TL;DR
Warm autoimmune hemolytic anemia (wAIHA) involves the immune system attacking red blood cells, primarily at body temperature.
- Pathophysiology ▾: Caused by autoantibodies (IgG) targeting red blood cells leading to premature red blood cell destruction (hemolysis), causing anemia.
- Causes ▾: It can be primary (idiopathic) or secondary to other conditions like autoimmune diseases, infections, or medications.
- Symptoms ▾: Fatigue, pallor, jaundice, and shortness of breath.
- Diagnosis ▾: Direct antiglobulin test (DAT) is crucial
- Treatment ▾: Corticosteroids, rituximab, and sometimes splenectomy are used.
- Variable Course: Can be acute or chronic, with potential complications like thrombosis and infection.
*Click ▾ for more information
Introduction
Warm antibody hemolytic anemia (warm AIHA) represents the most frequently encountered form of autoimmune hemolytic anemia (AIHA). This condition is characterized by the production of autoantibodies that exhibit optimal reactivity at or above normal human body temperature, within the range of 36.5 to 37.5°C. These autoantibodies target and attach to the surface of red blood cells (RBCs), effectively marking them for premature destruction (hemolysis) by the body’s immune system.
What is hemolytic anemia?
Hemolytic anemia is a condition characterized by the premature destruction of red blood cells (hemolysis), occurring at a rate faster than the bone marrow can replace them. This accelerated breakdown of red blood cells leads to a shortage of these oxygen-carrying cells, resulting in anemia.
| Inherited (Intrinsic) Causes | Acquired (Extrinsic) Causes |
| Red blood cell membrane defects Hereditary spherocytosis Hereditary elliptocytosis Hereditary stomatocytosis | Autoimmune hemolytic anemia (AIHA) Warm AIHA Cold agglutinin disease Paroxysmal cold hemoglobinuria |
| Enzyme deficiencies G6PD deficiency Pyruvate kinase deficiency | Drug-induced hemolytic anemia Certain medications can trigger immune-mediated or non-immune-mediated hemolysis |
| Hemoglobinopathies Sickle cell disease Thalassemia (alpha and beta) | Mechanical hemolytic anemia Heart valve prostheses Microangiopathic hemolytic anemia e.g. TTP, HUS, DIC March hemoglobinuria |
| Infections Malaria Clostridium perfringens sepsis Others | |
| Chemicals and toxins Lead poisoning Arsenic poisoning Snake venoms | |
| Hypersplenism | |
| Paroxysmal nocturnal hemoglobinuria (PNH) | |
| Transfusion reactions |
What is autoimmune hemolytic anemia (AIHA)?
Autoimmune hemolytic anemia (AIHA) is a condition where the body’s immune system mistakenly attacks and destroys its own red blood cells because the immune system produces autoantibodies that recognize red blood cells as foreign invaders. This premature destruction of red blood cells leads to anemia, a condition characterized by a deficiency of red blood cells.
Autoimmune hemolytic anemia (AIHA) is generally categorized into the following subtypes, primarily based on the temperature at which the autoantibodies are most active.
- Warm AIHA: This is the most common subtype, where autoantibodies (typically IgG) are most active at body temperature (37°C).
- Cold AIHA: This subtype involves autoantibodies (typically IgM) that are most active at colder temperatures. This includes:
- Cold agglutinin disease (CAD)
- Paroxysmal cold hemoglobinuria (PCH)
- Mixed AIHA: This subtype involves a combination of both warm and cold autoantibodies.
- Drug-induced AIHA: This sub type involves AIHA that is caused by reactions to certain medications.
Pathophysiology of warm AIHA
The pathophysiology of warm autoimmune hemolytic anemia (AIHA) centers around the aberrant production of autoantibodies, primarily of the IgG class, which target red blood cell (RBC) surface antigens.
Autoantibody Production
The immune system, for reasons often unknown (in primary AIHA), or due to underlying conditions (secondary AIHA), loses its tolerance to self-antigens on RBCs. This leads to the production of IgG autoantibodies that recognize and bind to these RBC surface antigens.
These antibodies are typically “warm-reacting,” meaning they are most active at body temperature (37°C).
RBC Sensitization
The IgG autoantibodies attach to the surface of the RBCs, “sensitizing” them. The number of autoantibodies bound and their affinity for the RBC antigen plays a significant role in the severity of hemolysis.
