Procedure-At-A-Glance
The myeloperoxidase reaction (MPO) stain works by utilizing the peroxidase in leukocyte granules to oxidize a chromogen (like benzidine) in the presence of hydrogen peroxide (H2O2), forming an insoluble, colored reaction product (colorless to blue or brown/green). It is primarily used as the most specific marker of the myeloid lineage to differentiate Acute Myeloid Leukemia (AML) from Acute Lymphoblastic Leukemia (ALL), as myeloid blasts are typically MPO-positive and lymphoid blasts are MPO-negative.
- Fix an air-dried peripheral blood or bone marrow smear slide in a fixative (e.g., 10% formal ethanol) at room temperature (typically for 60 seconds).
- Cover the slide with the staining solution and incubate (typically for 1 minute). Rinse the slide again.
- Counterstain with Giemsa stain at a 1:10 dilution (typically for 10 minutes).
- Wash off the counterstain, wipe the back of the slide, and air dry in a tilted position or with a hairdryer on the lowest speed.
- Mount the slide with Depex and a cover slip, making it ready for viewing.
Introduction
Myeloperoxidase is a lysosomal enzyme, located in the azurophilic granules of the neutrophils and its precursors, eosinophils and monocytes. This MPO stain is mainly used to differentiate acute myeloid leukaemia from acute lymphoblastic leukaemia. MPO stain can be a valuable tool for:
- Diagnosing and classifying leukemia: By differentiating between MPO-positive myeloid blasts (characteristic of AML) and MPO-negative lymphoid blasts (seen in ALL).
- Monitoring treatment response: Tracking changes in MPO expression in bone marrow samples can indicate the effectiveness of leukemia treatment.
- Identifying inflammatory processes: Increased MPO levels in tissues can suggest the presence of inflammation.
- Studying myeloid differentiation: MPO expression patterns can help visualize the differentiation process of myeloid stem cells.
Principle of Myeloperoxidase Stain
Peroxidase in leukocyte granules oxidizes benzidine to form insoluble, stable and non-diffusible reaction product in the presence of hydrogen peroxide (H2O2). This product is colourless to blue or brown derivatives which is localized at the site of the enzyme. Copper sulphate or nitrate can be used to enhance the staining.
