TL;DR
Chronic lymphocytic leukemia (CLL) is a slow progressing cancer due to overgrowth and accumulation of small incompetent mature-looking B-lymphocytes in the blood, bone marrow and lymphoid tissues. Small lymphocytic leukemia is a different clinical manifestation of this disorder.
Incidence
- Peak at > 65 years old
- Common in Western societies
- Male:female ratio is 2.8:1
Signs and symptoms ▾
- Discrete, non-tender symmetrical enlargement of the cervical, axillary or inguinal lymph nodes
- Splenomegaly
- Anemia
- Immunosuppression due to hypogammaglobulinemia
Laboratory investigations ▾
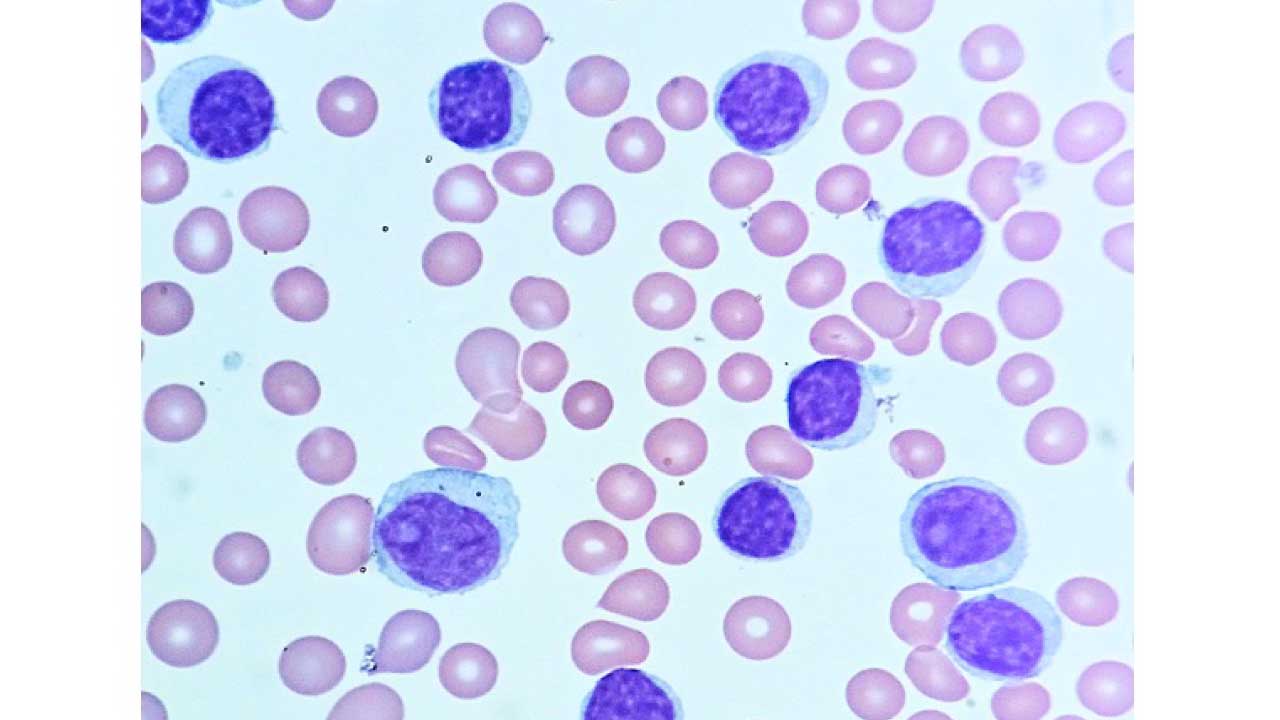
- PBF: Lymphocytosis with smudge cells. Normochromic normocytic anemia in bone marrow infiltration.
- Immunophenotyping: CD5+, CD19+, CD20+, CD23+ and surface immunoglobulin+
- FISH: Trisomy 12 (intermediate prognosis) , del17p (least favorable prognosis), del11q (least favorable prognosis), del13q (best prognosis)
- ↓ serum immunoglobulins
Staging ▾
| Rai stage | Characteristics | |||
| Rai 0 | Lymphocytosis only >15 x 109/L | |||
| Rai I | Lymphocytosis + lymphadenopathy | |||
| Rai II | Lymphocytosis + hepato-/splenomegaly ± lymphadenopathy | |||
| Rai III | Lymphocytosis + anemia (Hb < 11g/dL) ± lymphadenopathy ± organomegaly | |||
| Rai IV | Lymphocytosis + platelets < 100 x 109/L ± lymphadenopathy ± organomegaly | |||
| Binet stage | Lymphadenopathy/Organomegaly | Hemoglobin (g/dL) | Platelets (x 109/L) | |
| Binet A | ≤ 2 areas involved | > 10 | > 100 | |
| Binet B | ≥ 3 areas involved | ≥ 10 | ≥ 100 | |
| Binet C | Not considered | < 10 | < 100 | |
*Lymphadenopathy (> 1cm) /Organomegaly areas:
- Head and neck (uni- or bilateral)
- Axillar (uni- or bilateral)
- Inguinal (uni- or bilateral)
- Splenomegaly
- Hepatomegaly
Treatment and management ▾
- No treatment for Rai 0 – II stage and Binet A – B stage
- Chemoimmunotherapy e.g. purine analogs/ alkylating agents + anti-B cell monoclonal antibodies
- Oral kinase inhibitors
- Stem cell transplantation
*Click ▾ for more information
Introduction
Chronic lymphocytic leukemia (CLL) is a type of cancer that begins in the bone marrow. It is characterized clinically by the overproduction and accumulation of small mature-looking but functionally incompetent B-lymphocytes in blood, bone marrow and lymphoid tissues. The bone marrow is the spongy tissue inside bones where blood cells are made. Chronic lymphocytic leukemia (CLL) is a cancer of the B cells, which are a type of white blood cell that helps the body fight infection.