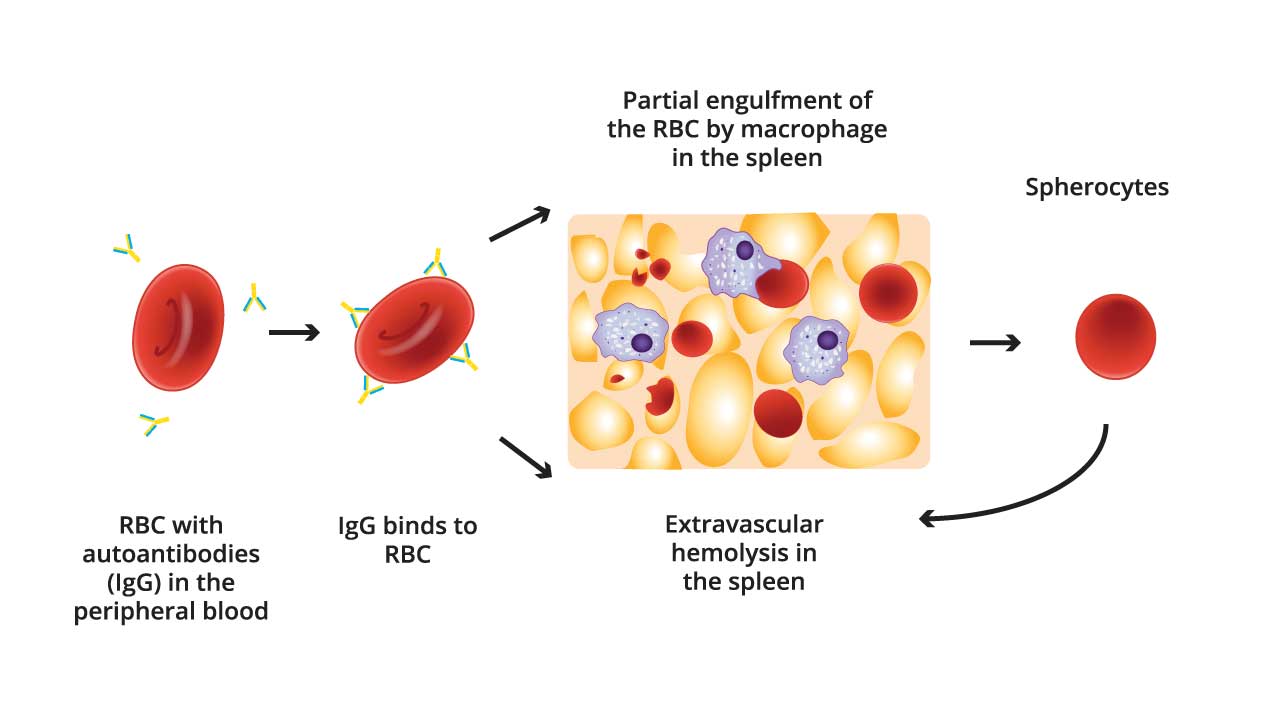
Extravascular Hemolysis
The primary mechanism of RBC destruction in warm AIHA is extravascular hemolysis, predominantly occurring in the spleen.
Splenic macrophages possess Fc receptors that recognize the Fc portion of the IgG autoantibodies bound to the RBCs. Macrophages then engulf and destroy these sensitized RBCs, leading to their premature removal from circulation. This process is called phagocytosis.
The reticuloendothelial system throughout the body also plays a role in this destruction, but the spleen is the major site.
Complement Activation (Less Common)
While less frequent than in cold AIHA, complement activation can also contribute to RBC destruction in warm AIHA.
In some cases, the bound IgG autoantibodies can activate the complement cascade, leading to the deposition of complement components (especially C3) on the RBC surface. This can further enhance macrophage-mediated destruction or, in rare severe cases, lead to direct lysis of the RBCs.
Consequences of Hemolysis
The accelerated destruction of RBCs results in anemia, characterized by a reduced number of circulating RBCs and decreased hemoglobin levels.
The breakdown of hemoglobin releases bilirubin, which can lead to jaundice. The bone marrow attempts to compensate for the RBC loss by increasing RBC production, resulting in reticulocytosis (increased immature RBCs). Haptoglobin, a protein which binds free hemoglobin, is decreased.
Factors Influencing Hemolysis Severity
The severity of hemolysis in warm autoimmune hemolytic anemia (AIHA) is not uniform and is influenced by a combination of factors.
Autoantibody Characteristics
- Antibody Affinity: The strength of the bond between the autoantibody and the RBC antigen significantly impacts hemolysis. Higher affinity antibodies lead to more robust RBC binding and, consequently, more efficient destruction.
- Antibody Quantity: The number of autoantibody molecules bound to each RBC is crucial. A higher density of bound antibodies increases the likelihood of macrophage recognition and phagocytosis.
- IgG Subclass: Different IgG subclasses (IgG1, IgG3, etc.) vary in their ability to activate complement and interact with macrophage Fc receptors. IgG1 and IgG3 generally mediate more severe hemolysis.
- Thermal Amplitude: While warm AIHA antibodies are most active at body temperature, some may retain activity at slightly lower temperatures, extending the duration of RBC destruction.
