TL;DR
Leukemia is a cancer of the blood and bone marrow. It affects the cells responsible for fighting infection and carrying oxygen throughout the body.
How does it occur? ▾
Normally, blood cells develop from stem cells in the bone marrow. In leukemia, the development goes wrong, leading to the production of abnormal cells called blasts. These blasts crowd out healthy blood cells, causing various problems.
Signs and Symptoms ▾
- Feeling tired and weak
- Frequent infections
- Easy bruising and bleeding
- Fever
- Swollen lymph nodes
Types of leukemia ▾
- Acute: Develops quickly and requires immediate treatment.
- Chronic: Develops slowly and may not have noticeable symptoms initially.
- Different types exist within each category (e.g., ALL, AML, CLL, CML).
How is it diagnosed? ▾
- Blood tests
- Bone marrow biopsy
- Other tests to understand the specific type
How is it treated? ▾
- Treatment depends on the type and severity
- Chemotherapy
- Radiation therapy
- Targeted therapy
- Stem cell transplant
*Click ▾ for more information
Introduction
Leukemia is a group of cancers that affect the blood and bone marrow. It arises when immature blood cells, called blasts, multiply uncontrollably in the bone marrow, crowding out healthy blood cell production. These abnormal cells often don’t function properly, leading to various health problems.
A Brief History of Leukemia Research and Understanding
While traces of leukemia were potentially observed in ancient times, our scientific understanding emerged much later. Here’s a glimpse into the key milestones:
Early Observations (18th-19th Centuries)
- 1700s: First descriptions of unusual blood findings resembling leukemia emerge.
- 1827: French Physician Jean Cruveilhier coins the term “leukemia” based on the white blood cell count observed in patients.
- 1845: Scottish physician John Hughes Bennett first uses the term “leucocythemia” to describe the disease.
- Mid-19th century: Rudolf Virchow distinguishes between lymphatic and myelogenous forms of leukemia based on the affected cell type.
Understanding the Disease (20th Century)
- 1900s: Advances in microscopy and blood analysis facilitate diagnosis and differentiation of leukemia subtypes.
- 1920s and 1930s: X-ray therapy emerges as an initial treatment option.
- 1940s: Nitrogen mustards, derived from chemical warfare agents, become the first chemotherapy drugs used for leukemia.
- 1950s: Bone marrow transplantation shows promise as a potential cure.
- 1960s and 1970s: Development of more effective chemotherapy regimens improves survival rates.
- 1980s and 1990s: Identification of chromosomal abnormalities and genetic mutations associated with specific leukemia types.
- Late 20th century: Targeted therapies based on specific genetic mutations begin to emerge.
Modern Era (21st Century)
- Focus on personalized medicine: Tailoring treatment based on individual patients’ genetic make-up and leukemia characteristics.
- Advances in immunotherapy: Harnessing the immune system to fight cancer cells.
- Gene therapy and stem cell-based therapies: Exploring approaches to repair or replace defective genes or eliminate malignant cells.
Continued progress
The field of leukemia research continues to rapidly evolve, with ongoing exploration of new therapies, early detection methods, and improved supportive care. Today, the outlook for many leukemia patients has significantly improved, thanks to the dedication of researchers and advancements in our understanding of this complex disease.
Global burden of leukemia
Leukemia poses a significant global health challenge, impacting individuals, families, and healthcare systems worldwide.
Incidence
- Globally, in 2020, 474,519 new cases of leukemia were estimated, constituting roughly 2.5% of all new cancer diagnoses.
- The exact number varies depending on various factors like age, sex, and geographical location.
- Although incidence might not be increasing dramatically, regional disparities exist. Countries with lower socioeconomic development tend to have higher rates, likely due to limited access to early diagnosis and preventative measures.
Prevalence
- Due to improved treatment options, the number of people living with leukemia is rising. Estimates suggest over 4 million individuals currently have the disease globally.
- This growing prevalence, especially in aging populations, adds to the healthcare burden and resource utilization.
Mortality
- In 2020, leukemia remained a significant cause of cancer-related deaths, claiming 311,594 lives, accounting for 3.1% of all cancer deaths.
- While mortality rates have been decreasing slowly in some regions due to advancements in diagnosis and treatment, disparities exist between developed and developing nations.
Additional Points
- Childhood leukemia: Remains the most common cancer in children under five years old, leading to high mortality rates in regions with limited access to pediatric cancer care.
- Type variations: Acute lymphoblastic leukemia (ALL) is the most prevalent type in children, while acute myeloid leukemia (AML) is more common in adults. Chronic lymphocytic leukemia (CLL) is the most common adult type overall.
- Future projections: Due to population growth and aging, the global burden of leukemia is expected to rise in the coming decades.
Classification of Leukemia
Leukemia classification helps healthcare professionals understand the specific type and characteristics of a patient’s disease, guiding treatment decisions and predicting potential outcomes.
By Cell Type
- Lymphoid leukemias (lymphoblastic): These arise from abnormal lymphoid progenitor cells, which normally develop into infection-fighting B or T lymphocytes. They are more common in children and young adults.
- Myeloid leukemias (myelocytic, myelogenous): These originate from abnormal myeloid progenitor cells, which typically mature into red blood cells, white blood cells (other than lymphocytes), and platelets. They are more prevalent in adults and older adults.
By Course
- Acute leukemias: These progress rapidly, with blasts multiplying quickly and disrupting healthy blood cell production within weeks or months.
- Chronic leukemias: These develop slowly over months or years, with an accumulation of abnormal cells but often maintaining some ability to produce mature blood cells.