Chronic lymphocytic leukemia (CLL) cells are abnormal and grow out of control in the bone marrow. Over time, the accumulation of chronic lymphocytic leukemia (CLL) cells can crowd out the healthy white blood cells, making it difficult for the body to fight infection. Chronic lymphocytic leukemia (CLL) can also spread to other parts of the body, such as the lymph nodes, liver, and spleen.
Chronic lymphocytic leukemia (CLL) is a slow-growing cancer, and many people with chronic lymphocytic leukemia (CLL) do not need treatment right away. However, when treatment is needed, there are a number of options available, including chemotherapy, immunotherapy, and targeted therapy.
The prognosis for people with chronic lymphocytic leukemia (CLL) is generally good. With treatment, most people with chronic lymphocytic leukemia (CLL) can live long and active lives.
Here are some of the key things to know about chronic lymphocytic leukemia (CLL):
- It is the most common type of leukemia in adults.
- It is more common in men than in women.
- It is typically diagnosed in people over the age of 50.
- The exact cause of chronic lymphocytic leukemia (CLL) is unknown, but it is thought to be caused by a combination of genetic and environmental factors.
- There are no known ways to prevent chronic lymphocytic leukemia (CLL).
- There are a number of effective treatments available for chronic lymphocytic leukemia (CLL).
Most patients present with leukemia whereas a small minority present with small lymphocytic lymphoma (SLL). In earlier classifications of lymphoid malignancies, chronic lymphocytic leukemia (CLL) and SLL were considered to be separate entities but they are now accepted to be different clinical manifestations of the same disease.

Chronic Lymphocytic Leukemia (CLL) Symptoms
The clinical signs and symptoms of chronic lymphocytic leukemia (CLL) can vary depending on the stage of the disease and the location of the chronic lymphocytic leukemia (CLL) cells. Many people with chronic lymphocytic leukemia (CLL) do not have any symptoms when they are first diagnosed. The disease is often found when a person’s doctor orders blood tests for an unrelated reason.
The most common clinical chronic lymphocytic leukemia (CLL) symptoms include:
- Enlarged lymph nodes: Chronic lymphocytic leukemia (CLL) cells can build up in the lymph nodes, causing them to become enlarged. The lymph nodes are small, bean-shaped organs that are located throughout the body. They help to filter bacteria and other harmful substances from the blood. Symmetrical enlargement of cervical, axillary or inguinal lymph nodes is the most frequent clinical sign. The nodes are usually discrete and non-tender.
- Fatigue: Chronic lymphocytic leukemia (CLL) cells can crowd out the healthy red blood cells in the bone marrow, which can lead to anemia. Anemia is a condition in which the blood does not have enough healthy red blood cells to carry oxygen to the body’s tissues. This can cause fatigue, weakness, and shortness of breath.
- Night sweats: Chronic lymphocytic leukemia (CLL) cells can produce substances that cause night sweats. Night sweats are episodes of excessive sweating that occur during sleep.
- Fever: A fever is a sign that the body is fighting an infection. However, people with chronic lymphocytic leukemia (CLL) may develop a fever even if they do not have an infection. Immunosuppression is a significant problem resulting from hypogammaglobulinemia and cellular immune dysfunction.
- Unexplained weight loss: Chronic lymphocytic leukemia (CLL) cells can use up the body’s energy stores, which can lead to unexplained weight loss.
- Pain or fullness in the abdomen: Chronic lymphocytic leukemia (CLL) cells can build up in the liver and spleen, causing them to become enlarged. This can cause pain or fullness in the abdomen.
Other clinical chronic lymphocytic leukemia (CLL) symptoms may include:
- Easy bruising and bleeding: Chronic lymphocytic leukemia (CLL) cells can crowd out the healthy platelets in the bone marrow, which can lead to thrombocytopenia. Thrombocytopenia is a condition in which the blood does not have enough platelets. Platelets are blood cells that help the blood to clot. People with thrombocytopenia may bruise and bleed easily.
- Frequent infections: Chronic lymphocytic leukemia (CLL) cells can crowd out the healthy white blood cells in the bone marrow, which can make it difficult for the body to fight infection. People with chronic lymphocytic leukemia (CLL) may experience frequent infections, including pneumonia, urinary tract infections, and skin infections.