Red Blood Cell Susceptibility
- RBC Antigen Density: The number of target antigens on the RBC surface can influence the amount of autoantibody binding. Higher antigen density may theoretically increase the number of antibodies that can bind, though the relationship between antigen density and hemolysis severity can be complex.
- RBC Membrane Integrity: Underlying RBC membrane abnormalities can increase susceptibility to hemolysis.
- RBC Age: Older RBCs are naturally more fragile and may be more susceptible to destruction when sensitized by autoantibodies.
Splenic Function
The spleen is the primary site of RBC destruction in warm AIHA. The efficiency of splenic macrophage activity plays a major role in the rate of hemolysis. Splenomegaly (enlarged spleen) can exacerbate hemolysis by increasing the volume of RBCs exposed to splenic macrophages. Conversely, in individuals with asplenia (absence of a spleen), hemolysis may be less severe, but they remain at a heightened risk for infections.
Complement Activation
Although less common in warm AIHA than cold AIHA, complement activation can contribute to hemolysis. The extent to which the complement cascade is activated influences the severity of RBC destruction.
Individual Patient Factors
Underlying health conditions, such as other autoimmune diseases or infections, can influence the immune system’s response and thus affect the severity of hemolysis.
The bone marrow’s ability to compensate for RBC loss by increasing RBC production (reticulocytosis) also plays a role in the degree of anemia.
Understanding these factors is crucial for predicting the clinical course of warm AIHA and tailoring appropriate treatment strategies.
What is the difference between warm and cold autoimmune hemolytic anemia?
The key difference between warm and cold autoimmune hemolytic anemia (AIHA) lies in the temperature at which the autoantibodies responsible for red blood cell destruction are most active.
Warm AIHA
- Autoantibodies (typically IgG) are most active at body temperature (37°C).
- This is the most common form of AIHA.
- Hemolysis primarily occurs in the spleen (extravascular hemolysis).
- Often associated with other autoimmune diseases, lymphoproliferative disorders, or certain medications.
Cold AIHA
- Autoantibodies (typically IgM) are most active at colder temperatures (below 37°C).
- This includes cold agglutinin disease (CAD) and paroxysmal cold hemoglobinuria (PCH). Cold agglutinin disease is more common in older patients and can be associated with certain infections or lymphoproliferative disorders. Paroxysmal cold hemoglobinuria is less common, and can be related to prior infection.
- Hemolysis can occur both extravascularly and intravascularly.
- Symptoms can be exacerbated by exposure to cold.
Causes of Warm AIHA
Primary (Idiopathic) warm AIHA
In approximately half of all cases of warm AIHA affecting adults, a specific underlying cause cannot be identified . These instances are categorized as primary or idiopathic warm AIHA.
Despite ongoing research efforts, the precise trigger that initiates the production of autoantibodies against red blood cells in these individuals remains elusive. It is hypothesized that a combination of genetic predisposition and subtle, yet unidentified, environmental factors might play a role in disrupting immune tolerance and leading to this autoimmune response.
The lack of a clear initiating event in primary warm AIHA underscores the complexity of the immune system and the challenges in fully understanding the development of autoimmune disorders.
Secondary warm AIHA
Secondary warm AIHA develops in association with a diverse array of underlying medical conditions. Identifying the specific underlying cause is of paramount importance for the overall management of the patient, as treatment strategies often need to address both the warm AIHA and the associated condition .
These conditions can include:
Autoimmune Diseases
A spectrum of other autoimmune diseases can be associated with the development of warm AIHA. These include systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjogren’s syndrome, ulcerative colitis, and various other autoimmune conditions . The occurrence of warm AIHA in a patient already diagnosed with another autoimmune disease might indicate a period of increased disease activity or a flare-up of the underlying condition.
Lymphoproliferative Disorders
Conditions such as chronic lymphocytic leukemia (CLL), various forms of lymphoma, particularly non-Hodgkin’s lymphoma, and other lymphoproliferative syndromes are frequently observed in conjunction with secondary warm AIHA .
In some instances, warm AIHA can even be the initial clinical manifestation of an otherwise undiagnosed lymphoproliferative disorder, highlighting the necessity for thorough investigations, especially in older patients presenting with new-onset warm AIHA .
Infections
Both viral and bacterial infections have been implicated in the pathogenesis of secondary warm AIHA.
Viral infections such as HIV, hepatitis C, Epstein-Barr virus, and certain bacterial infections like mycoplasmal pneumonia are among those that have been linked to this condition. Notably, infection with SARS-CoV-2, the virus responsible for COVID-19, has also emerged as a potential trigger for warm AIHA.
One proposed mechanism for infection-associated warm AIHA is molecular mimicry, where antigens present on the infectious agent bear structural similarities to antigens on red blood cells, leading the immune system to mistakenly target the body’s own cells .