Subtypes
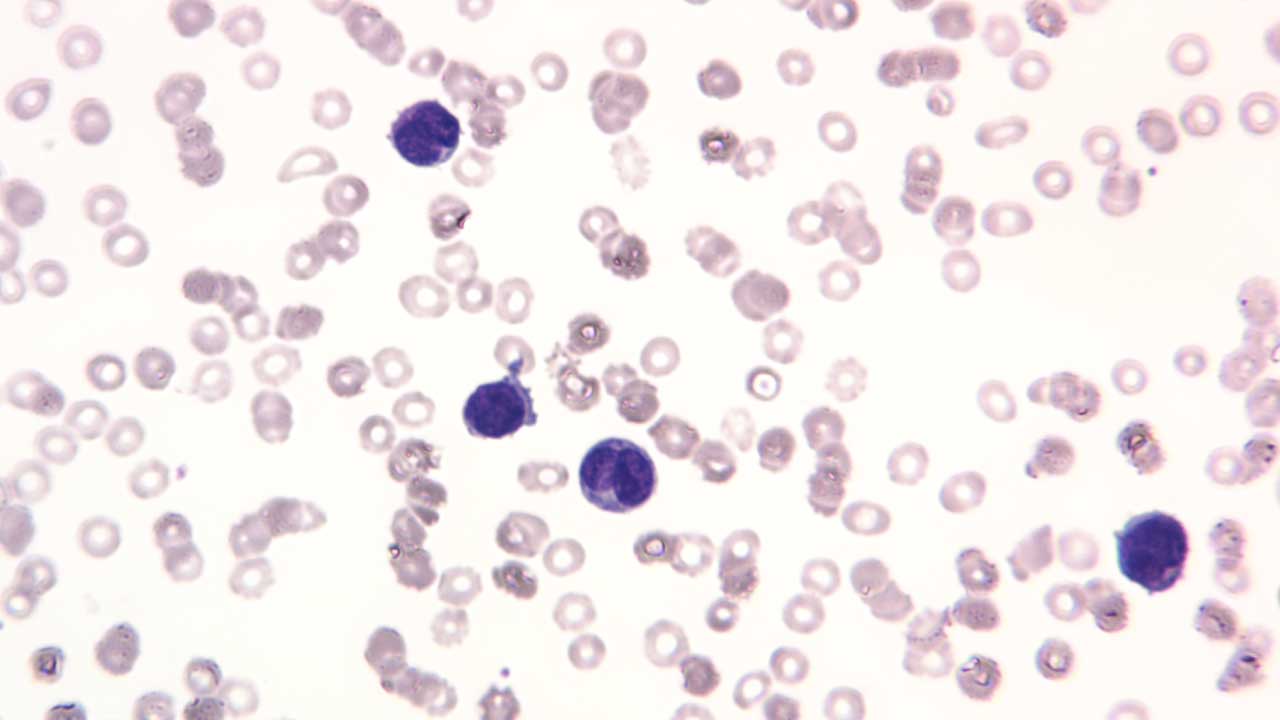
Acute Lymphoblastic Leukemia (ALL)
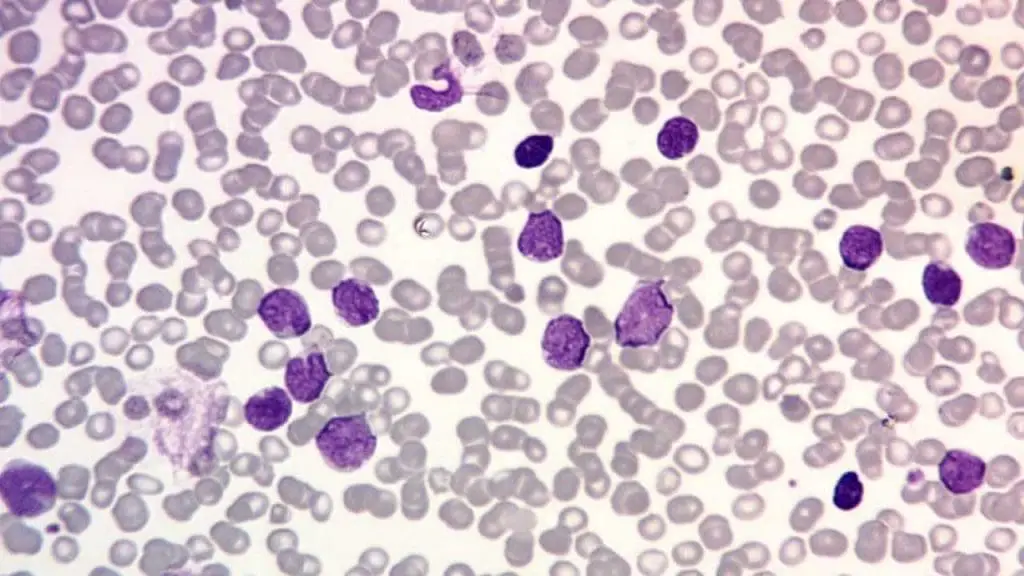
- B-cell ALL: More common in children, characterized by immature B-lymphocytes in the blood and bone marrow. Subtypes include:
- Early B-cell ALL: Diagnosed at an early stage of B-cell development.
- Late B-cell ALL: Diagnosed at a later stage of B-cell development.
- Philadelphia chromosome-positive (Ph+): Associated with a specific genetic abnormality and requires targeted therapy.
- Burkitt-type ALL: Aggressive subtype with unique features.
- T-cell ALL: Less common, affects T-lymphocytes. Subtypes include:
- Early T-cell precursor ALL: Affects very early T-cell precursors.
- Cortical T-cell ALL: Affects more mature T-cell precursors.