What are the laboratory investigations for CLL?
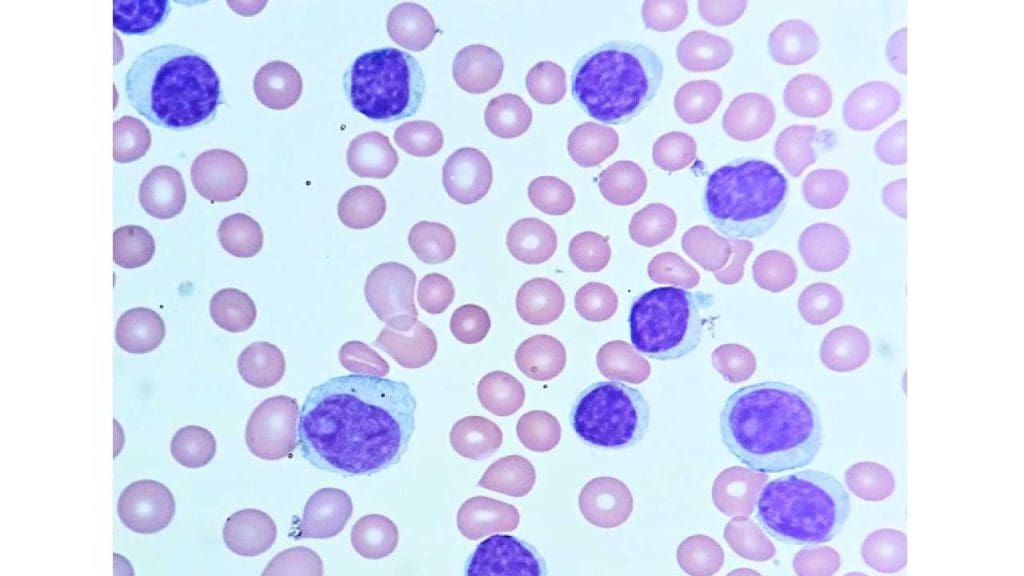
Laboratory investigations are used to diagnose chronic lymphocytic leukemia (CLL), monitor the progression of the disease, and assess the response to treatment. Some of the most common laboratory investigations used for chronic lymphocytic leukemia (CLL) include:
- Complete blood count (CBC) with differential: A CBC measures the number of different types of blood cells in the blood, including red blood cells, white blood cells, and platelets. A CBC can show anemia, thrombocytopenia, and lymphocytosis (an increased number of lymphocytes) in patients with chronic lymphocytic leukemia (CLL). Normocytic normochromic anemia is present in later stages as a result of marrow infiltration or hypersplenism
- Peripheral blood smear: A peripheral blood smear is a test that examines the different types of blood cells in a sample of blood under a microscope. A peripheral blood smear show lymphocytosis with smudge or smear cells
- Bone marrow biopsy: A bone marrow biopsy is a procedure in which a small sample of bone marrow is removed and examined under a microscope. A bone marrow biopsy can show the presence of chronic lymphocytic leukemia (CLL) cells and other abnormalities.
- Flow cytometry: Flow cytometry is a test that uses lasers to examine the characteristics of individual cells. Flow cytometry can be used to identify chronic lymphocytic leukemia (CLL) cells and to distinguish them from other types of white blood cells. In chronic lymphocytic leukemia (CLL), cells co‐express the surface antigen CD5 with the B‐cell antigens CD19, CD20, and CD23. The levels of surface immunoglobulin, CD20, and CD79b are characteristically low compared to those found on normal B cells. Each clone of leukemia cells is restricted to expression of either kappa or lambda immunoglobulin light chains. It should be noted that the expression of CD5 can also be observed in other lymphoid malignancies, such as mantle cell lymphoma. A recent, large harmonization effort has confirmed that a panel of CD19, CD5, CD20, CD23, kappa and lambda is usually sufficient to establish the diagnosis. In borderline cases, markers such as CD43, CD79b, CD81, CD200, CD10, or ROR1 may help to refine the diagnosis.
- Immunoglobulin studies: Immunoglobulin studies measure the levels of different types of immunoglobulins (antibodies) in the blood. There are reduced concentrations of serum immunoglobulins.
- Genetic testing: Genetic testing can be used to identify genetic mutations that are associated with chronic lymphocytic leukemia (CLL). Genetic testing can be helpful for diagnosing chronic lymphocytic leukemia (CLL) and for predicting the prognosis of patients with chronic lymphocytic leukemia (CLL).
In addition to the laboratory investigations listed above, other laboratory tests may be used to monitor the progression of chronic lymphocytic leukemia (CLL) and to assess the response to treatment. These tests may include:
- Serum beta-2 microglobulin (B2M): B2M is a protein that is released into the blood by chronic lymphocytic leukemia (CLL) cells and other types of cells. Elevated levels of B2M are associated with a more aggressive form of chronic lymphocytic leukemia (CLL).
- Lactate dehydrogenase (LDH): LDH is an enzyme that is released into the blood when cells are damaged. Elevated levels of LDH are associated with a more aggressive form of CLL.