But how does the stain differentiate between different cell types? The key lies in the selective expression of myeloperoxidase. Neutrophils and certain other myeloid cells like monocytes and some basophils are rich in myeloperoxidase, while lymphocytes lack it. Therefore, when applied to a tissue sample or blood smear, the stain paints only myeloperoxidase-containing cells with vibrant hues, leaving myeloperoxidase-negative cells unstained.
Function of Myeloperoxidase
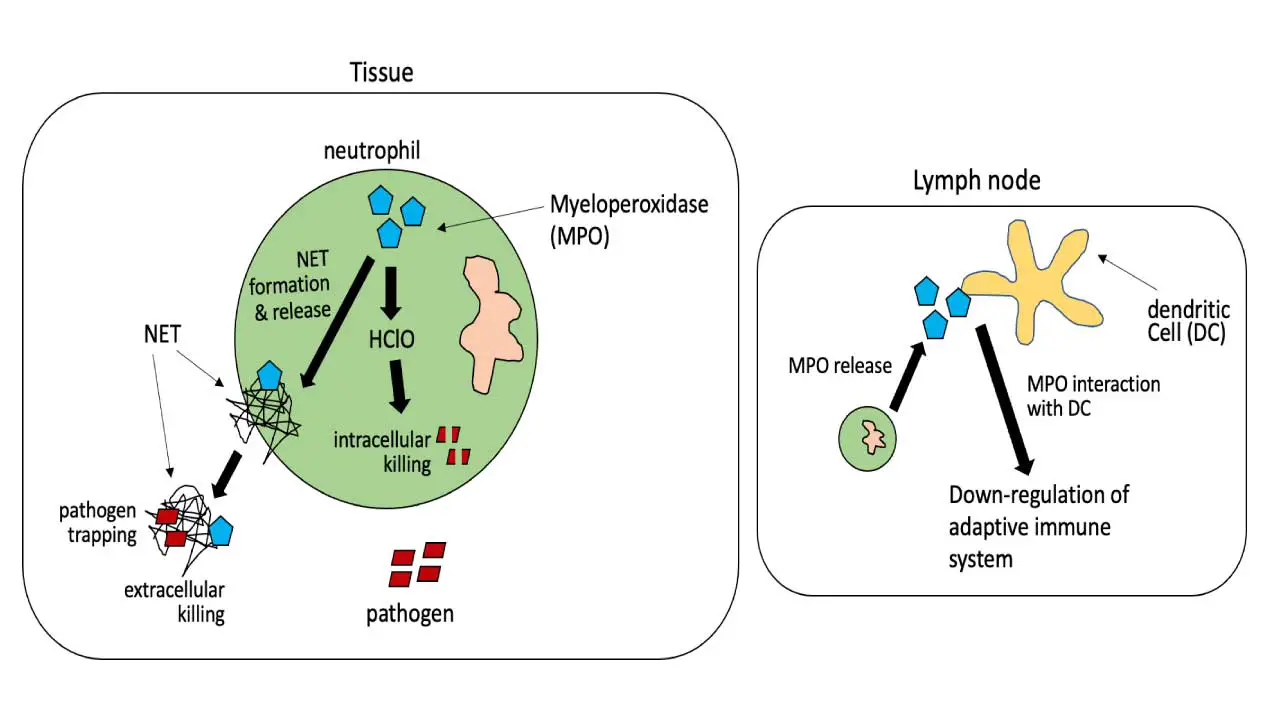
Myeloperoxidase (MPO) is a crucial enzyme primarily found in neutrophils and to a lesser extent in eosinophils and monocytes. Its main function is to play a fundamental role in innate immunity and the body’s defense against invading pathogens.
During a process called the “respiratory burst,” myeloperoxidase utilizes hydrogen peroxide (H2O2) and chloride ions (Cl–) to produce highly cytotoxic hypochlorous acid (HOCl), which is a potent antimicrobial agent. This hypochlorous acid is used by neutrophils to kill bacteria and certain types of fungi, such as Candida species.
Beyond its direct antimicrobial action, myeloperoxidase is also involved in other biological processes, including the breakdown of carbon nanotubes, and has been implicated in conditions like atherosclerosis and certain types of vasculitis.
Role of Myeloperoxidase (MPO) in Differentiating AML from ALL
Myeloperoxidase (MPO) staining plays a critical and specific role in differentiating acute myeloid leukemia (AML) from acute lymphoblastic leukemia (ALL) because MPO is considered the most specific marker of the myeloid lineage.
MPO is an enzyme found in the primary (azurophilic) granules of myeloid cells, including neutrophils and their precursors, as well as monocytes. When cytochemical staining for MPO is performed on bone marrow or peripheral blood blasts, a positive reaction (typically defined as ≥3% of blasts showing positivity by cytochemistry or ≥10% by flow cytometry) strongly indicates myeloid differentiation, thus supporting a diagnosis of AML.
In contrast, blasts in ALL are typically MPO-negative, as lymphoid cells lack this enzyme. This clear distinction is crucial for accurate diagnosis and subsequent treatment decisions, as AML and ALL require different therapeutic approaches.
While some rare cases of B-ALL can show isolated, usually dim, MPO expression, and advanced immunophenotyping and genetic analysis are often employed for a definitive diagnosis and to identify mixed phenotype acute leukemias, MPO staining remains a cornerstone in the initial differentiation due to its high specificity for myeloid lineage.
