to complete work or school tasks, engage in hobbies or social activities, and maintain household chores.
Accompanying Symptoms
Fatigue and weakness are often accompanied by other symptoms, such as:
- Pallor (pale skin)
- Shortness of breath
- Dizziness and lightheadedness
- Headaches
- Cold hands and feet
Pale Skin and Mucous Membranes
Pale skin and mucous membranes are common symptoms. The reduced oxygen-carrying capacity of the blood leads to a decrease in blood flow to the skin and mucous membranes, causing them to appear pale.
Pallor of the Skin
The skin, especially the face, lips, and nail beds, may appear pale or white in anemia. This pallor is due to the reduced amount of oxygenated blood circulating in the capillaries, the smallest blood vessels that supply oxygen and nutrients to the skin. The degree of pallor can vary depending on the severity of anemia and may be more noticeable in individuals with lighter skin tones.
Pallor of Mucous Membranes
Mucous membranes, the moist tissues that line the inside of the body, can also appear pale in anemia. This includes the inside of the mouth, lips, gums, tongue, and the inside of the eyelids. The pallor of mucous membranes is due to the same underlying mechanism as pallor of the skin, namely the reduced oxygenation of these tissues.
Significance of Pallor
Pallor is a non-specific sign, meaning it can be caused by a variety of factors, including anemia. However, it is an important clinical sign that should not be overlooked. In combination with other symptoms, such as fatigue, weakness, and shortness of breath, pallor can raise suspicion of anemia and prompt further investigation.
Underlying Causes of Pallor
The underlying causes of pallor in anemia be broadly categorized as:
- Reduced red blood cell production: This can be caused by iron deficiency, vitamin B12 deficiency, folic acid deficiency, or chronic diseases that affect bone marrow function.
- Increased red blood cell destruction: This can be caused by hemolytic anemias, which are characterized by the premature destruction of red blood cells.
- Blood loss: Significant blood loss, either acute or chronic, can also lead to anemia and pallor.
Shortness of Breath
Shortness of breath, also known as dyspnea, is a common symptom, a condition characterized by a deficiency in red blood cells or hemoglobin. The reduced oxygen-carrying capacity of the blood in anemia leads to a decreased ability to deliver oxygen to the body’s tissues, particularly during exertion, causing shortness of breath.
Mechanism of Shortness of Breath
During physical activity, the body’s oxygen demand increases to meet the increased energy demands of the muscles and other tissues. The reduced oxygen-carrying capacity of the blood limits the body’s ability to deliver adequate oxygen to these tissues, leading to shortness of breath.
Severity of Shortness of Breath
The severity of shortness of breath varies depending on the underlying cause, the degree of anemia, and the individual’s overall fitness level. In mild anemia, shortness of breath may only occur during moderate or strenuous exercise. However, as anemia worsens, shortness of breath can become more pronounced and may occur even during minimal exertion or at rest.
Impact on Daily Activities
Shortness of breath can significantly impact an individual’s ability to perform daily activities. It can make it difficult to exercise, climb stairs, walk for extended periods, or engage in other physical activities. This can lead to a decline in physical fitness, increased social isolation, and reduced quality of life.
Specific Signs Related to Certain Disorders
Splenomegaly and Hepatomegaly in Hemolytic Anemia
Splenomegaly and hepatomegaly, the enlargement of the spleen and liver, respectively, are specific signs that can be associated with hemolytic anemia, a condition characterized by the premature destruction of red blood cells. These enlargements occur due to the increased workload placed on these organs in response to the excessive breakdown of red blood cells.
Splenomegaly
The spleen plays a crucial role in filtering and removing damaged or abnormal red blood cells from circulation. The increased rate of red blood cell destruction overwhelms the spleen’s normal filtering capacity, leading to its enlargement. Splenomegaly can cause abdominal discomfort, a feeling of fullness in the upper left side of the abdomen, and occasionally, left shoulder pain.
Hepatomegaly
The liver also plays a role in processing and clearing bilirubin, a yellow pigment produced from the breakdown of hemoglobin. In hemolytic anemia, the excessive release of bilirubin due to increased red blood cell destruction can overload the liver’s ability to process and excrete it. This can lead to hepatomegaly, which may cause abdominal discomfort, nausea, and loss of appetite.
Association with Specific Hemolytic Anemias
The prominence of splenomegaly and hepatomegaly varies depending on the underlying type of hemolytic anemia.
For instance, splenomegaly is a common feature of hereditary spherocytosis, an inherited disorder characterized by abnormally shaped red blood cells that are more susceptible to destruction.
In contrast, hepatomegaly is more prominent in certain types of acquired hemolytic anemias, such as autoimmune hemolytic anemia, where antibodies attack and destroy healthy red blood cells.
Diagnostic Significance
The presence of splenomegaly and hepatomegaly in conjunction with other symptoms, such as jaundice, fatigue, and pale skin, can raise suspicion of hemolytic anemia. These enlargements can be detected through physical examination, imaging studies such as ultrasound or abdominal CT scans, and blood tests to assess liver and spleen function.
Management Strategies
The management of splenomegaly and hepatomegaly depends on the underlying cause and the severity of the enlargements. In some cases, splenectomy, the surgical removal of the spleen, may be considered to reduce the destruction of red blood cells and alleviate splenomegaly. For hepatomegaly, treatment focuses on managing the underlying hemolytic anemia to reduce the burden on the liver and prevent further enlargement.
Jaundice in Hemolytic Anemia
Jaundice, a yellowing of the skin and eyes, is a common sign. The excessive breakdown of red blood cells releases bilirubin, a yellow pigment, into the bloodstream. When the liver is unable to process and excrete bilirubin at a normal rate, it accumulates in the blood and tissues, leading to jaundice.
Mechanism of Jaundice
The increased rate of red blood cell destruction leads to an excessive release of bilirubin. This bilirubin, known as unconjugated bilirubin, is poorly soluble in the blood and cannot be directly excreted by the liver. The liver needs to convert unconjugated bilirubin into a form called conjugated bilirubin, which is more water-soluble and can be excreted through the bile ducts into the intestines.
However, in hemolytic anemia, the liver may be overwhelmed by the excessive amount of unconjugated bilirubin, leading to an accumulation of both unconjugated and conjugated bilirubin in the blood. This accumulation of bilirubin causes the yellowing of the skin and eyes, a characteristic sign of jaundice.
Severity of Jaundice
The severity of jaundice depends on the underlying cause and the degree of anemia. In mild cases, jaundice may be subtle and only noticeable in the whites of the eyes, known as scleral icterus. However, as anemia worsens and bilirubin levels rise, jaundice can become more pronounced, affecting the skin, eyes, and even mucous membranes.
Accompanying Symptoms
Jaundice is often accompanied by other symptoms, such as:
- Fatigue and weakness
- Pale skin
- Shortness of breath
- Splenomegaly (enlarged spleen)
- Hepatomegaly (enlarged liver)
Importance of Diagnosis and Treatment
Early diagnosis and treatment of hemolytic anemia are crucial for preventing complications, including jaundice. By addressing the underlying cause, the body’s ability to produce red blood cells and hemoglobin can be restored, leading to a significant improvement in jaundice and other symptoms.
In severe cases, phototherapy, a treatment that uses light to break down bilirubin, may be used to reduce jaundice and prevent bilirubin from reaching harmful levels. In rare cases, blood transfusions may be necessary to replenish red blood cells and alleviate the symptoms of hemolytic anemia.