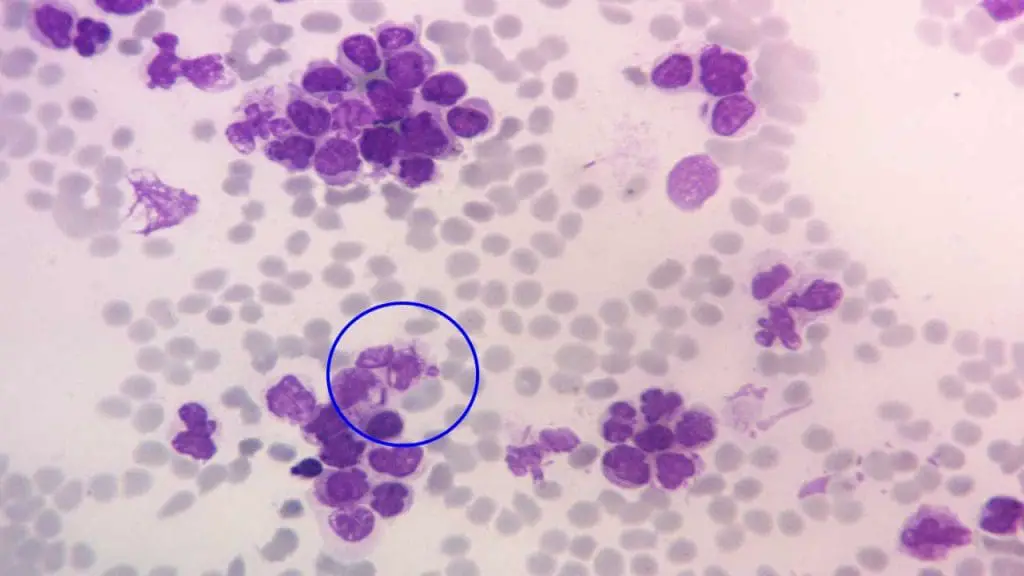
Acute Myeloid Leukemia (AML)
- Classification based on French-American-British (FAB) system: Categorizes AML into subtypes based on microscopic features of the blasts. For e.g.
- Acute myeloblastic leukemia with minimal differentiation (M0)
- Acute promyelocytic leukemia (APL) (M3)
- Genetic-based classification: Newer system identifies specific genetic mutations that influence treatment and prognosis. For e.g.
- AML with recurrent genetic abnormalities
- AML with myelodysplasia-related changes
- Therapy-related AML
Chronic Lymphocytic Leukemia (CLL)
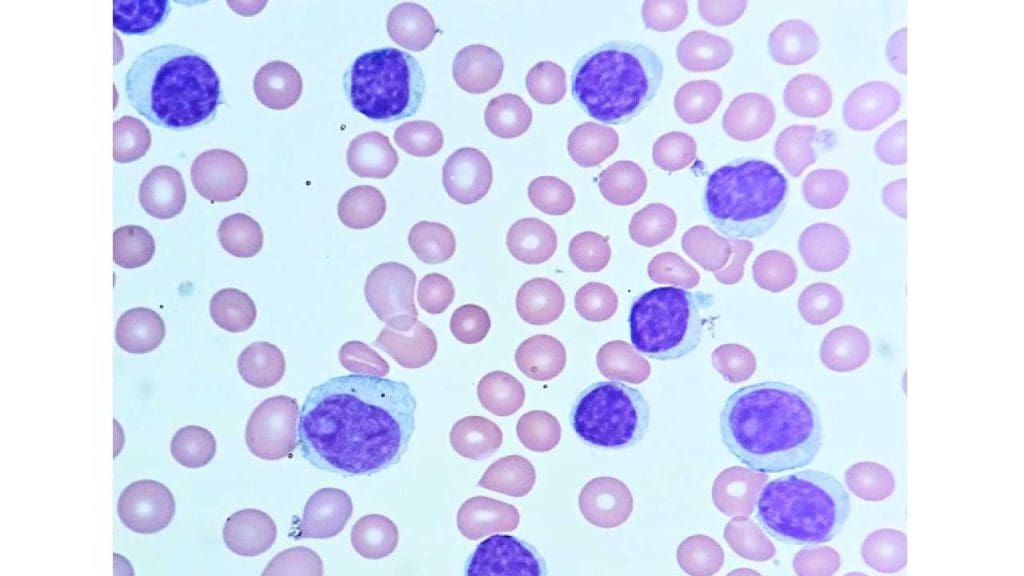
- Most common chronic leukemia, affecting mature B-lymphocytes.
- Slow progression, often diagnosed in older adults.
- Subtypes based on genetic characteristics and disease course.
Chronic Myeloid Leukemia (CML)
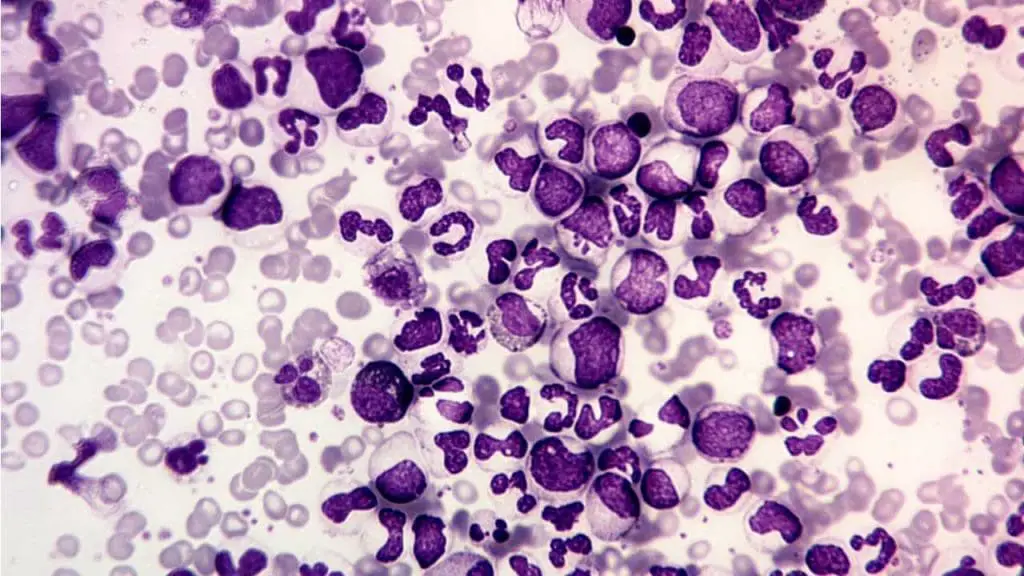
- Characterized by the Philadelphia chromosome, causing uncontrolled growth of myeloid progenitor cells.