- Imaging tests: Imaging tests, such as X-rays, computed tomography (CT) scans, and positron emission tomography (PET) scans, may be used to look for enlarged lymph nodes and other signs of chronic lymphocytic leukemia (CLL).
Chronic Lymphocytic Leukemia (CLL) Staging
Two widely accepted clinical staging systems co‐exist, named after the first authors of the original publications, Rai and Binet. Both systems describe three major prognostic groups with discrete clinical outcomes.
The Binet Staging System
The Binet staging system is based on two factors: the number of involved lymph node areas as defined by the presence of enlarged lymph nodes of greater than 1 cm in diameter or organomegaly and the presence of anemia or thrombocytopenia.
The Binet staging system has three stages:
- Stage A: Fewer than 3 lymph node areas are involved and there is no anemia or thrombocytopenia.
- Stage B: 3 or more lymph node areas are involved and there is no anemia or thrombocytopenia.
- Stage C: There is anemia or thrombocytopenia, regardless of the number of involved lymph node areas
The areas of involvement considered or lymph node regions are
- Head and neck, including the Waldeyer ring (this counts as one area, even if more than one group of nodes is enlarged)
- Axillae (involvement of both axillae counts as one area)
- Groins, including superficial femoral (involvement of both groins counts as one area)
- Palpable spleen
- Palpable liver (clinically enlarged)
The Binet staging system is important for determining the prognosis of patients with CLL and for guiding treatment decisions. Patients with early-stage CLL (Binet stage A) have a better prognosis than patients with advanced-stage CLL (Binet stage C).
The Modified Rai Staging System
The Rai staging system is based on three factors: the number of lymphocytes in the blood, the presence of enlarged lymph nodes, and the presence of anemia or thrombocytopenia.
The modified Rai staging system defines
Low‐risk disease: Patients who have lymphocytosis with leukemia cells in the blood and/or marrow (lymphoid cells >30%) (former Rai stage 0).
Intermediate Risk: Patients with lymphocytosis, enlarged nodes in any site, and splenomegaly and/or hepatomegaly (lymph nodes being palpable or not) (formerly considered Rai stage I or stage II).
High‐risk disease: Patients with disease‐related anemia (as defined by a hemoglobin [Hb] level less than 11 g/dL) (formerly stage III) or thrombocytopenia (as defined by a platelet count of less than 100 × 109/L) (formerly stage IV).
The Rai staging system is important for determining the prognosis of patients with CLL and for guiding treatment decisions. Patients with early-stage CLL (Rai stage 0 or I) have a better prognosis than patients with advanced-stage CLL (Rai stage III or IV).
What is the prognosis for CLL?
The prognosis for patients with chronic lymphocytic leukemia (CLL) is based on a number of factors, including the stage of the disease, the patient’s age and health, and the presence of certain genetic mutations. Cytogenetics is the study of chromosomes, and cytogenetic abnormalities can be found in up to 50% of patients with CLL.
The following cytogenetic abnormalities are associated with a poor prognosis in CLL:
- Deletion of 17p (del(17p)): Del(17p) is the most common cytogenetic abnormality in CLL, and it is associated with a poor prognosis. Patients with del(17p) have a shorter median survival time than patients without del(17p). Deletions of the short arm of chromosome 17 show marked resistance against genotoxic chemotherapies as the deletions almost always include band 17p13 where the prominent tumour suppressor gene TP53 is located
- Trisomy 12 (tris(12)): Tris(12) is the second most common cytogenetic abnormality in CLL, and it is also associated with a poor prognosis. Patients with tris(12) have a shorter median survival time than patients without tris(12).
- Deletion of 11q (del(11q)): Del(11q) is a less common cytogenetic abnormality in CLL, but it is associated with a very poor prognosis. Patients with del(11q) have a median survival time of less than 2 years. Deletions of the long arm of chromosome 11 typically show bulky lymphadenopathy, rapid progression and reduced overall survival.
- Complex karyotype: A complex karyotype is defined as the presence of 3 or more cytogenetic abnormalities in a single cell. Patients with a complex karyotype have a very poor prognosis, with a median survival time of less than 1 year.
- Expression of ZAP-70: A signaling protein that is not found in normal B cells and tumors with unmutated immunoglobulin genes.
Cytogenetic abnormalities associated with a good prognosis in chronic lymphocytic leukemia (CLL)
The following cytogenetic abnormalities are associated with a good prognosis in chronic lymphocytic leukemia (CLL):
- Deletion of 13q (del(13q)): Del(13q) is the most common cytogenetic abnormality in chronic lymphocytic leukemia (CLL), and it is associated with a good prognosis. Patients with del(13q) have a longer median survival time than patients without del(13q).
- Normal karyotype: Patients with a normal karyotype have the best prognosis of all patients with chronic lymphocytic leukemia (CLL). However, tumors with normal fluorescence in situ hybridization (FISH) cytogenetics and trisomy 12 have an intermediate prognosis.
- Mutated Ig variable region genes.