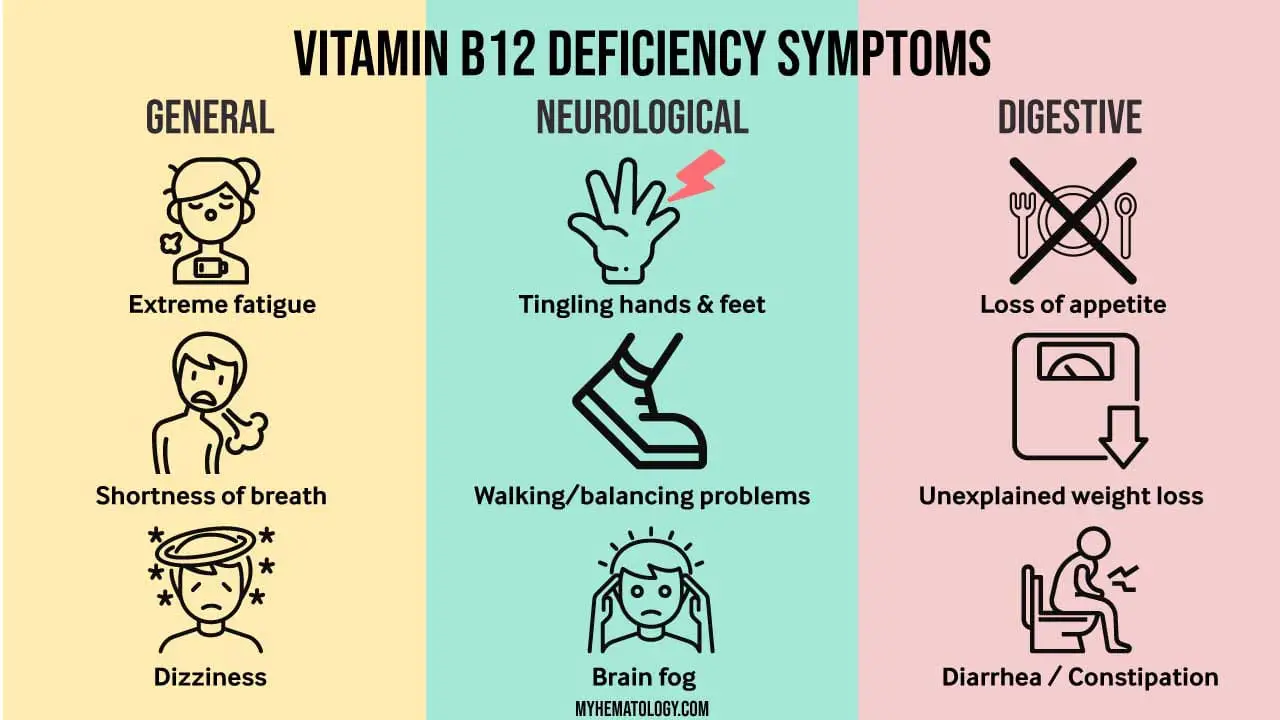
Neurological Symptoms in Vitamin B12 Deficiency Anemia
Neurological symptoms are a significant concern in vitamin B12 deficiency anemia, a condition characterized by a lack of vitamin B12, an essential nutrient for nerve cell function. Vitamin B12 plays a crucial role in the synthesis of myelin, the protective sheath that surrounds nerve fibers, ensuring proper transmission of nerve impulses. When vitamin B12 is deficient, myelin production is impaired, leading to damage to nerve fibers and the development of neurological symptoms.
Mechanism of Neurological Symptoms
Vitamin B12 deficiency can affect the central nervous system (CNS), the peripheral nervous system (PNS), and even the optic nerve. In the CNS, vitamin B12 deficiency can lead to demyelination, the loss of myelin from nerve fibers, causing disruption of nerve signaling and resulting in a range of neurological symptoms.
Types of Neurological Symptoms
The type and severity of neurological symptoms in vitamin B12 deficiency anemia vary depending on the extent of myelin damage and the affected nerve pathways. Common neurological symptoms include:
- Paresthesias: Abnormal sensations such as tingling, numbness, and burning pain in the hands and feet.
- Ataxia: Uncoordinated movements, difficulty walking, and balance problems.
- Cognitive impairment: Memory loss, confusion, and difficulty concentrating.
- Mood changes: Depression, irritability, and emotional lability.
- Optic neuropathy: Vision problems, including blurred vision, double vision, and loss of peripheral vision.
Severity and Progression
The severity of neurological symptoms in vitamin B12 deficiency anemia can range from mild to severe. In mild cases, symptoms may be subtle and may not be immediately recognized. However, as vitamin B12 deficiency progresses, symptoms can become more pronounced and can significantly impact an individual’s daily life and quality of life.
Importance of Early Diagnosis and Treatment
Early diagnosis and treatment of vitamin B12 deficiency are crucial for preventing irreversible neurological damage. Prompt initiation of vitamin B12 supplementation can halt myelin degeneration and improve neurological symptoms. In some cases, additional neurological interventions may be necessary to manage specific symptoms.
Skin Ulcer in Sickle Cell Anemia
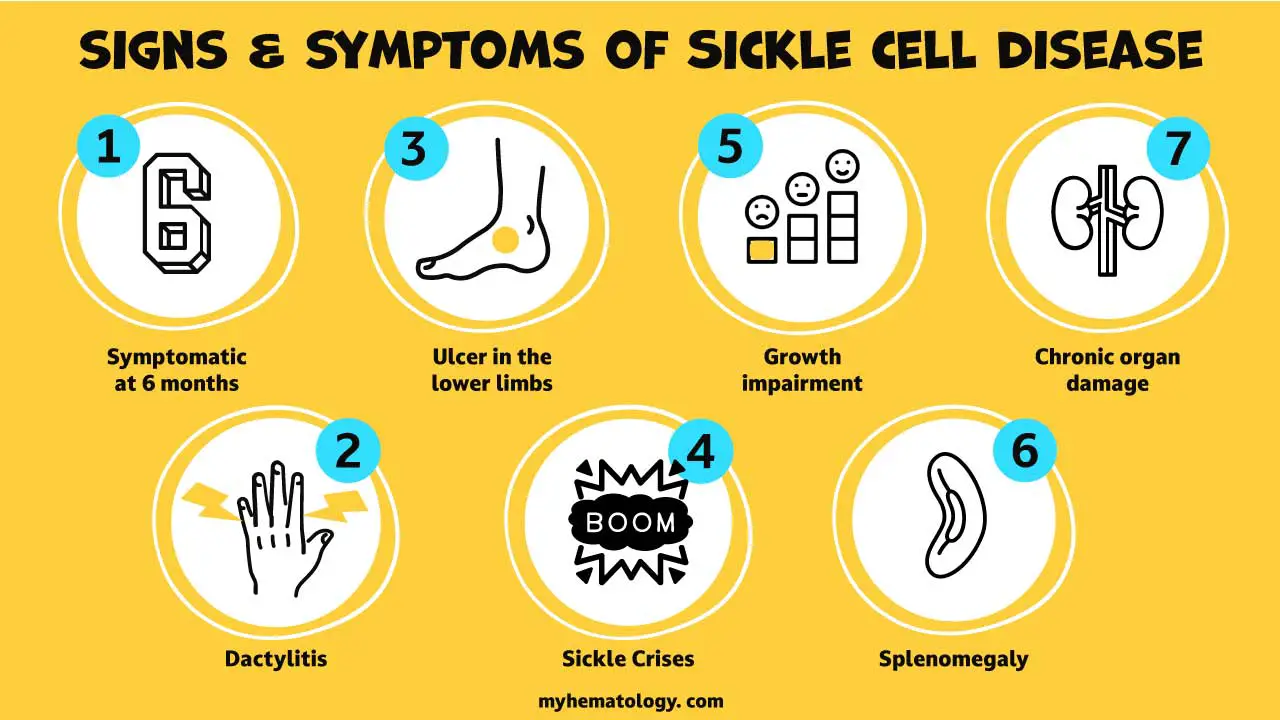
Skin ulcers, a common and debilitating complication of sickle cell anemia, are characterized by open sores that develop on the skin, particularly on the lower legs. These ulcers are caused by the sickle-shaped red blood cells, which are a hallmark of the disease, blocking blood flow to the skin and causing tissue damage.