Materials
- Air dried peripheral blood or bone marrow smear (within 24 hours of collection)
- Fixative – 10% formal ethanol (10 mL of 40% formalin and 90 mL of absolute ethanol)
- The staining solution:
- 30% Ethanol: 100 mL
- Benzidine dihydrochloride: 0.3 g
- 0.132M (3.8%) ZnSO4·7H2O: 1 mL
- Sodium acetate: 1 g
- 3% hydrogen peroxide: 0.7 mL
- 1 N sodium hydroxide: 1.5 mL
- Giemsa stain
Protocol
- Prepare air dried film – preferable within 24 hours of collection (peroxidase is unstable in the light).
- Fix slide in fixative at room temperature for 60 seconds.
- Rinse in slow running tap water for 15 – 30 seconds and air dry.
- Place slide on staining rack and fully cover the air-dried slide with the staining solution and incubate for 1 minute.
- Rinse in slow running tap water for 1 minute.
- Counterstain with Giemsa stain at 1:10 dilution for 10 minutes.
- Wash off counter stain.
- Wipe the back of the slide and edges with Kim wipes. Be careful not to touch the smear.
- Dry the slide using a hair dryer on the lowest speed (not more than 10 seconds at a time) or air dry in a tilted position.
- Mount the slide with Depex and cover the zone of morphology with a cover slip.
- This slide is now ready for viewing.
Enzyme activity may be preserved for as long as 3 weeks if preparations are stored in the dark.
Interpretation
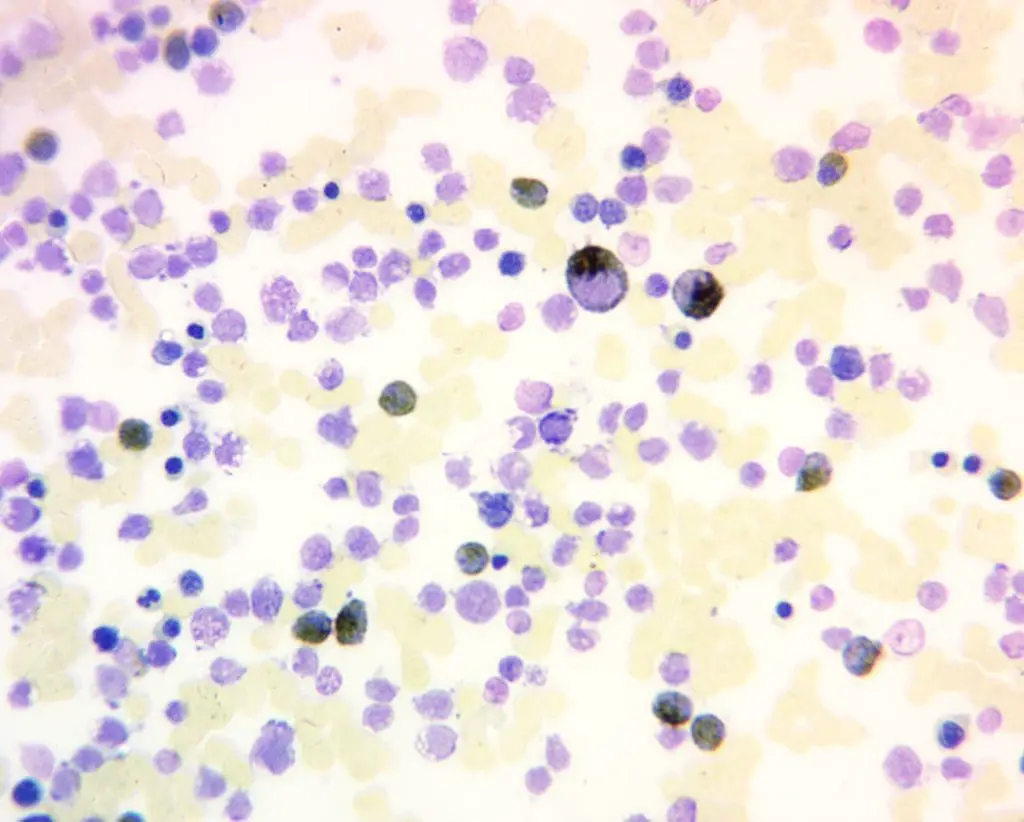
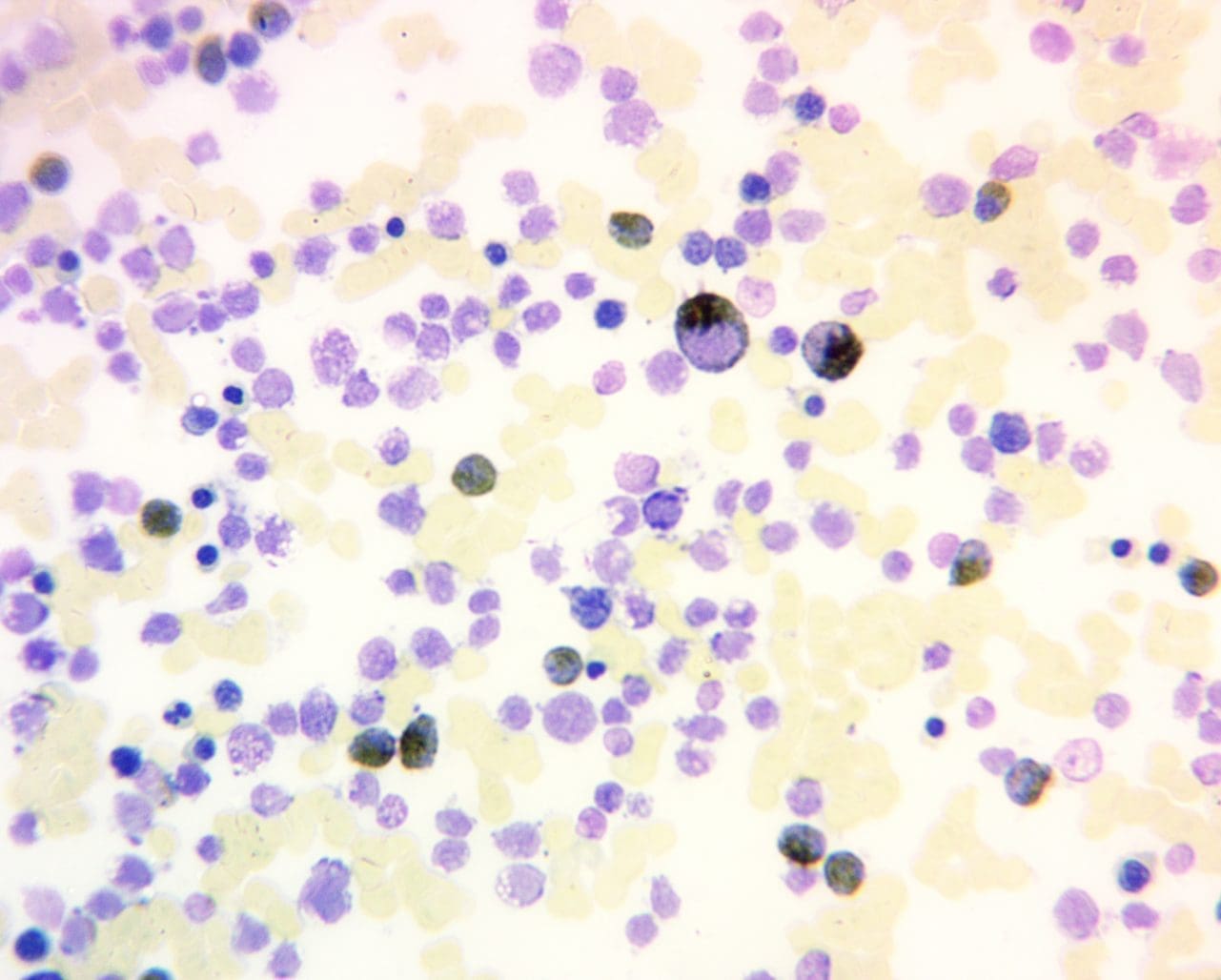
Positive Myeloperoxidase (MPO) Reaction: A positive myeloperoxidase (MPO) reaction is characterized by a distinct brown to green cytoplasmic staining in granulocytes, reflecting the presence and activity of the enzyme. This indicates the presence of mature neutrophils (positive control), eosinophils, and some basophils in the sample. It is also positive for myeloblasts (except those at a very primitive stage), promyelocytes and metamylocytes. A strong, consistent positive reaction suggests normal granulocyte maturation and function.