Chronic Lymphocytic Leukemia (CLL) Treatment
As for treatment, as many as 60% of chronic lymphocytic leukemia (CLL) patients never require treatment. Indications for chronic lymphocytic leukemia (CLL) treatment include cytopaenias like anemia and/or thrombocytopenia, bulky symptomatic lymphadenopathy and/or splenomegaly, presence of autoantibodies, presence of B symptoms like fever, night sweats and weight loss, and transformation to an aggressive lymphoma.
Recently, there have been significant advances in chronic lymphocytic leukemia (CLL) treatment with the development of new targeted therapies and immunotherapies. These new treatments have resulted in longer survival times and improved quality of life for patients with chronic lymphocytic leukemia (CLL).
Chronic lymphocytic leukemia (CLL) treatment depends on a number of factors, including the stage of the disease, the patient’s age and health, and the presence of certain genetic mutations. Some patients with CLL do not need treatment right away, while others need to start treatment right away.
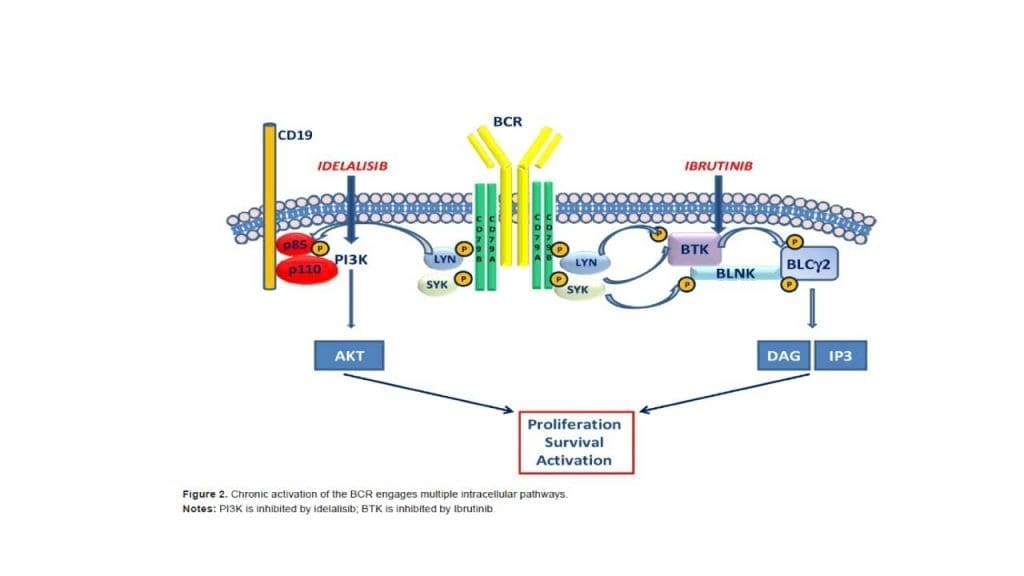
The current standard paradigm of treatment is to use chemoimmunotherapy, specifically with purine analogs and or alkylating agents combined with anti-B-cell monoclonal antibodies. Recent advances in therapy include the use of oral kinase inhibitors targeting Bruton tyrosine kinase and PI3 kinase.
Targeted Therapy
Targeted therapy is a type of treatment that uses drugs that target specific molecules on cancer cells. Targeted therapy drugs can help to kill chronic lymphocytic leukemia (CLL) cells while sparing healthy cells.
Some of the targeted therapy drugs that are used as chronic lymphocytic leukemia (CLL) treatment include:
- Ibrutinib (Imbruvica)
- Zanubrutinib (Brukinsa)
- Acalabrutinib (Calquence)
- Venetoclax (Venclexta)
Immunotherapy
Immunotherapy is a type of treatment that uses the patient’s own immune system to fight cancer. Immunotherapy drugs can help to activate the immune system to kill chronic lymphocytic leukemia (CLL) cells.
Some of the immunotherapy drugs that are used as chronic lymphocytic leukemia (CLL) treatment include:
- Rituximab (Rituxan)
- Obinutuzumab (Gazyva)
- Ofatumumab (Arzerra)
Chemotherapy
Chemotherapy is a type of treatment that uses drugs to kill cancer cells. Chemotherapy is often used as chronic lymphocytic leukemia (CLL) treatment that is progressing or that is causing chronic lymphocytic leukemia (CLL) symptoms.
Some of the chemotherapy drugs that are used to chronic lymphocytic leukemia (CLL) treatment include:
- Fludarabine
- Cyclophosphamide
- Bendamustine
Stem Cell Transplantation
Stem cell transplantation is a procedure in which healthy stem cells are transplanted into the patient’s body. Stem cells are the immature cells that develop into different types of blood cells.
Stem cell transplantation may be an option for patients with chronic lymphocytic leukemia (CLL) who have relapsed after initial treatment or who have not responded to initial treatment. Patients may eventually succumb due to the development of resistant disease, superimposed infections, or less commonly, transformation to a more aggressive lymphoma.
Clinical Trials
Clinical trials are research studies that test new treatments for chronic lymphocytic leukemia (CLL). Clinical trials can be a good option for patients with chronic lymphocytic leukemia (CLL) who have not responded to other treatments or who have relapsed after initial treatment.