The occurrence of skin ulcers in sickle cell anemia is a result of a combination of factors, including:
- Reduced blood flow: Sickle-shaped red blood cells can clog small blood vessels, preventing adequate oxygen and nutrients from reaching the skin tissues. This chronic deprivation of oxygen and nutrients leads to tissue damage and the formation of ulcers.
- Frequent infections: Individuals with sickle cell anemia are more susceptible to infections due to impaired immune function. These infections can further exacerbate skin ulcers and hinder their healing process.
- Leg pain: This disorder is often accompanied by severe pain in the legs, known as vaso-occlusive crises. This pain can make it difficult for individuals to walk and participate in physical activities, further contributing to the development of skin ulcers.
Skin ulcers in sickle cell anemia can range from small and superficial to large and deep, and can cause significant discomfort, pain, and social embarrassment. In severe cases, these ulcers can become infected and lead to complications such as bone infections and tissue death.
Early diagnosis and management of sickle cell anemia are crucial for preventing skin ulcers and other complications. Regular monitoring, prompt treatment of infections, and pain management are essential aspects of care. In addition, maintaining a healthy lifestyle, including a balanced diet, adequate hydration, and regular exercise, can help prevent and manage skin ulcers in sickle cell anemia.
Chipmunk Facies in Beta-Thalassemia Major
Chipmunk facies, also known as frontal bossing, is a characteristic facial feature observed in individuals with beta-thalassemia major, a severe form of thalassemia, a genetic disorder characterized by abnormalities in hemoglobin production. This facial feature is caused by the enlargement of the frontal bone, the bone forming the forehead, and the protrusion of the maxilla, the upper jawbone.
Mechanism of Chipmunk Facies in Beta-Thalassemia Major
In beta-thalassemia major, the production of beta-globin chains, a component of hemoglobin, is significantly reduced or absent. This deficiency in beta-globin chains leads to the formation of abnormal hemoglobin, which is less stable and more prone to destruction.
To compensate for the reduced production of functional hemoglobin, the body undergoes a process called erythropoiesis, the production of red blood cells, at an increased rate. This increased erythropoiesis stimulates bone marrow expansion, and the expansion of the bone marrow within the skull puts pressure on the frontal bone and maxilla, leading to their enlargement.
Appearance of Chipmunk Facies
Chipmunk facies are characterized by a prominent forehead, a flattened midface, and a forward-projecting chin. The combination of these features gives rise to the chipmunk-like appearance. The severity of chipmunk facies can vary among individuals and may become more pronounced with age.
Morphological Classification of Anemia
The morphological classification of anemia involves categorizing anemia based on the size and color of red blood cells observed in a blood smear. This classification provides valuable insights into the underlying cause of anemia and guides further diagnostic and treatment decisions.
The Three Primary Morphological Categories of Anemia
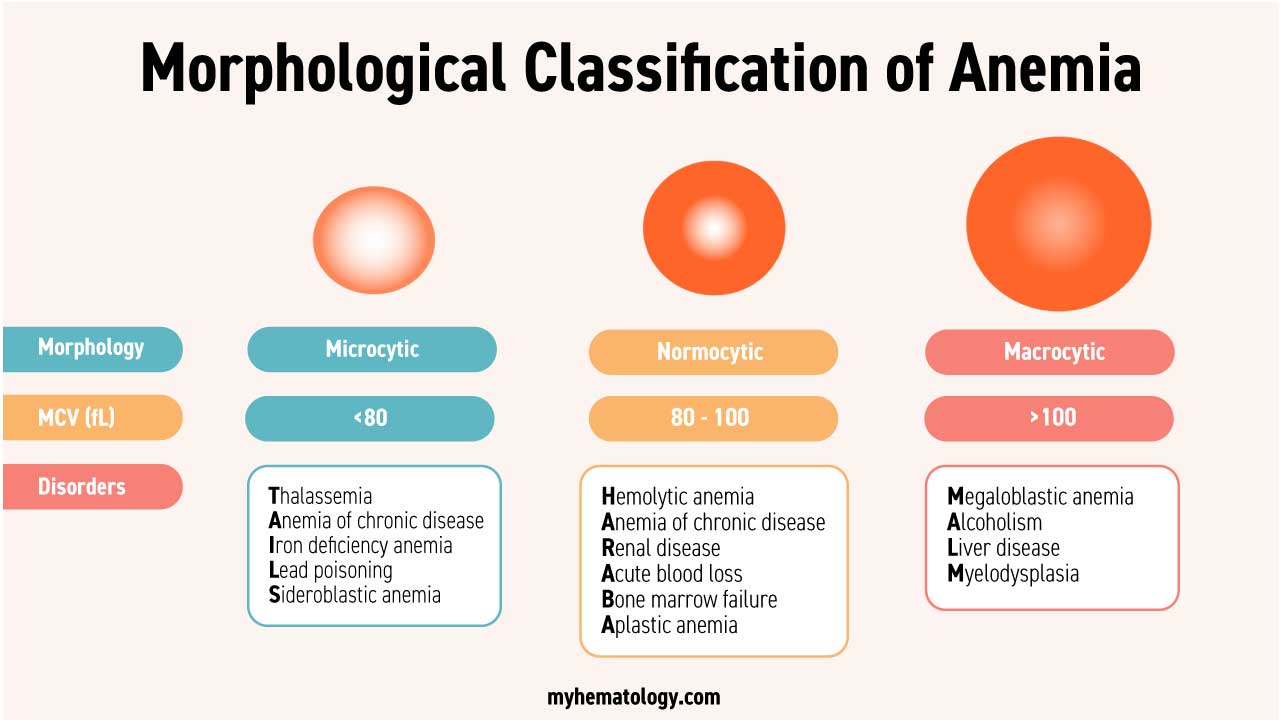
- Normocytic normochromic anemia: This type of anemia is characterized by red blood cells that are of normal size and color. It indicates a problem with red blood cell production or lifespan. The disorders commonly associated with this morphology are hemolytic anemia as well as aplastic anemia.
- Microcytic hypochromic anemia: This type of anemia is characterized by red blood cells that are smaller than normal and paler than normal. It is most commonly caused by iron deficiency and beta-thalassemia, both which affect hemoglobin production.
- Macrocytic anemia: This type of anemia is characterized by red blood cells that are larger than normal. It can be caused by a variety of factors, including vitamin B12 deficiency, folate deficiency, and alcohol abuse.
Additional Morphological Considerations
In addition to the three primary categories, other morphological features of red blood cells can provide further clues to the underlying cause of anemia. These features include:
- Anisocytosis: Variability in the size of red blood cells
- Poikilocytosis: Abnormalities in the shape of red blood cells
- Polychromasia: The presence of immature red blood cells with a bluish tinge
Clinical Significance of Morphological Classification
The morphological classification of anemia is an essential tool for clinicians in the diagnosis and management of anemia. By identifying the specific type of anemia, clinicians can narrow down the possible causes and initiate appropriate diagnostic tests and treatment strategies.
For instance, if a patient presents with microcytic hypochromic anemia, IDA is a strong possibility, and further testing to confirm iron deficiency would be warranted. Similarly, if a patient presents with macrocytic anemia, vitamin B12 or folate deficiency should be considered, and appropriate tests would be ordered.
Severity Classification
The severity of anemia is classified based on hemoglobin (Hb) levels, the primary protein in red blood cells responsible for carrying oxygen throughout the body. Hb levels below the normal range indicate anemia, and the severity increases as Hb levels decrease.