Medications
Certain pharmaceutical agents can also induce the development of warm AIHA, a phenomenon known as drug-induced AIHA. Examples of such medications include methyldopa, levodopa, cephalosporin antibiotics, and penicillins . In many cases of drug-induced warm AIHA, the condition resolves once the patient discontinues the use of the causative medication, emphasizing the importance of obtaining a comprehensive medication history from individuals diagnosed with warm AIHA.
Others
- Immunodeficiency Disorders
- Solid Tumors
- Transplants
- Immune check point inhibitors: Medications used in cancer treatment.
Clinical Presentation (Symptoms) of warm AIHA
Warm autoimmune hemolytic anemia (wAIHA) symptoms arise from the body’s destruction of red blood cells, leading to anemia. The severity and specific symptoms can vary greatly from person to person.
Symptoms Related to Anemia
- Fatigue: This is a very common symptom, as the reduced oxygen-carrying capacity of the blood leads to general tiredness and weakness.
- Pallor (pale skin)
- Shortness of breath (dyspnea).
- Dizziness or lightheadedness
- Headaches
- Chest pain
- Rapid heartbeat (tachycardia)
Other Potential Symptoms
- Jaundice (yellowing of the skin and eyes): This occurs when the liver cannot process the bilirubin released from the destroyed red blood cells.
- Dark urine (hemoglobinuria): The breakdown of red blood cells can lead to the excretion of hemoglobin in the urine, making it darker.
- Enlarged spleen (splenomegaly): The spleen is involved in removing damaged red blood cells, and it can become enlarged as it works harder.
- Abdominal discomfort: The enlarged spleen can cause discomfort in the left upper abdomen.
- Fever: this can occur, especially if the warm AIHA is secondary to an infection.
Symptoms of Underlying Conditions (if secondary AIHA)
When warm autoimmune hemolytic anemia (wAIHA) is secondary to an underlying condition, the symptoms of wAIHA itself can be compounded or masked by the symptoms of the primary disease.
1. Systemic Lupus Erythematosus (SLE)
- Joint pain and swelling: Arthritis is a common feature.
- Rash: Particularly a butterfly-shaped rash across the face.
- Fatigue: Often severe.
- Fever: Unexplained fevers.
- Kidney problems: Leading to swelling in the legs and feet.
- Chest pain: Due to pleurisy (inflammation of the lining of the lungs).
- Mouth ulcers
- Photosensitivity
2. Lymphoproliferative Disorders (e.g., CLL, lymphoma)
- Enlarged lymph nodes: Swollen glands in the neck, armpits, or groin.
- Night sweats
- Unexplained weight loss
- Fatigue
- Fever
- Frequent infections
- Enlarged spleen or liver: Causing abdominal discomfort.
3. Infections
Symptoms vary depending on the specific infection. Viral infections can cause fever, fatigue, and general malaise. COVID-19 can cause a wide array of symptoms including fever, cough, shortness of breath, fatigue, and many others.
4. Medications
Symptoms related to drug-induced warm AIHA may appear shortly after starting a new medication. Other side effects of the medication may be present.
5. Other Autoimmune Diseases (e.g., Rheumatoid Arthritis)
- Joint pain and stiffness: Particularly in the morning.
- Swelling in the joints
- Fatigue
- Fever
- Dry eyes and mouth
Acute vs. Chronic Presentation
| Feature | Acute wAIHA | Chronic wAIHA |
| Onset | Rapid (days or hours) | Gradual (weeks or months) |
| Severity of Symptoms | Severe (potentially life-threatening) | Mild to moderate |
| Typical Symptoms | Severe fatigue, pronounced pallor, significant shortness of breath, jaundice, dark urine, fever, chest pain, circulatory collapse (in extreme cases) | Persistent fatigue, mild pallor, some shortness of breath; may be asymptomatic |
| Hemoglobin Levels | Rapid and substantial decrease | Stable or slowly declining |
| Potential Triggers | Infections, certain medications | Often associated with underlying conditions (autoimmune diseases, lymphoproliferative disorders) |
| General prognosis | Requires immediate medical intervention. | Can require long term management |
| Speed of Diagnosis | Urgent | Slower, requiring thorough over time analysis |
Laboratory Investigations to Diagnose Warm AIHA
Diagnosing warm autoimmune hemolytic anemia (wAIHA) involves a combination of clinical evaluation and laboratory investigations.
Complete Blood Count (CBC)
Expected results in warm AIHA include anemia, reticulocytosis due to bone marrow compensation for red blood cell destruction and elevated mean corpuscular volume (MCV) may be present due to the high number of reticulocytes.