- Chronic phase with manageable symptoms but can progress to accelerated and blast crisis phases without treatment.
- Targeted therapy available for long-term management.
Less Common Types
- Hairy cell leukemia: Slow-growing leukemia affecting B-cells with hair-like projections.
- Adult T-cell leukemia-lymphoma (ATLL): Aggressive T-cell leukemia associated with human T-lymphotropic virus type 1 (HTLV-1) infection.
- Other rare types: Myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN) can sometimes transform into acute leukemias.
Pathogenesis and Pathophysiology
Normal blood cell development in the bone marrow (Hematopoiesis)
The journey of a blood cell begins with the versatile hematopoietic stem cell residing in the bone marrow. This remarkable cell acts as the mother of all blood cells, possessing the unique ability to both duplicate itself and differentiates into various specialized cells. Its initial differentiation creates two distinct paths: the myeloid and lymphoid lineages through the common myeloid progenitors and common lymphoid progenitors, respectively.
The lymphoid pathway leads to the development of immune warriors like lymphocytes and natural killer cells. The lymphoid progenitor cells mature into lymphoblasts, which eventually become the small lymphocytes patrolling our body, constantly vigilant against invaders.
Meanwhile, the myeloid progenitor embarks on a diverse journey giving rise to several crucial cell types. One path leads to the production of red blood cells (erythrocytes). Through a series of stages, erythroblasts mature into reticulocytes and finally functional erythrocytes, responsible for oxygen transport throughout the body.
Another branch of the myeloid lineage produces platelets, essential for blood clotting. Megakaryocytes, formed from megakaryoblasts, fragment into thousands of tiny platelets, ready to seal any breach and prevent bleeding.
Finally, the myeloid progenitor also gives rise to different types of white blood cells known as granular leukocytes (neutrophils, eosinophils, and basophils) and monocytes. Monocytes mature into macrophages, acting as scavengers and defenders within our tissues.
Genetic and molecular changes leading to leukemia
Leukemia isn’t just an overproduction of white blood cells; it arises from a complex interplay between genetic and molecular abnormalities that disrupt the delicate balance of blood cell production.
- Chromosomal Abnormalities: Translocations, deletions, and duplications in chromosomes can lead to uncontrolled cell growth and disrupt normal gene function. For example, the Philadelphia chromosome, a hallmark of chronic myeloid leukemia (CML), results in the fusion of two genes, promoting uncontrolled proliferation.
- Mutations: Point mutations in specific genes can activate oncogenes (promoting cell division) or inactivate tumor suppressor genes (controlling cell growth), leading to uncontrolled leukemic cell proliferation. Mutations in genes like FLT3 in AML or TP53 in CLL are examples.
- Epigenetic Alterations: Chemical modifications on DNA or histones can silence tumor suppressor genes or activate oncogenes, contributing to leukemogenesis.
Domino Effect on Bone Marrow
These genetic and molecular aberrations wreak havoc on the bone marrow, the factory responsible for blood cell production.
- Uncontrolled Proliferation: Abnormal cells, driven by genetic mutations, divide uncontrollably, crowding out healthy stem cells and disrupting their differentiation into mature blood cells.
- Impaired Differentiation: Mutations can impair the normal maturation process of blood cells, leading to the production of immature and dysfunctional blasts.
- Disrupted Microenvironment: The bone marrow environment, crucial for supporting healthy blood cell development, can be altered by abnormal cells, further hindering healthy blood cell production.
Consequences of Blood Cell Depletion
The decreased production of healthy blood cells leads to a cascade of devastating consequences.
- Infections: Reduced white blood cells (neutrophils, lymphocytes) weaken the immune system, making individuals susceptible to life-threatening infections.
- Anemia: Decreased red blood cells (erythrocytes) lead to reduced oxygen delivery throughout the body, causing fatigue, weakness, and shortness of breath.
- Bleeding: Low platelet counts impair blood clotting, increasing the risk of easy bruising and bleeding.
- Organ Damage: Infiltrating leukemic cells can damage vital organs like the liver, spleen, and lymph nodes, leading to organ dysfunction.
Epidemiology and Risk Factors
Leukemia, while affecting individuals of all ages and backgrounds, exhibits distinct patterns in its occurrence, offering valuable insights into potential causes.
Age and Gender Distribution
- Childhood Leukemia: Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, predominantly affecting children under five. Acute myeloid leukemia (AML) is less frequent but also occurs in children.
- Adult Leukemia: CLL and AML become more prevalent with age, with the highest rates observed in individuals over 65. CML typically affects adults between 45 and 55.
- Gender Variations: ALL shows a slight male predominance in childhood, while CLL is more common in men throughout life. Conversely, AML affects men and women relatively equally.
Geographic and Ethnic Disparities
- Incidence Variations: Rates of certain leukemia types vary geographically. For example, ALL and CML show higher rates in developed nations, while AML has a higher incidence in some developing countries.
- Ethnic Differences: Genetic variations and environmental exposures contribute to ethnic disparities in leukemia risk. For instance, certain ethnicities demonstrate higher susceptibility to specific genetic mutations associated with leukemia.