Hemoglobin Levels and Anemia Severity
The World Health Organization (WHO) guidelines classify anemia severity based on Hb levels in adults as follows:
- Mild anemia: Hb levels between 10.0 and 12.0 g/dL for women and between 11.0 and 13.5 g/dL for men.
- Moderate anemia: Hb levels between 8.0 and 9.9 g/dL.
- Severe anemia: Hb levels between 6.5 and 7.9 g/dL.
- Life-threatening anemia: Hb levels below 6.5 g/dL.
Factors Influencing Hb Levels
Hb levels can vary slightly from person to person due to factors such as age, gender, altitude, and pregnancy. However, a significant drop in Hb levels indicates anemia, and the severity classification based on Hb levels helps determine the appropriate course of treatment.
Anemia Severity Symptoms and Complications
The severity of symptoms and potential complications generally correlate with the degree of Hb deficiency. Mild anemia may cause subtle symptoms such as fatigue and pale skin, while moderate to severe anemia can lead to more pronounced symptoms such as shortness of breath, weakness, dizziness, and cognitive impairment. Severe anemia can also increase the risk of heart complications and other health problems.
Treatment Considerations Based on Severity
The treatment approach depends on the underlying cause and the severity of the condition. For mild anemia, lifestyle modifications such as dietary changes and iron supplementation may be sufficient. However, moderate to severe anemia may require more intensive treatment, such as blood transfusions or medications, depending on the underlying cause.
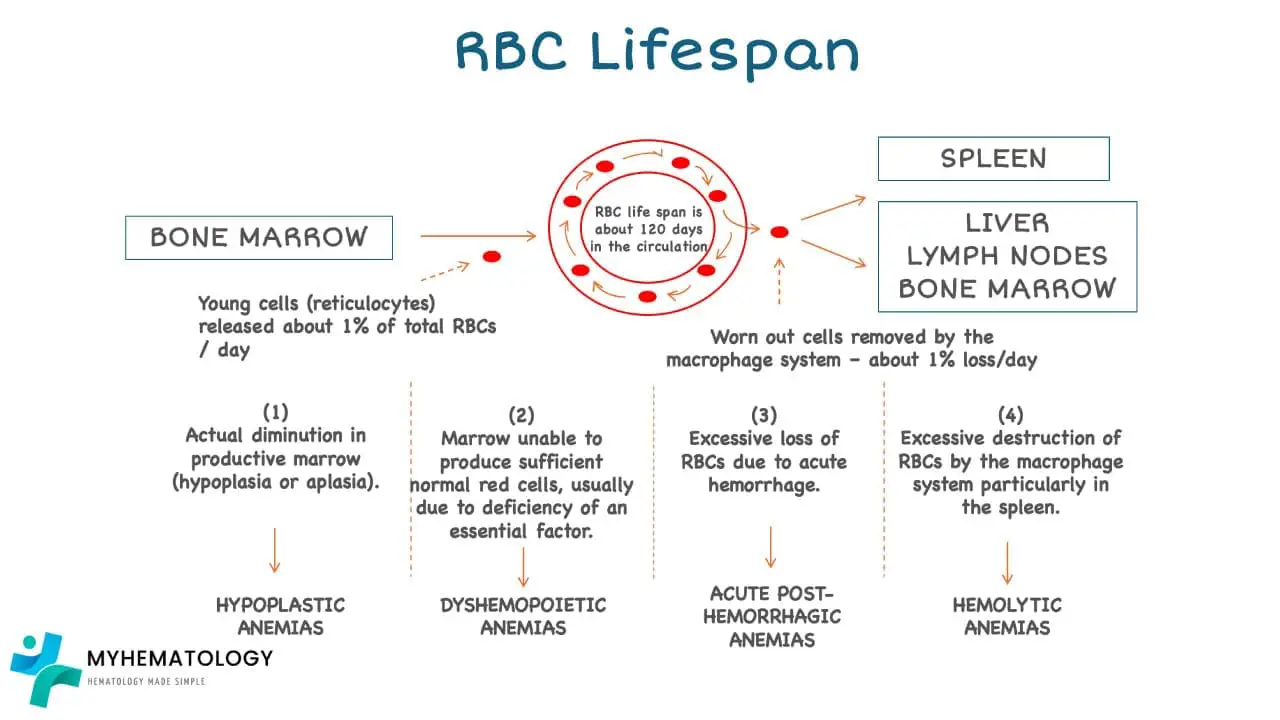
Red Cell Balance Sheet
Production vs. Destruction of Red Blood Cells
The maintenance of a healthy blood cell count is essential for human survival, and the production and destruction of red blood cells (RBCs) are closely regulated processes that work in balance to maintain this count. The red cell balance sheet, a concept used in hematology, tracks the production and destruction of RBCs to assess the overall health of the blood and identify potential underlying issues.
Production of Red Blood Cells
The production of RBCs, known as erythropoiesis, occurs primarily in the red bone marrow, where hematopoietic stem cells differentiate into mature RBCs. This process is stimulated by the hormone erythropoietin (EPO), which is produced by the kidneys in response to low oxygen levels in the blood.
RBC production involves several stages
- Commitment: Hematopoietic stem cells commit to the erythroid lineage, becoming erythroid precursors.
- Proliferation: Erythroid precursors divide rapidly to increase the number of RBCs.
- Maturation: Erythroid precursors undergo maturation, developing the necessary components for oxygen transport, such as hemoglobin.
- Enucleation: The nucleus is expelled from the erythrocyte, forming a mature RBC.
- Release: Mature RBCs are released from the bone marrow into the bloodstream.
Destruction of Red Blood Cells
The lifespan of RBCs is approximately 120 days, after which they are senescent and undergo destruction. This process, known as phagocytosis, primarily occurs in the spleen and liver, where macrophages engulf and break down senescent RBCs.
Several factors contribute to RBC destruction
- Aging: Senescent RBCs exhibit changes in their surface proteins and membrane structure, making them more susceptible to phagocytosis.
- Damage: RBCs damaged by physical or chemical insults, such as oxidative stress or infections, are also targeted for phagocytosis.
- Ineffective erythropoiesis: RBCs produced with abnormalities, such as abnormal hemoglobin or membrane defects, have a shorter lifespan and are more likely to be destroyed.
Red Cell Balance Sheet
The red cell balance sheet represents the equilibrium between RBC production and destruction. Under normal conditions, the rate of RBC production matches the rate of destruction, maintaining a stable RBC count. However, imbalances in either production or destruction can lead to anemia or other blood disorders.
Factors Affecting the Red Cell Balance Sheet
Various factors can disrupt the red cell balance sheet, leading to anemia or other RBC abnormalities:
- Nutritional deficiencies: Deficiencies in iron, vitamin B12, or folate can impair RBC production.
- Chronic diseases: Chronic conditions such as kidney disease, liver disease, or autoimmune disorders can affect RBC production or destruction.
- Bone marrow disorders: Diseases affecting the bone marrow, such as leukemia or aplastic anemia, can impair RBC production.
- Hemolytic disorders: Conditions that cause premature RBC destruction, such as sickle cell disease or hereditary spherocytosis, can lead to anemia.
- Blood loss: Acute or chronic blood loss can deplete RBCs, leading to anemia.
Clinical Significance of the Red Cell Balance Sheet
The red cell balance sheet represents a dynamic equilibrium between RBC production and destruction, maintaining a stable RBC count essential for human health. Disruptions in this balance lead to various blood disorders, and assessing the red cell balance sheet is critical for diagnosis, management, and improving patient outcomes.
Reticulocyte Count
Within this intricate balance, reticulocyte counts play a pivotal role in assessing the rate of RBC production and providing valuable insights into the overall health of the blood-forming system.