Chronic Lymphocytic Leukemia (CLL) Treatment Based on Stage
Chronic lymphocytic leukemia (CLL) treatment depends on the stage of the disease, the patient’s age and health, and the presence of certain genetic mutations.
- Patients with early-stage CLL (Rai stage 0 or I): Patients with early-stage CLL may not need treatment right away. However, some patients with early-stage CLL may benefit from early treatment, such as targeted therapy.
- Patients with intermediate-stage CLL (Rai stage II or III): Patients with intermediate-stage CLL will eventually need treatment. Treatment options for intermediate-stage CLL include targeted therapy, immunotherapy, and chemotherapy.
- Patients with advanced-stage CLL (Rai stage IV): Patients with advanced-stage CLL will need treatment right away. Treatment options for advanced-stage CLL include targeted therapy, immunotherapy, and chemotherapy.
Frequently Asked Questions (FAQs)
What are the first signs of chronic leukemia?
Chronic leukemia often progresses slowly, and in some cases, there might not be any noticeable symptoms in the early stages. However, some potential first signs of chronic leukemia to be aware of include:
- Feeling tired or fatigued: This can be a general feeling of exhaustion or lack of energy that persists even after rest.
- Unexplained weight loss: This can occur even if your appetite remains normal.
- Frequent infections: Chronic leukemia can weaken your immune system, making you more susceptible to infections.
- Easy bruising or bleeding: This can happen due to a decrease in platelets, which are important for blood clotting.
- Pain or fullness in the upper left abdomen: This can be caused by an enlarged spleen, a common finding in some types of chronic leukemia.
- Swollen lymph nodes: These are painless lumps that may appear in your neck, armpits, or groin.
It’s important to note that these symptoms can also be caused by other conditions.
What is the survival rate for lymphocytic leukemia?
The survival rate for chronic lymphocytic leukemia (CLL) depends on various factors, but here’s a general breakdown:
- Overall: Around 87% of people with chronic lymphocytic leukemia (CLL) live for 5 or more years following diagnosis. This statistic is based on data for people diagnosed in recent years, indicating potential improvements in treatment and outcomes.
- Age: Younger patients tend to have a better prognosis than older patients.
- Stage: Earlier stages of chronic lymphocytic leukemia (CLL) generally have a better outlook compared to advanced stages.
- Other health factors: Overall health and presence of other medical conditions can influence survival rates.
What is the main cause of chronic lymphocytic leukemia?
The exact cause of chronic lymphocytic leukemia (CLL) remains unknown in most cases. Researchers have identified several factors that contribute to the development of chronic lymphocytic leukemia (CLL), but there isn’t a single definitive cause.
- Genetic Mutations: Multiple genetic mutations occur in the DNA of blood-producing cells. These mutations disrupt the normal growth and maturation of lymphocytes (white blood cells), leading to the uncontrolled proliferation of abnormal lymphocytes characteristic of chronic lymphocytic leukemia (CLL).
- Risk Factors: While not direct causes, certain factors can increase the risk of developing chronic lymphocytic leukemia (CLL). These include:
- Age: chronic lymphocytic leukemia (CLL) is more common in older adults, with the average age of diagnosis being around 70 years.
- Sex: Men are more likely to develop chronic lymphocytic leukemia (CLL) compared to women.
- Family History: Having a close relative with chronic lymphocytic leukemia (CLL) slightly increases your risk.
- Environmental Exposures: Some studies suggest potential links between exposure to certain chemicals (e.g., herbicides, pesticides) and an increased risk of chronic lymphocytic leukemia (CLL), but more research is needed.
Is chronic lymphocytic leukemia (CLL) aggresive?
Chronic lymphocytic leukemia (CLL) can be aggressive in some cases, but it’s more commonly classified as a slow-growing cancer.
- Indolent CLL: This is the most common form of chronic lymphocytic leukemia (CLL), characterized by slow growth. Many patients with indolent chronic lymphocytic leukemia (CLL) may not experience any symptoms for years and might not even require immediate treatment. Regular monitoring is crucial for these patients.
- Aggressive CLL: This less frequent form of chronic lymphocytic leukemia (CLL) grows more rapidly and requires prompt treatment. Signs of aggressive chronic lymphocytic leukemia (CLL) might include:
- Increased number of abnormal lymphocytes in the blood
- Enlarged spleen or lymph nodes
- Low red blood cell count (anemia)
- Low platelet count (thrombocytopenia)
- Frequent infections
Factors Influencing Aggressiveness
- Genetic mutations: The specific genetic mutations present in the cancerous cells can impact their growth rate. Studies have revealed that chronic lymphocytic leukemia (CLL) patients with specific genetic abnormalities, including chromosomal translocations, complex karyotypes, and high genomic complexity detected by SNP arrays, tend to have a more aggressive form of the disease. This aggressive chronic lymphocytic leukemia (CLL) progresses rapidly and offers shorter remission periods after treatment.