Genetic Predisposition and Family History
- Inherited Mutations: Some individuals inherit genetic mutations that increase their risk of developing leukemia. Examples include Down syndrome, which elevates ALL risk, and specific gene mutations associated with familial CML.
- Family History: Having a close relative with leukemia slightly increases an individual’s risk, suggesting a possible genetic component.
Environmental Risk Factors
- Radiation Exposure: High-dose radiation exposure, either through medical procedures, occupational hazards, or accidents, can increase the risk of developing various leukemias.
- Chemicals: Exposure to certain chemicals like benzene, used in some industrial processes, has been linked to an increased risk of AML.
- Other Factors: Some studies suggest potential associations between specific infections and the development of certain leukemia types, but the evidence is still evolving.
Lifestyle Choices
- Smoking: Cigarette smoking is a well-established risk factor for AML, with increased risk proportional to smoking intensity and duration.
Clinical Presentation
Leukemia often presents with non-specific symptoms that can be easily overlooked. However, recognizing these early warning signs is crucial for prompt diagnosis and timely intervention.
General Signs and Symptoms
- Fatigue: This is the most common symptom, often described as persistent tiredness, weakness, and lack of energy. It arises from decreased oxygen delivery due to anemia caused by low red blood cells.
- Fever: Recurrent or persistent fevers can indicate infections due to a compromised immune system caused by low white blood cell counts.
- Frequent Infections: Increased susceptibility to infections like mouth sores, sore throat, and respiratory issues is a common sign due to impaired immune function.
- Easy Bruising and Bleeding: Low platelet counts can lead to easy bruising, nosebleeds, and bleeding gums, indicating impaired blood clotting.
- Lymphadenopathy: Swollen lymph nodes in the neck, armpits, or groin might occur due to the accumulation of abnormal cells within the lymphatic system.
Specific Signs and Symptoms
- Swollen Abdomen: Enlarged spleen or liver due to infiltration of leukemic cells might cause abdominal discomfort or fullness.
- Bone Pain: Infiltration of leukemic cells into the bone marrow can lead to bone pain and tenderness.
- Headaches and Seizures: Infiltration of the central nervous system by leukemic cells can cause neurological symptoms like headaches and seizures in rare cases.
- Skin Rashes: Specific types of leukemia, like hairy cell leukemia, might present with characteristic skin rashes.
Variation based on Type and Severity
- Acute Leukemias: Symptoms often progress rapidly and are more pronounced due to the aggressive nature of the disease.
- Chronic Leukemias: Symptoms may be subtle and develop slowly, sometimes even being absent initially, making early detection challenging.
Importance of Early Diagnosis and Screening
- Early diagnosis allows for prompt treatment initiation, improving the chance of successful remission and long-term survival.
- Early intervention can minimize complications like infections and bleeding.
- Screening programs targeting high-risk groups, though not currently standard practice for most leukemia types, are being explored for their potential in early detection.
Diagnosis and Prognosis
Accurately diagnosing leukemia and understanding its specific characteristics are essential for guiding treatment decisions and predicting potential outcomes.
Diagnostic Workup
- Blood Tests
- Complete Blood Count (CBC): Evaluates red blood cells, white blood cells, and platelets, identifying abnormalities indicative of leukemia.
- Peripheral Blood Smear: Microscopic examination of blood cells to visualize abnormal blast cells.
- Flow Cytometry: Identifies specific markers on the surface of cells to classify the type of leukemia.
- Bone Marrow Biopsy and Aspiration
- Extraction of bone marrow tissue and fluid to examine cell morphology and presence of abnormal blasts.
- Crucial for definitive diagnosis and classification of leukemia subtype.
- Cytogenetic Analysis
- Evaluates chromosomal abnormalities associated with specific leukemia types, providing prognostic information and guiding treatment options.
- Molecular Testing
- Identifies specific gene mutations within the leukemic cells, offering insights into the disease’s driving mechanisms and potential targeted therapies.
Staging and Risk Stratification
- Staging Systems: Different systems categorize patients based on disease extent and severity, like the French-American-British (FAB) system for AML or the Binet stage system for CLL.
- Risk Stratification: Factors like age, genetic mutations, and disease characteristics are combined to estimate individual risk and guide treatment intensity and prognosis.
Prognosis and Survival Rates
- Prognosis varies significantly depending on several factors
- Type of leukemia: Each type has a different prognosis, with acute leukemias generally having lower survival rates compared to chronic types.
- Age: Younger patients often have better outcomes compared to older patients.
- Genetic mutations: Certain mutations can influence prognosis and response to treatment.
- Overall health: Underlying medical conditions can impact treatment tolerance and outcomes.
- Survival rates: While constantly improving, they vary based on the factors mentioned above. For example, the 5-year survival rate for childhood ALL is around 90%, while for adult AML, it is approximately 30%.
Treatment and Management
Leukemia treatment encompasses a multi-pronged approach, tailoring various strategies to the specific type, stage, and individual characteristics of the patient.
General Treatment Principles
- Chemotherapy: This cornerstone therapy uses potent drugs to kill rapidly dividing cancer cells, including leukemic blasts. Different combinations of drugs are used depending on the leukemia type.
- Radiation Therapy: High-energy beams target and kill cancer cells, often used for localized disease or to prevent central nervous system involvement.
- Targeted Therapy: These newer drugs focus on specific molecular abnormalities or pathways in leukemic cells, offering more precise treatment with potentially fewer side effects. Examples include tyrosine kinase inhibitors for CML and FLT3 inhibitors for AML.
- Stem Cell Transplantation: Healthy stem cells are infused to replace damaged bone marrow after high-dose chemotherapy or radiation, allowing for blood cell regeneration.