Reticulocytes: Immature Red Blood Cells
Reticulocytes are young RBCs that still contain remnants of RNA, giving them a characteristic blue-tinged appearance when stained with BCB or methylene blue dye. These immature cells emerge from the bone marrow, the site of RBC production, and complete their maturation within the bloodstream over a period of approximately one day.
Reticulocyte Count: A Measure of RBC Production
The reticulocyte count, expressed as a percentage of total RBCs, serves as a direct measure of the rate of RBC production. This count reflects the activity of erythropoiesis, the process by which stem cells in the bone marrow differentiate into mature RBCs.
Normal Reticulocyte Count Ranges
In healthy individuals, the reticulocyte count typically ranges from 0.5 to 2.0%. This range indicates a balanced rate of RBC production, ensuring a steady supply of oxygen-carrying cells to the body’s tissues.
Interpreting Reticulocyte Counts in Anemia
Deviations from the normal reticulocyte count range provide valuable clues to the underlying cause of anemia. A low reticulocyte count, known as hyporegenerative anemia, suggests that RBC production is impaired, while an elevated reticulocyte count, known as regenerative anemia, indicates that the bone marrow is attempting to compensate for increased RBC destruction.
Hyporegenerative Anemia
Hyporegenerative anemia arises from a variety of factors that hinder erythropoiesis. These factors include:
- Nutritional deficiencies: Inadequate intake of iron, vitamin B12, or folate can impede RBC production.
- Chronic diseases: Kidney disease, liver disease, or autoimmune disorders can disrupt erythropoiesis.
- Bone marrow disorders: Conditions such as leukemia or aplastic anemia can suppress RBC production.
Regenerative Anemia
Regenerative anemia occurs when the body attempts to counteract increased RBC destruction by boosting RBC production. This compensatory response is often observed in hemolytic disorders, such as sickle cell anemia or hereditary spherocytosis.
Reticulocyte Maturation Index (RMI)
The reticulocyte maturation index (RMI) provides further insights into the effectiveness of erythropoiesis. The RMI, based on the proportion of reticulocytes in different stages of maturation, helps determine whether RBC production is appropriately responsive to the body’s needs.
Hemoglobinuria and Fecal Occult Blood Tests
Hemoglobinuria and the fecal occult blood test (FOBT) play crucial roles in assessing the extent of RBC destruction and identifying potential underlying disorders.
Hemoglobinuria: A Sign of Intravascular RBC Destruction
Hemoglobinuria, the presence of hemoglobin in the urine, is a hallmark of intravascular RBC destruction. When RBCs rupture within the bloodstream, hemoglobin, the protein responsible for oxygen transport, is released into the circulation. Under normal conditions, the kidneys filter out hemoglobin and prevent it from entering the urine. However, when RBC destruction exceeds the kidneys’ filtration capacity, hemoglobin spills into the urine, causing it to appear red or brown.
Causes of Hemoglobinuria
Several factors can lead to hemoglobinuria:
- Hemolytic anemias: Conditions that cause premature RBC destruction, such as sickle cell anemia or hereditary spherocytosis, can result in hemoglobinuria.
- Acute intravascular hemolysis: Rapid destruction of RBCs due to infections, toxins, or certain medications can trigger hemoglobinuria.
- Rhabdomyolysis: Severe muscle damage can release excessive amounts of myoglobin, a protein similar to hemoglobin, into the bloodstream, leading to hemoglobinuria.
Fecal Occult Blood Test (FOBT): Detecting Occult Gastrointestinal Bleeding
The fecal occult blood test (FOBT) is a non-invasive screening tool that detects occult (hidden) blood in the stool. Occult blood may be present due to bleeding from the gastrointestinal (GI) tract, including the esophagus, stomach, small intestine, colon, or rectum.
Causes of Fecal Occult Blood
Several conditions can cause occult GI bleeding:
- Hemorrhoids: Swollen veins in the lower rectum can bleed during bowel movements, leading to occult blood in the stool.
- Anal fissures: Small tears in the lining of the anus can cause bleeding during bowel movements, resulting in occult blood in the stool.
- Colon polyps: Noncancerous growths in the colon can bleed, leading to occult blood in the stool.
- Colorectal cancer: Early stages of colorectal cancer may not cause visible blood in the stool, but occult blood may be detected by FOBT.
Clinical Significance of Hemoglobinuria and FOBT
Hemoglobinuria and FOBT are valuable diagnostic tools in assessing RBC destruction and identifying potential underlying disorders.
Hemoglobinuria provides immediate evidence of intravascular RBC destruction, prompting further investigation to determine the underlying cause. FOBT, while less specific, serves as a screening tool for occult GI bleeding, which may be a sign of various conditions, including colorectal cancer.
Interpretation of Hemoglobinuria and FOBT Results
A positive hemoglobinuria test requires immediate medical attention to identify the underlying cause of intravascular RBC destruction. A positive FOBT result warrants further evaluation to rule out serious GI conditions such as colorectal cancer.
Causes of Anemia in Major Categories
The causes of anemia can be loosely divided into 4 major categories.
Blood Loss Anemia
Among the diverse causes of anemia, blood loss plays a substantial role, contributing to both acute and chronic forms of the condition. Understanding the causes of blood loss anemia is crucial for effective diagnosis, management, and prevention.
Acute Blood Loss Anemia
Acute blood loss anemia arises from a sudden and rapid loss of a large amount of blood, typically within a short period. This sudden depletion of RBCs leads to a sharp decline in hemoglobin levels, causing symptoms such as weakness, dizziness, and shortness of breath.
Causes of Acute Blood Loss Anemia
Several factors can trigger acute blood loss anemia:
- Traumatic injuries: Severe trauma from accidents, falls, or gunshot wounds can cause significant blood loss and lead to acute anemia.
- Gastrointestinal bleeding: Heavy bleeding from ulcers, hemorrhoids, or esophageal varices can rapidly deplete RBCs and result in acute anemia.
- Obstetric complications: Hemorrhage during childbirth or placental abruption can cause substantial blood loss and lead to acute anemia.
- Postoperative bleeding: Excessive bleeding following surgery can contribute to acute anemia.
Chronic Blood Loss Anemia
Chronic blood loss anemia develops gradually over time due to persistent, low-level blood loss. This ongoing loss of RBCs leads to a gradual decline in hemoglobin levels, often without noticeable symptoms in the early stages.
Causes of Chronic Blood Loss Anemia
Several factors can contribute to chronic blood loss anemia:
- Menstrual bleeding: Heavy menstrual bleeding, known as menorrhagia, can cause significant iron loss over time, leading to chronic iron deficiency anemia.
- Gastrointestinal bleeding: Chronic bleeding from ulcers, hemorrhoids, or colon polyps can lead to gradual depletion of RBCs and iron deficiency anemia.
- Certain medications: Long-term use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) can irritate the stomach lining and cause chronic blood loss, leading to anemia.
- Unexplained bleeding: In some cases, chronic blood loss may occur without an obvious cause, requiring further investigation to identify the underlying condition.
Comparison of Acute and Chronic Blood Loss Anemia
Acute and chronic blood loss anemia differ in their onset, severity, and clinical presentation:
- Onset: Acute blood loss anemia develops rapidly, while chronic blood loss anemia develops gradually.
- Severity: Acute blood loss anemia can be severe and life-threatening, while chronic blood loss anemia may be mild or moderate.
- Clinical presentation: Acute blood loss anemia often presents with noticeable symptoms such as weakness, dizziness, and shortness of breath, while chronic blood loss anemia may have subtle symptoms or no noticeable symptoms in the early stages.