- Lymphocyte doubling time: This refers to the time it takes for the number of cancerous lymphocytes to double. A shorter doubling time suggests a more aggressive form of chronic lymphocytic leukemia (CLL).
- Presence of certain proteins: The presence or absence of specific proteins on the surface of the cancerous lymphocytes can indicate a more aggressive course. For example, patients with unmutated IGHV CLL cells typically have more aggressive disease than patients with CLL cells that express a mutated IGHV.
Can a patient live a normal life with CLL?
Yes, many patients with chronic lymphocytic leukemia (CLL) can live normal or near-normal lives, especially those diagnosed with the indolent form.
Type of chronic lymphocytic leukemia (CLL)
- Indolent CLL: This is the most common form, characterized by slow growth. Many patients with this type might not experience symptoms for years and may not require immediate treatment. Regular monitoring allows them to maintain a normal life with minimal disruption.
- Aggressive CLL: This less frequent form requires prompt treatment, which can sometimes involve side effects that might impact daily activities. However, with advancements in treatment, many patients with aggressive chronic lymphocytic leukemia (CLL) can still achieve a good quality of life.
What happens if chronic lymphocytic leukemia (CLL) is not treated?
If chronic lymphocytic leukemia (CLL) is left untreated, the potential consequences can vary depending on the initial aggressiveness of the cancer and the individual’s overall health.
- Progression of the Disease: Untreated, chronic lymphocytic leukemia (CLL) will likely progress over time. The abnormal lymphocytes in the bone marrow continue to multiply, crowding out healthy blood cells.
- Impact on Blood Counts: This can lead to a decrease in red blood cells (anemia), causing fatigue, weakness, and shortness of breath. There might also be a decrease in white blood cells (leukopenia), making the body more susceptible to infections. Additionally, a reduction in platelets (thrombocytopenia) can increase the risk of bleeding.
- Enlarged Spleen and Lymph Nodes: As the number of abnormal lymphocytes increases, the spleen and lymph nodes, which are part of the immune system, can become enlarged due to an overactive attempt to filter the cancerous cells.
- Increased Risk of Infections: The decrease in healthy white blood cells can significantly weaken the immune system, making the body less able to fight off infections. These infections can become severe and even life-threatening.
- Bone Pain and Discomfort: In some cases, the cancerous cells can infiltrate the bones, leading to bone pain and discomfort.
- Transformation to a More Aggressive Form: Although rare, untreated chronic lymphocytic leukemia (CLL) can transform into a more aggressive type of leukemia over time. This aggressive form requires more intensive treatment and might have a poorer prognosis.
Importance of Early Intervention
Early diagnosis and treatment of chronic lymphocytic leukemia (CLL) are crucial to manage the disease effectively and prevent potential complications. Treatment can help control the growth of cancerous cells, minimize symptoms, and improve overall well-being.
Is there a cure for chronic lymphocytic leukemia (CLL)?
Currently, there isn’t a definitive cure for chronic lymphocytic leukemia (CLL) in the traditional sense of eliminating all cancerous cells from the body. However, significant advancements in treatment approaches offer hope for long-term management and potentially achieving a state similar to a cure for many patients.
How painful is chronic lymphocytic leukemia (CLL)?
The experience of pain in chronic lymphocytic leukemia (CLL) can vary greatly from person to person. Some individuals might experience minimal or no pain throughout the course of the disease, while others might have more frequent or severe pain episodes.
However, as the disease progresses, some potential sources of pain can arise:
- Enlarged Spleen or Lymph Nodes: When the spleen and lymph nodes become enlarged due to an increased number of abnormal lymphocytes, they can press on surrounding organs or nerves, causing discomfort or dull aches in the upper left abdomen or other areas depending on the location of the enlarged lymph nodes.
- Bone Marrow Infiltration: In some cases, cancerous cells can infiltrate the bone marrow, leading to bone pain. This pain can be dull or achy and might worsen with activity.
- Secondary Conditions: Chronic lymphocytic leukemia (CLL) can increase the risk of infections, and some infections themselves can be painful. Additionally, anemia, a common complication of chronic lymphocytic leukemia (CLL), can cause fatigue and a general feeling of weakness.
Can chronic lymphocytic leukemia (CLL) turn into brain cancer?
Chronic lymphocytic leukemia (CLL) itself isn’t known to directly turn into brain cancer. However, there are rare instances where chronic lymphocytic leukemia (CLL) can affect the central nervous system (CNS), which includes the brain and spinal cord.
CLL Involvement in the CNS
- In rare cases (between 0.8% and 7% depending on autopsy studies), chronic lymphocytic leukemia (CLL) cells can migrate to the CNS and cause neurological symptoms. This isn’t necessarily due to cancerous transformation, but rather the presence of abnormal lymphocytes within the brain or spinal cord.
- Symptoms can vary depending on the location of the chronic lymphocytic leukemia (CLL) cells within the CNS and might include headaches, seizures, weakness, numbness, or problems with balance.
- This CNS involvement is often treatable with medications or radiation therapy directed at the brain or spinal cord.