Peripheral Blood Smear
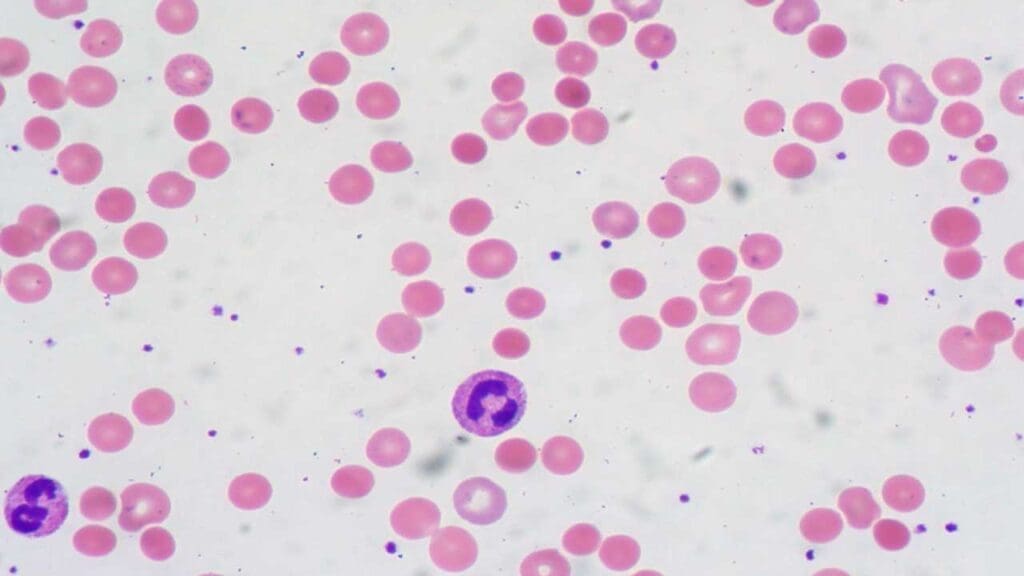
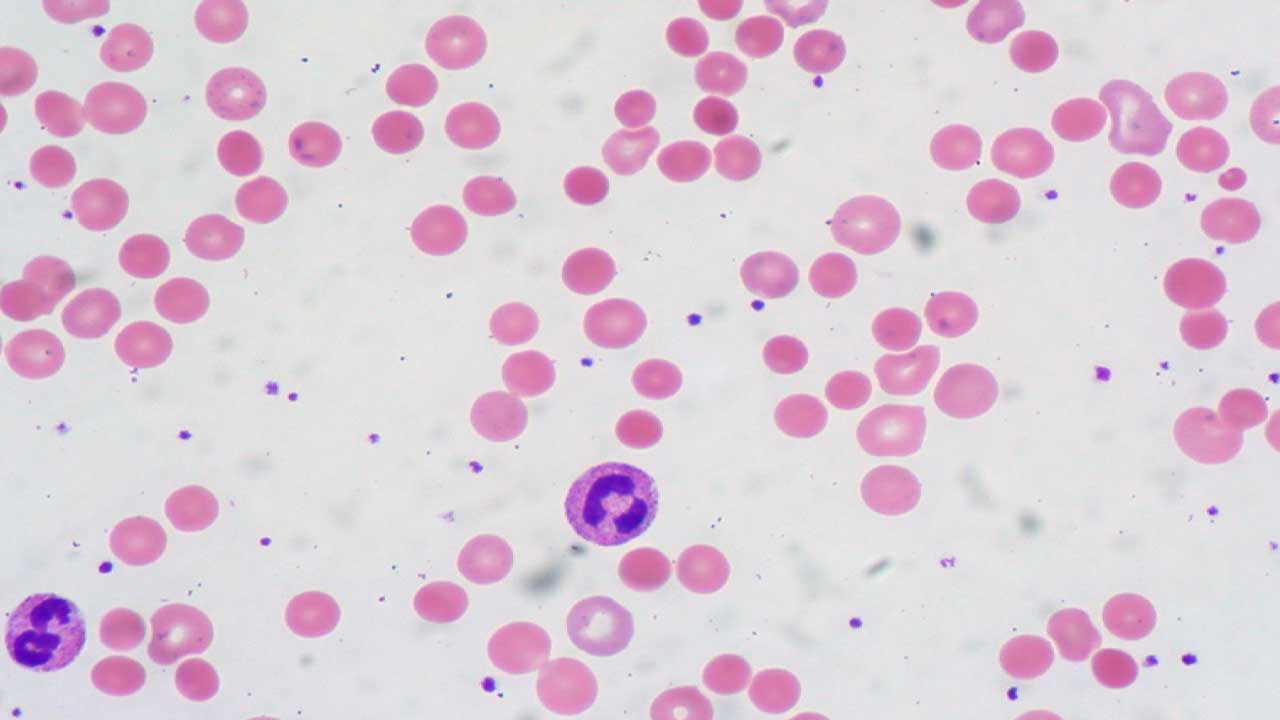
A common finding is the presence of microspherocytes, which are small, round red blood cells that have lost their typical biconcave shape and lack the central pallor seen in normal RBCs.