Nutritional Deficiency Anemia
Nutritional deficiencies play a substantial role, contributing to a significant proportion of anemia cases worldwide. The most common ones in this category are iron deficiency anemia (IDA), vitamin B12 deficiency anemia and folic acid deficiency anemia. Detailed explanations of each disorder can be found in other posts.
Iron Deficiency Anemia (IDA)
Iron deficiency anemia (IDA), the most common type of anemia, arises from a deficiency in iron, an essential component of hemoglobin. Iron is required for the production of heme, the protein that binds oxygen in red blood cells. Without adequate iron, hemoglobin production is impaired, leading to a reduction in oxygen-carrying capacity and the development of IDA.
Causes of IDA
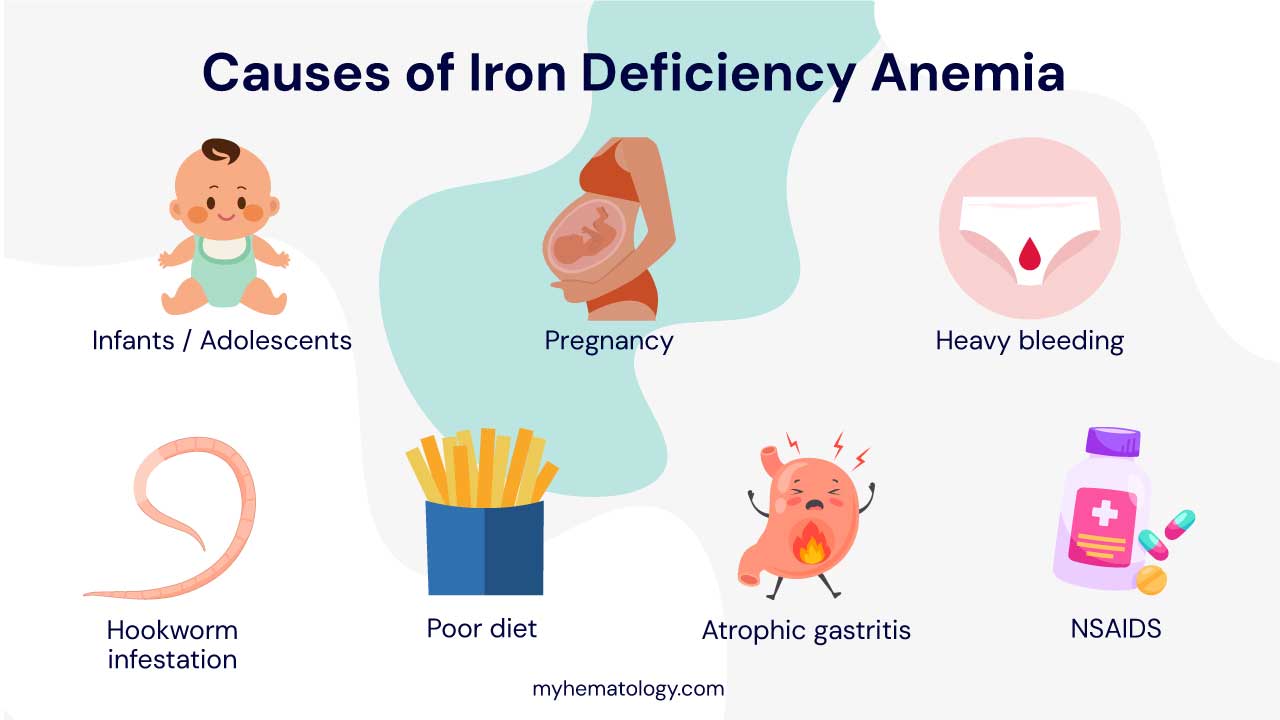
Several factors can contribute to IDA:
- Inadequate dietary intake: Insufficient iron intake from food sources, particularly in populations with limited access to iron-rich foods or restricted diets, can lead to IDA.
- Increased iron requirements: Individuals with certain conditions, such as pregnancy, growth spurts, or chronic blood loss, have higher iron requirements and are more susceptible to IDA.
- Impaired iron absorption: Certain conditions, such as celiac disease or inflammatory bowel disease, can impair the absorption of iron from food, increasing the risk of IDA.
Vitamin B12 Deficiency Anemia
Vitamin B12, also known as cobalamin, is an essential nutrient involved in DNA synthesis and red blood cell formation. A deficiency in vitamin B12 can lead to a type of anemia characterized by large, misshapen red blood cells known as megalocytes.
Causes of Vitamin B12 Deficiency Anemia

Primary vitamin B12 deficiency anemia is caused by a lack of intrinsic factor, a protein produced by the stomach that is necessary for the absorption of vitamin B12 from food. This condition often affects older adults due to age-related changes in stomach acid production.
Secondary vitamin B12 deficiency anemia can arise from various factors, including:
- Dietary deficiencies: Strict vegetarian or vegan diets may lack adequate vitamin B12, increasing the risk of deficiency.
- Malabsorption disorders: Conditions that impair nutrient absorption, such as celiac disease or Crohn’s disease, can lead to vitamin B12 deficiency.
- Medications: Certain medications, such as proton pump inhibitors used for acid reflux, can interfere with vitamin B12 absorption.
Folate Deficiency Anemia
Folate, also known as folic acid, is a B vitamin that plays a crucial role in DNA synthesis and red blood cell formation. A deficiency in folate can lead to a type of anemia characterized by large, immature red blood cells known as megaloblasts.
Causes of Folate Deficiency Anemia
Several factors can contribute to folate deficiency anemia:
- Inadequate dietary intake: A diet lacking adequate folate-rich foods, such as leafy green vegetables, legumes, and fortified cereals, can lead to folate deficiency.
- Increased folate requirements: Pregnant women, breastfeeding mothers, and individuals with certain medical conditions, such as anemia or inflammatory bowel disease, have higher folate requirements and are more susceptible to deficiency.
- Malabsorption disorders: Conditions that impair nutrient absorption, such as celiac disease or Crohn’s disease, can lead to folate deficiency.
Prevention of Nutritional Deficiency Anemia
Fortunately, preventive measures can help reduce the risk of nutritional deficiency anemia:
- Dietary modifications: Consuming a balanced diet rich in iron-rich foods, vitamin B12-rich foods, and folate-rich foods can help prevent nutritional deficiencies.
- Supplementation: In cases where dietary intake is inadequate or individual needs are higher, supplementation with iron, vitamin B12, or folate may be recommended.
- Early diagnosis and treatment of underlying conditions: Addressing conditions that can impair nutrient absorption or increase nutrient requirements can help prevent nutritional deficiency anemia.
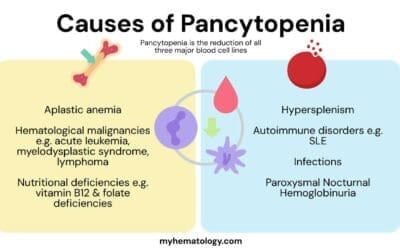
Bone Marrow Failure
Bone Marrow Failure Syndromes
Bone marrow failure syndromes (BMFS) are a group of disorders characterized by impaired production of blood cells, including red blood cells, white blood cells, and platelets. This impairment can be caused by various factors, leading to a reduction in the number of mature blood cells in the circulation and the development of anemia.