Negative Myeloperoxidase (MPO) Reaction: Conversely, a negative positive myeloperoxidase (MPO) reaction reveals the absence of brown to green cytoplasmic staining for example in lymphoblasts and lymphocytes. Monoblasts and monocytes may be positive or negative.
Pathological Variations: Deviations from the expected staining pattern can unveil underlying pathologies for example in congenital deficiency of neutrophil, where results will be negative or in dysplastic neutrophils.
MPO Staining Interpretation in Leukemia
While it’s not a formal “grading” system like that used for solid tumors (e.g., Grade 1, 2, 3), the interpretation of MPO staining involves quantitative and qualitative aspects.
Percentage of Positive Blasts
For diagnosing AML, a key criterion is the percentage of blasts that stain positive for MPO.
- Cytochemistry: Traditionally, by cytochemical staining (on a slide), a positive result for AML requires ≥3% of blasts to show MPO positivity.
- Flow Cytometry: When MPO is detected by flow cytometry (which is more sensitive), the cut-off for positivity is often higher, with ≥10% of blasts being considered positive. Some studies suggest even higher thresholds (e.g., 20% or 28%) for optimal discrimination between AML and ALL.
Intensity of Staining
The intensity of MPO staining can also be noted:
- Strong, granular positivity: This is typically seen in more mature myeloid cells and in many cases of AML (especially M1, M2, M3 subtypes). Auer rods, which are pathognomonic for AML, are strongly MPO positive.
- Weak or finely granular positivity: Monocytic cells often show a less intense, finely granular MPO reaction. Early myeloblasts might show dim or weak positivity, with intensity increasing as they mature.
- Negative: Lymphoid cells (blasts in ALL) are MPO-negative.
MPO Staining Pattern in Different Tissues
comprehensive diagnostic aid and reinforcing its E-E-A-T by providing clear, actionable data.
| Cell Type/Tissue | MPO Staining Result/Pattern | Clinical Significance/Notes |
| Neutrophilic Granulocytes | Intensive black granular cytoplasmic staining | Indicative of myeloid lineage; activity increases with maturation |
| Eosinophils | Positive staining | Presence of MPO |
| Monocytes | Faint red granular cytoplasmic staining | Presence of MPO; activity increases with maturation |
| Early Myeloblasts | Generally negative, granular positivity appears with maturation | Immature myeloid cells; helps differentiate from mature forms |
| Lymphoblasts | Negative staining | Indicative of lymphoid lineage; crucial for AML/ALL differentiation |
| Basophils | Weakly positive or negative | Variable MPO presence |
| Spleen (Red Pulp) | Numerous MPO positive granulocytes | Normal tissue control for MPO staining |
| Colon Epithelial Cells | Absent MPO immunostaining | Normal tissue negative control; MPO positive granulocytes may be present in capillaries/stroma |
| Tumor Microenvironment | Variable MPO positive granulocytes seen; MPO expression by cancer cells not seen | Granulocyte infiltration, not cancer cell expression; critical for accurate diagnosis |
| Aggressive Periodontitis (Neutrophils) | Grade 2 or 3 intensity (Moderate to Intense) | Higher MPO levels indicate hyperfunctional neutrophils; diagnostic potential |
| Chronic Periodontitis/Healthy (Neutrophils) | Grade 1 intensity (Mild) | Baseline MPO levels |
For Optimal Result
- Adherence to standardized protocols for sample collection, handling, and processing is crucial.