Polychromasia, an increased variation in the color of red blood cells, reflecting the presence of reticulocytes is also frequently observed. In some instances, red blood cell agglutination, or clumping, may also be noted on the peripheral smear.
The combination of anemia and the presence of microspherocytes on the peripheral smear strongly suggests extravascular hemolysis, the primary mechanism of red blood cell destruction in warm AIHA.
Direct Antiglobulin Test (DAT) (Coombs Test)
This is the cornerstone of warm AIHA diagnosis. A positive DAT indicates the presence of antibodies (IgG) and/or complement (C3) on the surface of red blood cells.
While a positive DAT is a hallmark of warm AIHA, it is important to note that in a small percentage of cases, the DAT may be negative. This can occur due to very low levels of autoantibodies bound to the red blood cells, the presence of low-affinity antibodies that are not easily detected by standard DAT reagents, or, rarely, when the autoantibodies involved are of the IgA isotype . Therefore, a negative DAT does not entirely exclude the diagnosis of warm AIHA in a patient with clinical and other laboratory findings suggestive of the condition.
Hemolysis Markers
- Lactate Dehydrogenase (LDH): Elevated LDH levels, indicating red blood cell destruction.
- Haptoglobin: Decreased or absent haptoglobin levels, as it binds to free hemoglobin released during hemolysis.
- Bilirubin: Increased indirect (unconjugated) bilirubin levels, a product of hemoglobin breakdown.
Other Investigations
- Indirect Antiglobulin Test: The indirect antiglobulin test, another component of the Coombs testing, is used to detect the presence of unbound autoantibodies circulating freely in the patient’s serum or plasma that have the potential to react with normal red blood cells. A positive result on the indirect antiglobulin test with a negative direct antiglobulin test typically suggests the presence of alloantibodies, which are antibodies directed against foreign red blood cell antigens (e.g., in the context of blood transfusions or pregnancy), rather than the autoantibodies that cause hemolysis in warm AIHA.
- Monospecific Antibody Testing: Following a positive DAT result obtained with a polyspecific reagent (which detects both IgG and complement), further testing with monospecific antisera is usually performed. These antisera are specific for individual immunoglobulin isotypes (such as anti-IgG, anti-C3d, and sometimes anti-IgA and anti-IgM) and are used to identify the specific types of antibodies or complement components that are bound to the patient’s red blood cells . Identifying the specific immunoglobulin isotype involved, such as IgG, IgA, or IgM, can provide additional insights into the nature of the autoimmune response and may have implications for treatment strategies.
- Identifying underlying conditions: Depending on the clinical picture, further tests may be needed to identify underlying conditions associated with secondary wAIHA, such as:
- Autoantibody testing (e.g., antinuclear antibodies [ANA] for SLE).
- Lymphocyte subset analysis using flow cytometry.
- Imaging studies (e.g., CT scans) to detect lymphadenopathy or splenomegaly.
- Testing for viral infections.
Key Laboratory Investigations for Warm AIHA
| Test | Expected Findings in Warm AIHA | Significance |
| Complete Blood Count (CBC) | Anemia (low hemoglobin, hematocrit), MCV normal or increased | Indicates reduced red blood cell count |
| Peripheral Smear | Microspherocytes, polychromasia, possible RBC agglutination | Suggests extravascular hemolysis and bone marrow response |
| Reticulocyte Count | Elevated (reticulocytosis), may be low in severe cases (reticulocytopenia) | Indicates increased RBC production by bone marrow |
| Lactate Dehydrogenase (LDH) | Elevated | Marker of red blood cell destruction |
| Bilirubin (Indirect) | Elevated | Product of hemoglobin breakdown |
| Haptoglobin | Decreased or undetectable | Protein consumed during hemolysis |
| Direct Antiglobulin Test (DAT) | Positive for IgG, may also have complement (C3) | Detects antibodies or complement on red blood cell surface |
| Indirect Antiglobulin Test | Usually negative | Detects free-circulating antibodies in serum |
Differential Diagnosis
When considering a diagnosis of warm autoimmune hemolytic anemia (wAIHA), it’s crucial to differentiate it from other conditions that can cause similar symptoms or laboratory findings.