Causes of Bone Marrow Failure Anemia
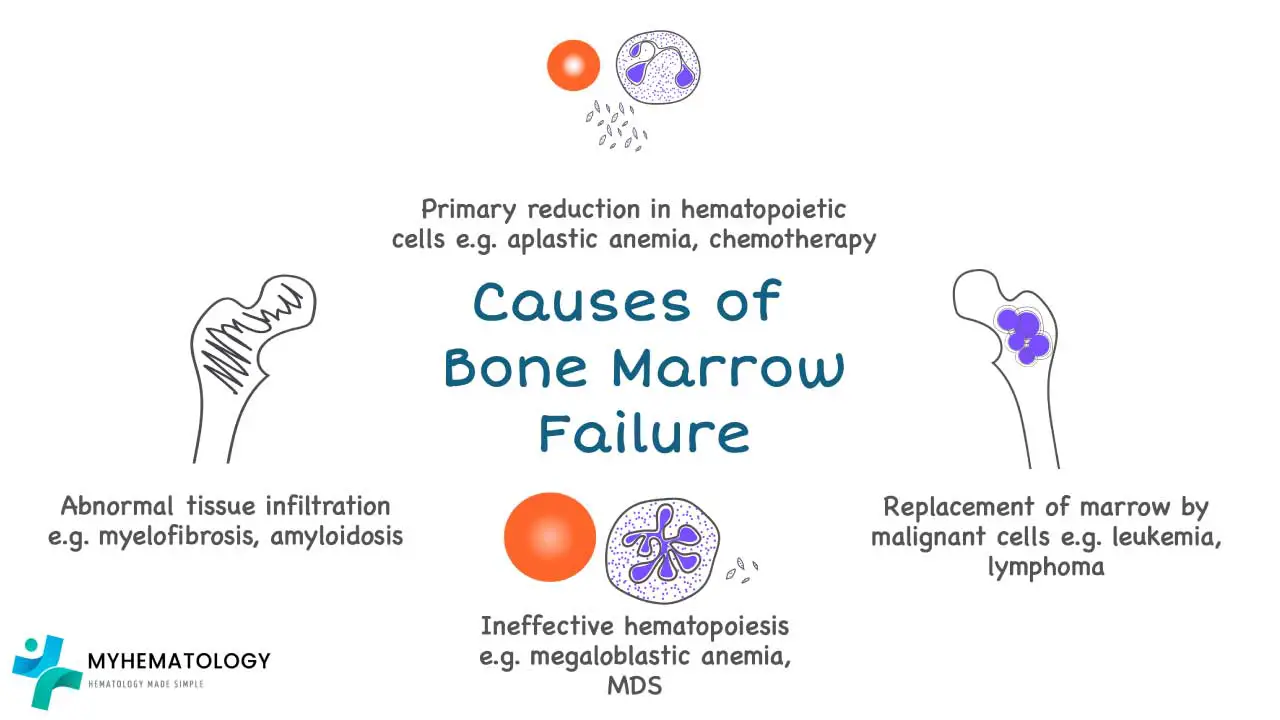
- Aplastic anemia: This severe form of bone marrow failure is characterized by a near-complete failure of the bone marrow to produce blood cells. The exact cause of aplastic anemia is often unknown, but it is thought to involve an autoimmune attack on the bone marrow or exposure to certain toxins or medications.
- Myelodysplastic syndromes (MDS): These are a group of disorders characterized by the production of abnormal blood cells and a gradual decline in the production of healthy blood cells. The causes of MDS are diverse and may include genetic factors, exposure to certain chemicals or radiation, and age-related changes in the bone marrow.
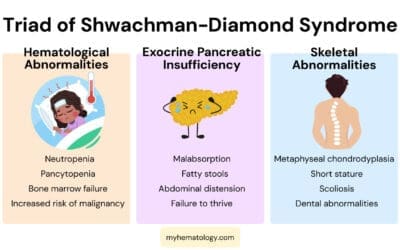
- Inherited bone marrow failure syndromes: These are rare genetic disorders that cause impaired bone marrow function from birth. Examples include Fanconi anemia, Shwachman-Diamond syndrome, and Dyskeratosis congenita.
Clinical Presentation of Bone Marrow Failure Anemia
The clinical presentation of bone marrow failure anemia depends on the specific underlying cause and the severity of the condition. Common symptoms include:
- Fatigue: Due to reduced oxygen-carrying capacity of the blood
- Pale skin: Due to a deficiency in hemoglobin
- Shortness of breath: Due to decreased oxygen supply to the tissues
- Increased susceptibility to infections: Due to impaired white blood cell production
- Easy bruising and bleeding: Due to impaired platelet production
Diagnosis and Management of Bone Marrow Failure Anemia
Diagnosing bone marrow failure anemia requires a comprehensive evaluation, including medical history, physical examination, and complete blood count (CBC) with blood cell morphology analysis. Bone marrow aspiration and biopsy are often performed to assess the bone marrow’s ability to produce blood cells.
Treatment of bone marrow failure anemia depends on the underlying cause and the severity of the condition. In some cases, supportive care with blood transfusions and growth factors may be sufficient. However, in more severe cases, stem cell transplantation may be the only curative option.
Preventive Strategies
While not always possible to prevent bone marrow failure anemia, certain measures can help reduce the risk:
- Avoiding exposure to known toxins or radiation: Reducing exposure to chemicals or radiation known to damage the bone marrow can help lower the risk of bone marrow failure.
- Prompt diagnosis and treatment of underlying conditions: Early identification and management of conditions that may increase the risk of bone marrow failure, such as certain infections or autoimmune disorders, can help prevent complications.
- Genetic counseling: For individuals with a family history of inherited bone marrow failure syndromes, genetic counseling can provide information about the risks and options for carrier testing and prenatal diagnosis.
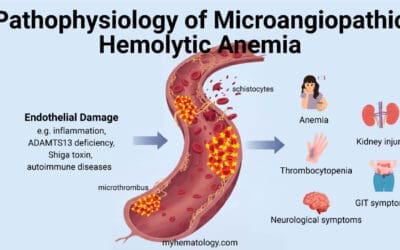
Hemolytic Anemia
In normal physiology, red blood cells have an average lifespan of approximately 120 days. However, in hemolytic anemia, red blood cells are destroyed prematurely, leading to a decline in their number and a decrease in oxygen-carrying capacity. This premature destruction can occur within the bloodstream (intravascular hemolysis) or within the spleen and liver (extravascular hemolysis).
Causes of Hemolytic Anemia: A Diverse Spectrum
The causes of hemolytic anemia are diverse and can be broadly categorized into intrinsic abnormalities, autoimmune causes, and infectious triggers.
Intrinsic Abnormalities of Red Blood Cells
Intrinsic abnormalities of red blood cells, also known as inherited hemolytic anemias, are caused by genetic defects that affect the structure, function, or membrane integrity of red blood cells. These defects make red blood cells more susceptible to premature destruction. Common examples include:
- Sickle cell anemia: This genetic disorder causes red blood cells to take a sickle-like shape, leading to increased fragility and destruction.
- Thalassemias: These genetic disorders result in the production of abnormal or insufficient amounts of hemoglobin, leading to impaired oxygen-carrying capacity and red blood cell destruction.
- Hereditary spherocytosis: This genetic disorder causes red blood cells to have a spherical shape, making them more susceptible to destruction by the spleen.
Autoimmune Hemolytic Anemia
Autoimmune hemolytic anemias arise when the body’s immune system mistakenly attacks and destroys red blood cells. These antibodies can bind to red blood cells, marking them for destruction by macrophages. Common examples include:
- Warm autoimmune hemolytic anemia (WAHA): This type of hemolytic anemia is caused by IgG antibodies that bind red blood cells at normal body temperature.
- Cold agglutinin disease: This type of hemolytic anemia is caused by IgM antibodies that bind red blood cells at lower temperatures.
- Paroxysmal nocturnal hemoglobinuria (PNH): This rare condition is characterized by the presence of abnormal red blood cells that are susceptible to complement-mediated destruction.
Infectious Causes of Hemolytic Anemia
Infections can trigger hemolytic anemia by various mechanisms, including:
- Direct invasion of red blood cells: Certain pathogens, such as malaria parasites, can directly invade and destroy red blood cells.
- Production of toxins: Some bacteria produce toxins that can damage red blood cells, leading to their destruction.