Richter’s Syndrome
- In even rarer instances (2-10% of chronic lymphocytic leukemia (CLL) patients), chronic lymphocytic leukemia (CLL) can transform into an aggressive lymphoma called Richter’s syndrome. This is a distinct type of cancer, and while it can affect the lymph nodes and other organs, it can also develop within the brain.
- Richter’s syndrome typically progresses rapidly and requires specific treatment approaches for aggressive lymphomas.
Is chronic lymphocytic leukemia fatal?
While chronic lymphocytic leukemia (CLL) itself might not be fatal, if left untreated, it can lead to complications that can be life-threatening. These include:
- Severe infections due to a weakened immune system
- Uncontrolled bleeding due to low platelet count
- Anemia causing shortness of breath and fatigue
- Bone pain and damage from infiltration by cancerous cells
Is chronic lymphocytic leukemia genetically inherited?
Chronic lymphocytic leukemia has a genetic basis, but it’s not typically inherited in a straightforward manner. Multiple factors, including genetic predisposition and environmental influences, work together to increase the risk of developing chronic lymphocytic leukemia (CLL). Research continues to explore the complex interplay of genetics and other factors in chronic lymphocytic leukemia (CLL) development.
What are the signs that the chronic lymphocytic leukemia (CLL) is getting worse?
Chronic Lymphocytic Leukemia (CLL) progresses slowly, and some people with indolent chronic lymphocytic leukemia (CLL) might not experience any worsening symptoms for years. However, there are signs that can indicate the chronic lymphocytic leukemia (CLL) might be getting worse. Here are some to watch for:
- Increased Fatigue: Extreme fatigue and persistent weakness can be a sign that the cancerous cells are multiplying and crowding out healthy blood cells in the bone marrow. This can significantly impact daily activities and energy levels.
- Frequent Infections: A healthy immune system relies on white blood cells to fight infections. As chronic lymphocytic leukemia (CLL) progresses, white blood cell count can decrease, making the patient more susceptible to infections. These infections might be more frequent, severe, and take longer to heal.
- Uncontrolled Bleeding: A decrease in platelets (thrombocytopenia) is a potential complication of worsening chronic lymphocytic leukemia (CLL). Platelets are essential for blood clotting, and a low count can lead to easy bruising or bleeding, even from minor injuries.
- Weight Loss: Loss of appetite and difficulty absorbing nutrients can occur due to an enlarged spleen or other complications associated with advancing chronic lymphocytic leukemia (CLL). This can lead to unintended weight loss.
- New or Worsening Bone Pain: Bone pain can be a sign of cancerous cells infiltrating the bone marrow. This pain might be persistent, worsen with activity, and be more pronounced in specific areas like the hips, ribs, or back.
- Enlarged Spleen or Lymph Nodes: While enlargement can occur in early stages of CLL, a noticeable increase in the size of the spleen or lymph nodes might indicate worsening of the disease. These enlarged organs can cause discomfort or pain and potentially affect nearby organs.
- Shortness of Breath or Dizziness: Anemia, a common complication of worsening chronic lymphocytic leukemia (CLL), can lead to shortness of breath, dizziness, and pale skin due to a lack of healthy red blood cells to carry oxygen throughout the body.
It’s important to note that these signs can have other causes as well.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ; International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008 Jun 15;111(12):5446-56. doi: 10.1182/blood-2007-06-093906. Epub 2008 Jan 23. Erratum in: Blood. 2008 Dec 15;112(13):5259. PMID: 18216293; PMCID: PMC2972576.
- Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, Gregor M, Cymbalista F, Buske C, Hillmen P, Hallek M, Mey U; ESMO Guidelines Committee. Electronic address: [email protected]. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021 Jan;32(1):23-33. doi: 10.1016/j.annonc.2020.09.019. Epub 2020 Oct 19. PMID: 33091559.
- Mukkamalla SKR, Taneja A, Malipeddi D, et al. Chronic Lymphocytic Leukemia. [Updated 2023 Mar 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
- Shadman M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA. 2023 Mar 21;329(11):918-932. doi: 10.1001/jama.2023.1946. PMID: 36943212.
- National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients® Chronic Lymphocytic Leukemia. 2022.
- Hallek M, Eichhorst B, Catovsky D. Chronic Lymphocytic Leukemia (Hematologic Malignancies) 1st ed. 2019 Edition (Springer).
- Carr JH. Clinical Hematology Atlas 6th Edition (Elsevier).
- Ouillette P, Fossum S, Parkin B, Ding L, Bockenstedt P, Al-Zoubi A, Shedden K, Malek SN. Aggressive chronic lymphocytic leukemia with elevated genomic complexity is associated with multiple gene defects in the response to DNA double-strand breaks. Clin Cancer Res. 2010 Feb 1;16(3):835-47. doi: 10.1158/1078-0432.CCR-09-2534. Epub 2010 Jan 19. PMID: 20086003; PMCID: PMC2818663.
- Michael Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating M, Montserrat E, Chiorazzi N, Stilgenbauer S, Rai KR, Byrd JC, Eichhorst B, O’Brien S, Robak T, Seymour JF, Kipps TJ. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. 2018; 131 (25): 2745–2760.