| Condition | Key Differentiating Features | Key Laboratory Findings |
| Cold Agglutinin Disease (CAD) | Symptoms related to cold exposure (acrocyanosis, Raynaud’s), Antibody reaction at cold temperatures | DAT positive for C3, often negative for IgG |
| Hereditary Hemolytic Anemias (e.g., spherocytosis, thalassemia, G6PD deficiency) | Family history, specific red cell morphology, genetic abnormalities | Abnormal red cell indices, specific enzyme deficiencies, genetic testing |
| Microangiopathic Hemolytic Anemia (MAHA) (e.g., TTP, HUS, DIC) | Fragmented red blood cells (schistocytes), thrombocytopenia, organ dysfunction | Schistocytes on peripheral blood smear, abnormal coagulation parameters |
| Paroxysmal Nocturnal Hemoglobinuria (PNH) | Nocturnal hemoglobinuria, thrombosis, bone marrow failure | Flow cytometry demonstrating lack of CD55 and CD59 on red blood cells |
| Iron Deficiency Anemia | Lack of iron stores, microcytic anemia | Low iron, low ferritin, low MCV |
| Anemia of Chronic Disease | Association with chronic inflammatory conditions | Normal or increased ferritin, mildly decreased hemoglobin |
| Aplastic Anemia | Pancytopenia (low counts of all blood cell types), bone marrow failure | Pancytopenia, hypocellular bone marrow on biopsy |
| Drug-induced hemolytic anemia (non-autoimmune) | Temporal association with drug exposure, can have various red cell morphologies | DAT usually negative |
| Post-transfusion reactions | Occurs after transfusion, often fever, chills, and change in urine color. | Positive DAT, potentially with a mixed field reaction indicating donor cells, and free hemoglobin in serum/urine |
| Warm AIHA | Autoantibodies react at body temperature | DAT positive (typically for IgG and/or C3), spherocytes |
Treatment and Management of Warm AIHA
The treatment of warm autoimmune hemolytic anemia (wAIHA) aims to stop the destruction of red blood cells, manage anemia symptoms, and address any underlying conditions.
- Initial Management and Supportive Care: The management of warm AIHA involves addressing any underlying causes, providing supportive care to alleviate symptoms, and employing specific therapies to suppress the autoimmune destruction of red blood cells.
- Addressing Underlying Causes: In cases of secondary warm AIHA, the initial and often most critical step is to identify and treat the underlying medical condition that is associated with the development of the autoimmune response. This may involve treating an underlying lymphoma or leukemia, managing an infection, addressing another autoimmune disease, or discontinuing a medication known to induce warm AIHA . Effective management of the primary condition can sometimes lead to the resolution of the secondary warm AIHA.
- Corticosteroids: This is typically the first-line treatment. Prednisone is commonly used to suppress the immune system’s attack on red blood cells. The dosage is gradually tapered as the patient’s condition improves to minimize side effects. Long term use can lead to many side effects, so other treatment options are often needed.
- Rituximab: This is a monoclonal antibody that targets B cells, which are involved in producing autoantibodies. It’s often used in patients who don’t respond well to corticosteroids or who experience significant side effects. It can also be used as a first line treatment in combination with corticosteroids.
- Splenectomy: Surgical removal of the spleen may be considered if other treatments are ineffective. The spleen is a major site of red blood cell destruction in warm AIHA. Vaccinations against encapsulated bacteria (e.g., pneumococcus, meningococcus, Haemophilus influenzae) are crucial before splenectomy to reduce the risk of infections.
- Immunosuppressant Medications: Other immunosuppressants, such as azathioprine, mycophenolate mofetil, or cyclosporine, may be used to suppress the immune system. These medications are often used in combination with corticosteroids or rituximab.
- Blood Transfusions: Blood transfusions may be necessary in severe cases of anemia to quickly increase red blood cell counts. However, finding compatible blood can be challenging due to the presence of autoantibodies. Transfusions are generally used as a temporary measure until other treatments become effective. It is important to remember that blood transfusions can exacerbate hemolysis.
Treatment Modalities for Warm AIHA
| Treatment | Mechanism of Action | Indications | Potential Side Effects |
| Corticosteroids (e.g., Prednisone) | Suppresses immune system, reduces autoantibody production and macrophage activity | First-line treatment for most patients | Weight gain, mood changes, increased risk of infection, elevated blood sugar, osteoporosis |
| Rituximab | Depletes CD20-positive B cells, reducing autoantibody production | Steroid-refractory or dependent cases, sometimes first-line in severe disease | Infusion reactions, late-onset neutropenia, hypogammaglobulinemia, reactivation of infections |
| Other Immunosuppressants (e.g., Azathioprine, Mycophenolate Mofetil, Cyclophosphamide, Cyclosporine) | Suppress immune system through various mechanisms | Patients who fail corticosteroids and rituximab | Vary depending on the agent; myelosuppression, gastrointestinal issues, organ toxicity |
| Splenectomy | Surgical removal of the spleen, a major site of RBC destruction | Refractory or relapsing cases after failure of medical therapies | Risk of infection (especially with encapsulated organisms), thrombosis |
| Blood Transfusion | Provides temporary increase in red blood cell count | Severe, symptomatic anemia or cardiopulmonary compromise | Transfusion reactions, volume overload |
Prognosis and Complications
The prognosis and potential complications of warm autoimmune hemolytic anemia (wAIHA) can vary significantly depending on several factors, including the severity of the disease, the presence of underlying conditions, and the individual’s response to treatment.