- Using high-quality validated reagents is essential.
- Maintaining proper laboratory practices and quality control measures helps ensure consistent and reliable results.
- Experienced pathologists who are familiar with myeloperoxidase (MPO) staining patterns should interpret the results.
- Correlation with other diagnostic tests and clinical findings is necessary for accurate diagnosis and management decisions.
Comparison of Myeloperoxidase (MPO) Stain with Other Common Hematological Stains
| Stain Name | Principle | Specific Cellular Components/Molecules Stained | Primary Diagnostic Applications |
| Myeloperoxidase (MPO) | Enzyme-catalyzed oxidation of substrate by MPO (in presence of H2O2) to colored precipitate. | MPO in primary (azurophilic) granules of myeloid cells. | Differentiating AML from ALL: Most specific myeloid marker. Strong positivity in neutrophil granulocytes, faint in monocytes, red-brown in eosinophils. Auer rods are MPO positive. |
| Periodic Acid-Schiff (PAS) | Periodic acid oxidizes carbohydrates to aldehydes, which react with Schiff’s reagent for color. | Carbohydrates (glycogen, glycoproteins, mucopolysaccharides). | ALL: Coarse block-like/granular positivity in blasts. Erythroleukemia (AML-M6): Diffuse positivity in erythroid precursors. |
| Perl’s Prussian Blue (Iron) | Ferric iron reacts with potassium ferrocyanide to form blue precipitate. | Non-heme iron, primarily hemosiderin. | Sideroblastic Anemia: Identifies ring sideroblasts (iron-laden mitochondria around nucleus in erythroblasts). Assesses bone marrow iron stores (e.g., in iron deficiency, hemochromatosis). |
| Leukocyte Alkaline Phosphatase (LAP) | Detects alkaline phosphatase enzyme activity in neutrophils. | Alkaline phosphatase in secondary (specific) granules of mature neutrophils. | Differentiating CML from Leukemoid Reactions: Low/absent LAP score in CML, high LAP score in leukemoid reactions. Helps differentiate CML from other myeloproliferative neoplasms (e.g., polycythemia vera, primary myelofibrosis, which have high LAP scores). |
Frequently Asked Questions (FAQs)
What cells are myeloperoxidase (MPO) positive?
The primary cells that are MPO positive are neutrophils, a crucial type of white blood cell responsible for battling infections. MPO is the most abundant enzyme within these cells, playing a key role in their antimicrobial activity.
However, MPO isn’t exclusive to neutrophils.
Myeloid Lineage
- Monocytes: These white blood cells, involved in inflammation and antigen presentation, can also express MPO, although typically in lower levels than neutrophils.
- Eosinophils: While their primary function isn’t directly linked to MPO, a small subset of eosinophils might exhibit MPO positivity.
- Myeloid blasts: In acute myeloid leukemia (AML), the abnormal blasts arising from myeloid stem cells are typically MPO-positive, making the MPO stain crucial for diagnosis and subclassification.
Other Cells
- Myeloid precursors: During the differentiation process of myeloid stem cells into mature cells, MPOexpression gradually increases, making some precursor cells also MPO-positive.
- Rare cases: In exceptional circumstances, MPO expression has been observed in cells outside the myeloid lineage, such as some subsets of lymphocytes or even certain epithelial cells. However, these instances are not typical and require further investigation.
What color does myeloperoxidase (MPO) stain positive?
Myeloperoxidase (MPO) itself is colorless, but when detected using an MPO stain, it produces a colored product that allows visualization under a microscope. The specific color of the stained cells depends on the chromogen used in the staining procedure.