- Activation of the immune system: Infections can activate the immune system, leading to the production of antibodies that may cross-react with red blood cells, causing hemolytic anemia.
Clinical Presentation and Diagnosis of Hemolytic Anemia
The clinical presentation of hemolytic anemia depends on the underlying cause and severity of the condition. Common symptoms include:
- Fatigue: Due to reduced oxygen-carrying capacity of the blood
- Pale skin: Due to a deficiency in hemoglobin
- Jaundice: Yellowing of the skin and eyes due to excess bilirubin, a breakdown product of heme
- Splenomegaly: Enlargement of the spleen, a consequence of the increased destruction of red blood cells
- Dark urine: Due to the presence of excess bilirubin in the urine
Diagnosis of hemolytic anemia requires a comprehensive evaluation, including medical history, physical examination, complete blood count (CBC) with blood cell morphology analysis, and reticulocyte count. Additional tests, such as direct antiglobulin test (DAT) and hemoglobin electrophoresis, may be performed to identify specific causes.
How is Anemia Tested?
Initial Steps in Anemia Diagnosis
The evaluation of anemia begins with a comprehensive medical history, including a detailed assessment of anemia symptoms, dietary habits, medication use, and family history of anemia or other blood disorders. This initial assessment provides valuable clues about potential causes and helps tailor subsequent diagnostic procedures.
Complete Blood Count (CBC): A Cornerstone of Anemia Diagnosis
The complete blood count (CBC) is a cornerstone of anemia diagnosis. This comprehensive blood test provides a detailed analysis of the cellular components of blood, including red blood cells, white blood cells, and platelets.
Hemoglobin and Hematocrit: Quantifying Red Blood Cell Parameters
Hemoglobin, the protein in red blood cells responsible for oxygen transport, is measured as part of the CBC. Hematocrit, the proportion of blood volume occupied by red blood cells, is also determined. Low hemoglobin or hematocrit levels are hallmark indicators of anemia.
Reticulocyte Count: Assessing Red Blood Cell Production
Reticulocytes, immature red blood cells, are measured as part of the CBC. The reticulocyte count reflects the rate of red blood cell production. A low reticulocyte count suggests impaired red blood cell production, while an elevated reticulocyte count may indicate increased destruction of red blood cells.
Red Blood Cell Morphology
The peripheral blood smear provides information on red blood cell morphology, the size, shape, and color of red blood cells. Alterations in red blood cell morphology can provide clues about the underlying cause of anemia.
Further Diagnostic Tests
In addition to the CBC, further diagnostic tests may be employed to identify the specific cause of anemia. These tests may include:
- Iron studies: To assess iron levels in the blood and identify iron deficiency anemia.
- Vitamin B12 and folate levels: To evaluate the levels of these essential nutrients, which are crucial for red blood cell production.
- Bone marrow aspiration and biopsy: To directly assess the bone marrow, the site of red blood cell production, for abnormalities.
- Hemoglobin electrophoresis: To identify specific types of hemoglobin abnormalities, such as sickle cell anemia or thalassemia.
- Antibody testing: To detect the presence of antibodies that may be causing hemolytic anemia.
Interpretation of Test Results
The interpretation of test results requires a collaborative approach between the attending physician and a hematologist, a specialist in blood disorders. By carefully evaluating the clinical presentation, CBC findings, and results of additional tests, the underlying cause of anemia can be accurately identified.
How is Anemia Treated?
The treatment depends on the underlying cause. The primary goal of anemia treatment is to increase the production of red blood cells or hemoglobin, thereby improving oxygen transport and alleviating symptoms.
Nutritional Deficiency Anemia
For IDA, oral iron supplements are typically sufficient to restore iron stores and hemoglobin levels. In severe cases or for individuals with poor absorption, intravenous iron may be administered.
Vitamin B12 deficiency anemia is treated with vitamin B12 injections or supplements. Folic acid deficiency anemia is treated with folic acid supplements.
Blood Loss Anemia
Acute blood loss anemia may require blood transfusions to replenish red blood cells and stabilize vital signs. Chronic blood loss anemia is addressed by identifying and treating the underlying cause of bleeding. This may involve surgical interventions, medications, or lifestyle modifications.
Bone Marrow Failure
Bone marrow failure anemia may require supportive care with blood transfusions and growth factors to stimulate red blood cell production. In severe cases, stem cell transplantation may be the only curative option.
Hemolytic Anemia
Hemolytic anemia treatment depends on the specific cause. For autoimmune hemolytic anemia, corticosteroids or other immunosuppressive drugs may be used to suppress antibody production and reduce red blood cell destruction. In some cases, splenectomy may be considered to reduce the destruction of red blood cells.
Symptom Management and Preventive Strategies
In addition to addressing the underlying cause, various measures can help manage anemia symptoms and improve overall health:
- Rest: Adequate rest can help reduce fatigue and improve energy levels.
- Dietary modifications: A balanced diet rich in iron, vitamin B12, and folate can support red blood cell production.
- Hydration: Maintaining proper hydration is essential for overall health and can help reduce fatigue.
- Regular monitoring: Regular checkups and blood tests allow for monitoring of anemia and early detection of any complications.
By tailoring treatment to the underlying cause and implementing supportive measures, anemia can be effectively managed, improving quality of life and overall health outcomes.
Frequently Asked Questions (FAQs)
Is anemia threatening?
Yes, anemia can be threatening as it signifies a reduced oxygen-carrying capacity of the blood, which can lead to various complications. While mild cases might only cause fatigue, severe or untreated anemia can result in significant organ damage, heart problems (due to the heart working harder to compensate for low oxygen), impaired cognitive function, and increased risk of infections.
Can anemia cause hair loss?
Yes, anemia, particularly iron deficiency anemia, can cause hair loss.
Iron is essential for the production of hemoglobin, the protein in red blood cells that carries oxygen to all the body’s tissues, including hair follicles. When there isn’t enough iron, the body prioritizes oxygen delivery to vital organs, diverting it away from non-essential functions like hair growth. This lack of oxygen and nutrients to the hair follicles can disrupt the hair growth cycle, leading to premature shedding (often manifesting as a condition called telogen effluvium, which is diffuse hair thinning) and can also make hair brittle and weak. Fortunately, hair loss caused by iron deficiency anemia is often reversible with appropriate treatment to restore iron levels.
Can anemia cause pain?
Yes, anemia can cause pain, although it’s not always a direct or primary symptom like fatigue. The most common type of pain associated with anemia is often a headache, resulting from reduced oxygen delivery to the brain. In specific types of anemia, such as sickle cell anemia, severe pain is a hallmark symptom due to “pain crises” where misshapen red blood cells block blood flow in small vessels, causing intense tissue ischemia. Additionally, some anemic conditions can lead to bone pain or joint pain, as the bone marrow works harder to produce more red blood cells, or due to underlying inflammatory conditions causing the anemia.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Turner J, Parsi M, Badireddy M. Anemia. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499994/
- Anaemia of chronic disease – References | BMJ Best Practice. Available from: https://bestpractice.bmj.com/topics/en-gb/95
- Warner MJ, Kamran MT. Iron Deficiency Anemia. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448065/
- Assessment of anaemia – References – BMJ Best Practice. Available from: https://bestpractice.bmj.com/topics/en-gb/93
- Anemia in Pregnancy – ACOG. Available from: https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/08/anemia-in-pregnancy
- David R. Thomas, Anemia and Quality of Life: Unrecognized and Undertreated, The Journals of Gerontology: Series A, Volume 59, Issue 3, March 2004, Pages M238–M241, https://doi.org/10.1093/gerona/59.3.M238
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Understanding Anemia (Understanding Health and Sickness Series) (Uthman, 1998).