Prognosis
- Variable Response: Many patients respond well to initial treatment with corticosteroids or rituximab. However, some individuals may experience relapses or require long-term immunosuppressive therapy. Those with secondary warm AIHA generally have a prognosis tied to the underlying condition.
- Chronic Nature: Warm AIHA can be a chronic condition, requiring ongoing management. With appropriate treatment and monitoring, many patients can lead relatively normal lives.
- Mortality: Mortality is generally low, but it can be higher in severe cases or when arm AIHA is associated with serious underlying conditions. Complications from treatment, such as infections related to immunosuppression, can also contribute to mortality.
Complications
- Severe Anemia: Severe anemia can lead to heart failure, particularly in individuals with pre-existing heart conditions. It can also cause fatigue, shortness of breath, and other debilitating symptoms.
- Thromboembolic Events: Patients with warm AIHA have an increased risk of blood clots (thromboembolism), particularly in the venous system.
- Infections: Immunosuppressive medications, such as corticosteroids and rituximab, increase the risk of infections. Splenectomy also increases the risk of infections, especially with encapsulated bacteria.
- Complications from Underlying Conditions: If warm AIHA is secondary to an underlying condition, such as lupus or lymphoma, complications related to that condition can occur.
- Treatment-Related Complications: Long-term corticosteroid use can lead to side effects such as osteoporosis, weight gain, diabetes, and increased susceptibility to infections. Other immunosuppressant medications also have their own set of potential side effects.
- Transfusion Complications: Though sometimes necessary, blood transfusions can lead to transfusion reactions, and other complications.
- Organ Damage: In severe cases, and when left untreated, organ damage can occur as a result of chronic oxygen deprivation.
Key Considerations
- Regular monitoring and follow-up are essential to detect relapses and manage potential complications.
- Prompt treatment of infections is crucial in patients receiving immunosuppressive therapy.
- The management of underlying conditions is essential for improving the prognosis of secondary wAIHA.
- A risk vs benefit analysis should be performed when deciding on treatment options, due to the side effect profiles of many of the medications used.
Frequently Asked Questions (FAQs)
Why does warm AIHA cause spherocytes?
In warm AIHA, IgG autoantibodies bind to the surface of red blood cells. When these antibody-coated red cells pass through the spleen, splenic macrophages partially phagocytose portions of the red cell membrane. This piecemeal removal of membrane, without a proportional loss of the cell’s internal contents, results in a decreased surface area-to-volume ratio. Consequently, the red blood cells lose their normal biconcave disc shape and become spherical, or spherocytes. These spherocytes are less deformable and more prone to being trapped and destroyed in the spleen, contributing to the hemolytic anemia characteristic of warm AIHA.
What is the difference between IgG and IgM?
IgG and IgM are both antibodies, but they differ in structure and function. IgM is the first antibody produced during an initial immune response, forming a large pentamer structure that’s efficient at activating complement. IgG, the most abundant antibody in the blood, appears later in the immune response, is a smaller monomer, and excels at opsonization (marking pathogens for destruction) and neutralizing toxins, providing longer-term immunity.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Kalfa TA. Warm antibody autoimmune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):690-697. doi: 10.1182/asheducation-2016.1.690. PMID: 27913548; PMCID: PMC6142448.
- Branch DR. Warm autoimmune hemolytic anemia: new insights and hypotheses. Curr Opin Hematol. 2023 Nov 1;30(6):203-209. doi: 10.1097/MOH.0000000000000779. Epub 2023 Jul 27. PMID: 37497853; PMCID: PMC10552839.
- Yui JC, Brodsky RA. Updates in the Management of Warm Autoimmune Hemolytic Anemia. Hematol Oncol Clin North Am. 2022 Apr;36(2):325-339. doi: 10.1016/j.hoc.2021.11.005. Epub 2022 Mar 11. PMID: 35282949.
- Fattizzo B, Barcellini W. New Therapies for the Treatment of Warm Autoimmune Hemolytic Anemia. Transfus Med Rev. 2022 Oct;36(4):175-180. doi: 10.1016/j.tmrv.2022.08.001. Epub 2022 Sep 6. PMID: 36182620.
- Kuter DJ, Piatek C, Röth A, Siddiqui A, Numerof RP, Dummer W; FORWARD study group. Fostamatinib for warm antibody autoimmune hemolytic anemia: Phase 3, randomized, double-blind, placebo-controlled, global study (FORWARD). Am J Hematol. 2024 Jan;99(1):79-87. doi: 10.1002/ajh.27144. Epub 2023 Nov 6. PMID: 37929318.