Here are some common chromogens and the colors they produce with MPO:
- 3,3′-diaminobenzidine (DAB): This is the most widely used chromogen, and it reacts with MPO to produce a brown precipitate.
- Benzidine: This chromogen is no longer commonly used due to its carcinogenic properties, but it also produces a brown precipitate with MPO.
- 3,3′-diaminobenzidine tetrahydrochloride (DAB-TH): This is a variant of DAB that produces a black precipitate, offering better contrast for microscopic observation.
- Alpha-naphthyl acetate esterase (ANAE): This is a different type of stain that targets specific enzymes within neutrophils, including MPO. It produces a red precipitate when MPO is present.
Therefore, depending on the chosen chromogen, MPO-positive cells can appear brown, black, or red when stained. It’s crucial to know the specific chromogen used in the context of your inquiry to interpret the color correctly.
What are the components of myeloperoxidase (MPO) stain?
The content of an MPO stain typically consists of the following components:
Antibody
This is the most crucial component of the stain and is specifically designed to recognize and bind to the MPO enzyme. Antibodies are highly specific proteins produced by the immune system in response to foreign substances. In the case of MPO stains, the antibody is engineered to target and bind only to the MPO) enzyme, leaving other cellular components unlabeled.
Chromogen
Once the antibody binds to myeloperoxidase (MPO), a chromogen is introduced. This is a colorless compound that gets activated by the enzyme, undergoing a chemical reaction that produces a visible colored product. The specific color of the product depends on the chosen chromogen. Some common chromogens used in myeloperoxidase (MPO) stains include:
- 3,3′-diaminobenzidine (DAB): This produces a brown precipitate.
- 3,3′-diaminobenzidine tetrahydrochloride (DAB-TH): This is a variant of DAB that produces a black precipitate, offering better contrast for microscopic observation.
- Alpha-naphthyl acetate esterase (ANAE): This targets specific enzymes within neutrophils, including MPO, and produces a red precipitate.
Buffer
The staining solution usually contains a buffer to maintain a specific pH and ionic strength, which are crucial for optimal antibody and enzyme activity.
Other components
Depending on the specific protocol, the staining solution might also include additional components like preservatives, stabilizers, or detergents to optimize the staining process and prevent degradation of the reagents.
The exact composition of an MPO stain might vary depending on the manufacturer, the intended application, and the specific needs of the laboratory. However, the core components mentioned above are essential for the stain to function effectively and specifically detect MPO expression in cells.
Do monoblasts stain with myeloperoxidase (MPO)?
In general, monoblasts typically do not stain positively for MPO.
- MPO and Myeloid Differentiation: MPO is primarily expressed in mature myeloid cells like neutrophils, monocytes, and dendritic cells. During myeloid differentiation, the process by which stem cells mature into specific myeloid cells, MPO expression gradually increases. Early precursors, including monoblasts, generally express very little or no MPO.
- Monoblast MPO Staining: While monoblasts are considered part of the myeloid lineage, they are the earliest stage and haven’t fully differentiated yet. Therefore, most monoblasts do not exhibit positive MPO staining. In rare cases, particularly with certain rare types of leukemia, some monoblasts might show weak or partial MPO positivity. However, this is not typical and requires further investigation in such instances.
Importance of Context
MPO staining is used in various contexts, primarily for differentiating leukemias. When interpreting MPO staining results, it’s crucial to consider the specific cell type, disease context, and patient history. Absence of MPO staining in monoblasts is consistent with their early stage in differentiation.
What are the limitations of the MPO stain?
The MPO stain, while valuable in various clinical settings, has several limitations that need to be considered for accurate diagnosis and interpretation.
Specificity
- Not entirely specific: While MPO is primarily found in myeloid cells, trace amounts can be present in other cell types like some lymphocytes or even epithelial cells in rare cases. This can lead to misinterpretation, especially in challenging diagnostic scenarios.