Specific Treatment Approaches
- Acute Leukemias: Intensive multi-agent chemotherapy is the mainstay, often followed by consolidation therapy and maintenance therapy. Stem cell transplantation might be incorporated depending on risk factors.
- Chronic Leukemias: Treatment aims for long-term disease control and may involve targeted therapies, chemotherapy, or combinations depending on the specific type and stage.
- Treatment individualization: Advancements in personalized medicine allow tailoring treatment based on specific genetic mutations and risk factors, potentially improving outcomes and reducing side effects.
Supportive Care and Management of Complications
- Supportive care plays a crucial role in managing side effects of treatment and improving quality of life.
- Transfusions: To replenish red blood cells, platelets, or white blood cells as needed.
- Infection prevention: Due to compromised immunity, preventive measures and prompt treatment of infections are vital.
- Nutritional support: Maintaining adequate nutrition is crucial for healing and overall well-being.
- Pain management: Addressing pain associated with treatment or disease progression is essential.
- Psychological support: Coping with the emotional challenges of diagnosis and treatment requires professional support and counseling.
Importance of Patient Education and Psychosocial Support
- Empowering patients through education about their disease, treatment options, and potential outcomes is crucial. This fosters informed decision-making and active participation in their care.
- Psychosocial support groups and individual therapy can provide invaluable emotional and social support, helping patients navigate the challenges of living with leukemia.
Future Directions and Advances
The fight against leukemia continues to evolve, with exciting new avenues emerging in personalized medicine, targeted therapies, and novel treatment approaches.
Personalized Medicine and Targeted Therapies
- Going beyond standard treatment: Traditionally, leukemia treatment relied on broad-spectrum approaches like chemotherapy. Personalized medicine focuses on identifying specific genetic mutations and molecular abnormalities within an individual’s leukemia cells. This allows for targeted therapies that attack these unique vulnerabilities, offering more precise treatment with potentially fewer side effects.
- Examples of targeted therapies: Tyrosine kinase inhibitors (TKIs) for CML and FLT3 and BCL-2 inhibitors for AML are some examples of targeted therapies already revolutionizing treatment.
- Continuous advancements: Research is ongoing to develop even more targeted therapies, including antibody-drug conjugates, CAR T-cell therapies, and small molecule inhibitors, aiming for even greater efficacy and personalized treatment strategies.
Immunotherapy and its Potential
- Harnessing the immune system: Immunotherapy has emerged as a powerful tool in cancer treatment, and its potential in leukemia is being actively explored. This approach aims to activate or enhance the body’s own immune system to recognize and destroy cancer cells.
- CAR T-cell therapy: This innovative approach involves genetically engineering a patient’s T cells to recognize and attack specific antigens on leukemia cells, demonstrating remarkable results in certain types of ALL.
- Immune checkpoint inhibitors: These drugs unleash the immune system’s natural ability to fight cancer by blocking checkpoints that normally prevent excessive immune response. While still under investigation for leukemia, they show promise in some cases.
- Challenges and future directions: Optimizing immunotherapy for different leukemia types, managing potential side effects, and combining it with other therapies are areas of ongoing research.
Gene Therapy and Stem Cell-Based Therapies
- Correcting the genetic blueprint: Gene therapy aims to introduce healthy genes into leukemia cells to correct the mutations causing uncontrolled growth. This approach is still in early stages but holds potential for long-term disease control or even cure.
- Stem cell therapies: These therapies involve using healthy stem cells to replace damaged bone marrow or boost the immune system. While stem cell transplantation is already used in some leukemia treatments, research is exploring novel applications like using gene-edited stem cells for targeted therapy delivery.
- Ethical considerations and challenges: The long-term safety and efficacy of these therapies are still being evaluated, and addressing ethical concerns surrounding gene editing is crucial.
Importance of Clinical Trials and Patient Participation
- Advancing the future: Clinical trials are essential for testing and evaluating new treatment approaches. Participating in clinical trials allows patients to access potentially life-saving therapies while contributing to scientific progress.
- Empowered decision-making: Understanding the risks and benefits of participating in a clinical trial is crucial for informed decision-making. Consulting with healthcare professionals and patient advocacy groups can empower patients to make choices that align with their individual needs and goals.
Frequently Asked Questions (FAQs)
What is leukemia caused by?
The exact cause of leukemia is unknown in most cases. However, researchers have identified several contributing factors that seem to play a role in its development:
Genetic abnormalities: Certain genetic mutations inherited from parents or acquired during life can disrupt blood cell development and lead to uncontrolled growth of abnormal cells, potentially leading to leukemia.
Environmental factors: Exposure to some chemicals like benzene (found in gasoline and some industrial processes) and radiation (high doses from medical procedures or accidents) can increase the risk of developing certain types of leukemia.
Other factors: While the evidence is less conclusive, some studies suggest potential links between specific infections and the development of certain leukemia types, and certain medical conditions like Down syndrome can also increase the risk.
It’s important to note:
- These are risk factors, and having these factors does not guarantee that someone will develop leukemia.
- The specific causes can vary depending on the type of leukemia.
- Ongoing research continues to explore the complex interplay of various factors contributing to leukemia development.
Who is most at risk for leukemia?
While anyone can develop leukemia, certain groups have a higher risk than others.
Age
- Children under 5: More susceptible to acute lymphoblastic leukemia (ALL).
- Adults over 65: More susceptible to chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML).
Gender
- Men: Slightly higher risk compared to women.
Family history
- Having a close relative (parent, sibling, child) with leukemia slightly increases an individual’s risk, suggesting a possible genetic component.
Genetic conditions
- Down syndrome is associated with an increased risk of ALL.
- Other specific genetic mutations can also increase susceptibility.
Environmental factors
- High-dose radiation exposure: From medical procedures, accidents, or occupational settings.
- Exposure to certain chemicals: Benzene (found in gasoline and some industrial processes).
Lifestyle factors
- Smoking: Significantly increases the risk of AML.
It’s important to remember
- Risk factors do not guarantee someone will develop leukemia.
- The specific risk factors can vary depending on the type of leukemia.
Is leukemia curable if caught early?
Whether leukemia is curable when caught early depends on several factors, including:
- The specific type of leukemia: Different types have different prognoses (chances of recovery) and response rates to treatment. Generally, acute leukemias have a higher chance of cure compared to chronic leukemias.
- The stage of the disease at diagnosis: Early detection plays a crucial role. Earlier stages often respond better to treatment and offer a higher chance of complete remission (disappearance of all signs of cancer).
- Individual characteristics: Factors like age, overall health, and response to treatment also influence the outcome.
While it’s not entirely accurate to say all leukemias can be cured even with early detection, here’s a general breakdown:
- Acute Leukemias
- Chronic Leukemias
- CLL: While not typically considered curable, early diagnosis allows for long-term disease control through management strategies.
- CML: While not technically curable, early detection allows for effective treatment with targeted therapies that can achieve deep remission and significantly prolong life expectancy.
What are red flags of leukemia?
Leukemia can present with various symptoms, but some stand out as potential red flags that warrant prompt medical attention. These symptoms often indicate a more critical condition and require immediate evaluation:
Rapidly worsening symptoms
- Fast and progressive fatigue: Feeling extremely tired and weak, even with minimal activity, and experiencing a rapid decline in energy levels.
- Severe and persistent infections: Frequent infections that are difficult to treat or recur quickly, suggesting a significantly compromised immune system.
- Significant bleeding: Unprovoked bleeding episodes, such as heavy nosebleeds, prolonged bleeding from minor cuts, or bleeding gums without obvious cause.
- Rapid weight loss: Unexplained and rapid weight loss, not attributed to dietary changes or exercise.
Alarming physical changes
- Enlarged lymph nodes: Swollen lymph nodes, particularly in the neck, armpits, or groin, that are firm, painless, and rapidly growing.
- Swollen abdomen: A noticeable enlargement of the abdomen due to an enlarged spleen or liver, often accompanied by discomfort or pain.
- Bone pain: Persistent and severe pain in the bones or joints, especially without any apparent injury or trauma.
- Neurological symptoms: Headaches, seizures, or changes in mental clarity, which could indicate central nervous system involvement in some cases.
Remember: These red flags are not exclusive to leukemia and can occur due to other conditions. However, their presence, especially when multiple symptoms occur together or worsen rapidly, necessitates seeking immediate medical attention for proper diagnosis and prompt intervention.
How long can a person live with leukemia?
Here’s a general overview of the life expectancy for some common leukemia types:
Acute Leukemias
- Childhood ALL: Despite remaining the leading cause of death by disease for children past infancy in the United States, childhood cancer mortality dropped by over 50% between 1975 and 2020. This positive trend is reflected in improved survival rates for Acute Lymphoblastic Leukemia (ALL), where the 5-year survival rate for children under 15 climbed from 60% to nearly 90%, and for adolescents aged 15-19, skyrocketed from 28% to over 75%. These advancements offer hope for continued progress in treating childhood cancer.
- Adult AML: The 5-year relative survival rate for people 20 and older with AML is 28%. This means that 28 out of every 100 people diagnosed with AML at this age will be alive 5 years after their diagnosis. For people younger than 20, the 5-year relative survival rate is 69%.
Chronic Leukemias
- CLL: While there is no current cure for chronic lymphocytic leukemia (CLL), the outlook is encouraging. Around 87% of individuals diagnosed with CLL go on to live for at least 5 years. This signifies that many people with CLL can live well and manage the disease for an extended period.
- CML: Thanks to advancements in treatment, the five-year survival rate for chronic myeloid leukemia (CML) has more than doubled, with 70% of patients now surviving for five years or longer. Previously, the typical survival rate for CML was only three to five years.
What does leukemia pain feel like?
Describing leukemia pain can be challenging as the experience varies significantly depending on the individual and the source of the pain. However, here are some common types of pain associated with leukemia:
Bone pain: In leukemia, the abnormal accumulation of white blood cells in the bone marrow can cause it to expand, leading to bone pain. The long bones of the legs and arms are the most common location to experience this pain. It can be described as:
- Sharp and stabbing: This is often felt in the long bones of the arms and legs, ribs, sternum (breastbone), or back.
- Dull and aching: This might be a persistent, throbbing discomfort.
- Worse at night: Pain often intensifies at night, disrupting sleep and causing significant discomfort.
Joint pain: This can occur when leukemia cells accumulate around joints, causing inflammation and discomfort. It can feel similar to:
- Arthritis pain: Aching and stiffness in the joints, making movement difficult.
- General muscle soreness: A dull ache or tightness in the muscles surrounding the affected joint.
Does leukemia affect sperm?
Yes, leukemia can affect sperm production and quality in several ways.
- Direct damage: Leukemia cells can infiltrate the testicles, the organs responsible for sperm production. This infiltration can directly damage sperm-producing cells (spermatogonia) and disrupt spermatogenesis, the process of sperm formation.
- Chemotherapy: Many chemotherapy drugs used to treat leukemia can have side effects on sperm production. These drugs can damage spermatogonia, reduce sperm count, motility (movement), and morphology (shape).
- Hormonal imbalances: Leukemia can disrupt the production of hormones like testosterone, which plays a crucial role in sperm production and function.
Can leukemia stop you from having kids?
Leukemia itself doesn’t directly stop you from having kids, but the treatments used to fight it can impact fertility in both men and women.
- Chemotherapy, commonly used to treat leukemia, can damage egg and sperm cells, potentially reducing fertility or even causing temporary or permanent infertility.