- Variable expression: MPO expression levels can vary within different subtypes of the same cell type, adding a layer of complexity to interpreting stain intensity.
Sensitivity
- Weak expression: Some AML subtypes have inherently low MPO expression, potentially leading to false negatives if the stain sensitivity is insufficient.
- Technical factors: Improper tissue processing, suboptimal staining protocols, or inadequate observer experience can further affect sensitivity, potentially missing positive cases.
Diagnostic Limitations
- Not definitive alone: MPO staining should always be interpreted alongside other diagnostic tests like flow cytometry, cytogenetics, and molecular analysis for a comprehensive picture.
What are the alternative tests to the MPO stain?
While the MPO stain remains a valuable tool in various clinical settings, particularly for leukemia diagnosis and monitoring, it has limitations like potential lack of specificity and sensitivity. In some cases, alternative tests might offer additional insights or overcome these limitations.
Flow cytometry
This technique utilizes lasers and fluorescent antibodies to analyze individual cells, offering detailed information about their size, granularity, and surface marker expression.
In leukemia diagnosis, flow cytometry can precisely identify and characterize blasts based on their specific markers, including myeloperoxidase (MPO). Compared to MPO stain, flow cytometry provides quantitative data on MPO expression levels and can detect even small populations of MPO-positive blasts.
Immunohistochemistry (IHC) with different markers
While MPO is a helpful myeloid marker, other IHC markers can provide complementary information depending on the specific diagnostic question. Examples include myeloperoxidase receptor (MPOR), which can be expressed in some MPO-negative leukemias, or CD34, a marker for progenitor cells helpful in specific diagnostic scenarios.
Combining MPO staining with IHC for other markers can improve diagnostic accuracy and aid in subclassifying certain leukemias.
Cytogenetics and molecular analysis
These techniques analyze the genetic makeup of cells, identifying chromosomal abnormalities or specific gene mutations associated with different types of leukemia.
While not directly detecting MPO, these tests can provide crucial information about the underlying cause of leukemia and guide treatment decisions. In some cases, cytogenetic or molecular abnormalities might help differentiate between MPO-positive and negative leukemias with overlapping features.
Emerging techniques
Newer technologies like mass cytometry and next-generation sequencing are constantly evolving and hold promise for even more detailed and precise characterization of cells, potentially offering alternatives to traditional MPO staining in the future.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Bain BJ. A Practical Guide. 6th Edition (Wiley). 2022.
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- Carr JH. Clinical Hematology Atlas 6th Edition (Elsevier). 2021.
- Fatemeh E. Mahjoub, Fahimeh Firouzjaie Karder, Issa Jahanzad, Saghi Vaziri, Ramezan Ali Sharifian, Zahra Farahani. Introducing a Rapid and Safe Method for Myeloperoxidase Staining/ Open Journal of Pathology, Vol.5 No.2, 2015.
- Saksena, A., Desai, P., Chauhan, V., Gupta, N., & Singh, T. (2017). Revisiting Cytochemical Myeloperoxidase (MPO) Stain for Early Diagnosis of Acute Promyelocytic Leukemia (APML) in Areas with Limited Resources: An Experience from a Tertiary Care Center in India. Blood, 130(Supplement 1), 5096-5096.
- Singh, S., Acharya, A. B., & Kumar, S. C. (2011). Myeloperoxidase staining in the diagnosis of aggressive periodontitis. Journal of Indian Society of Periodontology, 15(2), 152–155. https://doi.org/10.4103/0972-124X.84385
- Valerón-Almazán, P., Bastida, J., Vilar, J., Santana, N., Medina, C., & Carretero, G. (2011). Utility of myeloperoxidase stain in the differential diagnosis of leukemia cutis vs. hystiocitoid Sweet syndrome. Dermatology online journal, 17(4), 11.