- Radiation therapy directed to the pelvic area can also significantly reduce fertility potential.
However, fertility preservation methods like sperm banking or egg freezing before treatment can offer options for having biological children in the future.
What is silent leukemia?
The term “silent leukemia” is not a specific medical term referring to a distinct type of leukemia. However, it’s sometimes used informally to describe chronic lymphocytic leukemia (CLL) in its early stages because it often doesn’t present with noticeable symptoms.
Here’s why CLL might be referred to as “silent”:
- Slow-growing: CLL is a slow-growing cancer, meaning symptoms often develop gradually and might not be readily apparent in the early stages.
- Non-specific symptoms: Even when symptoms do arise, they can be vague and non-specific, such as fatigue, mild infections, or weight loss, which can be easily attributed to other causes.
Why does leukemia cause splenomegaly?
Leukemia causes splenomegaly, or an enlarged spleen, due to a combination of factors.
1. Infiltration by abnormal cells
- Leukemia involves the uncontrolled growth of abnormal white blood cells. These cells can infiltrate the spleen, which is responsible for filtering blood and removing old or damaged cells.
- As the abnormal cells accumulate in the spleen, it enlarges to accommodate the increased cellular load.
2. Increased workload
- The spleen plays a crucial role in the immune system, fighting infections and removing abnormal cells from the bloodstream.
- In leukemia, the increased production of abnormal white blood cells puts a significant strain on the spleen, causing it to work harder and potentially enlarge to meet the increased demand.
3. Extramedullary hematopoiesis
- Normally, blood cell production occurs in the bone marrow. However, in some cases of leukemia, the bone marrow becomes overwhelmed by abnormal cells, and blood cell production can start occurring in other organs, including the spleen. This additional production can contribute to the enlargement of the spleen.
Why do leukemia patients need blood transfusions?
Leukemia patients commonly need blood transfusions for these main reasons:
- To combat anemia: Leukemia and its treatments can damage the bone marrow, limiting its ability to produce healthy red blood cells. This leads to anemia, causing fatigue, weakness, and shortness of breath. Red blood cell transfusions replenish these cells, improving oxygen delivery and alleviating symptoms.
- To prevent bleeding: Leukemia often leads to thrombocytopenia, a dangerously low platelet count. Platelets are critical for blood clotting. Platelet transfusions increase platelet levels, minimizing the risk of excessive bleeding or bruising.
- To fight infections: Some leukemias result in low white blood cell counts (neutropenia), weakening the immune system. While less common, transfusions of specific white blood cells can temporarily boost the body’s ability to combat infections.
- To support treatment: Chemotherapy and some leukemia treatments further damage bone marrow function. Transfusions provide an immediate supply of essential blood cells, helping patients tolerate and recover from treatments.
Important to note
- The type and frequency of transfusions depend on the leukemia type, how it affects the patient’s blood cells, their treatment plan, and their response.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Du M, Chen W, Liu K, Wang L, Hu Y, Mao Y, Sun X, Luo Y, Shi J, Shao K, Huang H, Ye D. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J Oncol. 2022 Apr 25;2022:1612702. doi: 10.1155/2022/1612702. PMID: 35509847; PMCID: PMC9061017.
- Dong Y, Shi O, Zeng Q, Lu X, Wang W, Li Y, Wang Q. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020 Jun 19;9:14. doi: 10.1186/s40164-020-00170-6. PMID: 32577323; PMCID: PMC7304189.
- Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020 Jul 31;55(S1):S1-S4. doi: 10.5045/br.2020.S001. PMID: 32719169; PMCID: PMC7386892.
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian RP, Larson RA, Levine RL, Miyazaki Y, Niederwieser D, Ossenkoppele G, Röllig C, Sierra J, Stein EM, Tallman MS, Tien HF, Wang J, Wierzbowska A, Löwenberg B. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022 Sep 22;140(12):1345-1377. doi: 10.1182/blood.2022016867. PMID: 35797463.
- Ishii H, Yano S. New Therapeutic Strategies for Adult Acute Myeloid Leukemia. Cancers (Basel). 2022 Jun 5;14(11):2806. doi: 10.3390/cancers14112806. PMID: 35681786; PMCID: PMC9179253.
- Sekeres MA. When Blood Breaks Down: Life Lessons from Leukemia (Mit Press). 2021
- Bhushan B. AML (Acute Myeloid Leukemia): A survival guide for patients. 2021.
- Greiner J. Immunotherapies for Acute Myeloid Leukemia (MDPI AG). 2020.
- Daniel J. DeAngelo, Elias Jabbour, and Anjali Advani. Recent Advances in Managing Acute Lymphoblastic Leukemia. American Society of Clinical Oncology Educational Book 2020 :40, 330-342.
- Yilmaz M, Kantarjian H, Ravandi-Kashani F, Short NJ, Jabbour E. Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: current treatments and future perspectives. Clin Adv Hematol Oncol. 2018 Mar;16(3):216-223. PMID: 29742077.
- Saleh K, Fernandez A, Pasquier F. Treatment of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia in Adults. Cancers (Basel). 2022 Apr 1;14(7):1805. doi: 10.3390/cancers14071805. PMID: 35406576; PMCID: PMC8997772.
- Carr JH. Clinical Hematology Atlas 6th Edition (Elsevier).
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ; International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008 Jun 15;111(12):5446-56. doi: 10.1182/blood-2007-06-093906. Epub 2008 Jan 23. Erratum in: Blood. 2008 Dec 15;112(13):5259. PMID: 18216293; PMCID: PMC2972576.
- National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients® Chronic Lymphocytic Leukemia. 2022.
- Hallek M, Eichhorst B, Catovsky D. Chronic Lymphocytic Leukemia (Hematologic Malignancies) 1st ed. 2019 Edition (Springer).