Introduction
Immunohistochemistry (IHC) staining is a powerful technique that allows us to peek into the intricate world of protein expression within tissues. IHC staining transcends the limitations of traditional histological stains like hematoxylin and eosin (H&E) that primarily assess tissue morphology. IHC staining allows us to visualize specific proteins in situ, providing invaluable insights into the diagnosis, prognosis, and even treatment of various diseases.
Role and purpose of IHC staining
- Diagnosing diseases: In hematopathology, IHC plays a crucial role in differentiating various types of lymphomas, leukemias, and other blood malignancies. Identifying specific protein markers using IHC, such as CD3 for T-cell lymphomas or CD20 for B-cell lymphomas, allows for accurate diagnosis and classification, guiding appropriate treatment strategies.
- Subclassify malignancies: Within the spectrum of lymphomas, IHC plays a vital role in classifying subtypes. By detecting specific markers like CD20, CD3, and BCL2 using IHC, we can accurately subdivide lymphomas, each with distinct clinical features and prognoses, paving the way for personalized therapy.
- Prognosticating disease course: IHC staining can reveal the expression of proteins associated with disease progression or treatment resistance. For instance, detecting elevated Ki-67 expression (a proliferation marker) in a lymphoma sample can indicate a more aggressive disease course.
- Understanding disease mechanisms: By visualizing the spatial distribution of proteins in tissues, IHC staining sheds light on cellular interactions and biological processes involved in disease development. In the context of hematological disorders, IHC can help elucidate the role of specific protein pathways in lymphomagenesis or leukemogenesis.
Hematological Disorders
Hematological malignancies, particularly lymphomas, present a prime example of where IHC shines brightest. These cancers, characterized by abnormal proliferation of lymphocytes, often require specific protein markers for definitive diagnosis and subclassification. IHC plays a critical role in identifying these markers, including:
- B-cell lymphomas: Detection of CD20, BCL2, and CD3 is crucial for differentiating B-cell lymphomas from T-cell lymphomas and other types, guiding appropriate treatment strategies.
- T-cell lymphomas: IHC markers like CD3, CD4, and CD8 help distinguish various subtypes of T-cell lymphomas, each with distinct clinical courses and treatment needs.
- Hodgkin lymphoma: Identifying specific markers like CD30 and CD15 helps differentiate Hodgkin lymphoma from other lymphoma subtypes, leading to accurate diagnosis and prognosis.
Principle of IHC
Preparation
Thin slices of tissue are fixed to preserve their structure and then treated to permeabilize cell membranes, allowing antibodies access to their targets. This process, called antigen retrieval, ensures the protein is accessible for its pursuer.
The Antibody Hunt
The primary antibody, Y-shaped molecules designed to recognize and bind to specific proteins or other antigens, tailored to the protein of interest, is applied to the tissue.
Amplifying the Signal
To visualize the binding, we need a secondary antibody linked to a reporter molecule. A secondary antibody, tagged with a fluorescent dye or enzyme, is introduced. This secondary antibody binds to the first antibody-antigen complex.
Visualization using a Reporter Molecule
If a fluorescent dye is used, the dye attached to the secondary antibody emits light of a specific wavelength when excited by a light source. the bound protein glows under a microscope, revealing its location in the tissue
If an enzyme is attached, a substrate is added, triggering a color-changing reaction. The protein’s presence becomes evident as a brown, red, or blue stain, marking its hiding place. This can be an enzyme like horseradish peroxidase (HRP). Upon receiving a specific signal, the enzyme converts a colorless substrate into a vibrant brown precipitate, marking the location of the antigen-antibody complex.
Interpretation
Finally, the tissue is mounted and examined under a microscope. By interpreting the brown stains or fluorescent signals, we can pinpoint the distribution and abundance of specific molecules within the tissue, providing valuable insights into disease processes and aiding in diagnosis and treatment.
Materials
Important Note: This protocol is a general guideline and may need to be adapted based on specific antibodies, tissues, and desired staining methods. Always consult the datasheets and recommendations of the antibody and reagent manufacturers before proceeding.
- Tissue sections on positively charged slides
- Xylene
- Ethanol solutions (100%, 95%, 70%, 50%)
- Deionized water
- Antigen retrieval buffer (specific to your target antigen)
- Blocking buffer (e.g., 1% BSA in PBS or 5% serum from the secondary antibody’s host)
- Primary antibody diluted in antibody diluent (concentration varies, refer to datasheet)
- Secondary antibody conjugated to reporter molecule (HRP, fluorescent dye)
- DAB chromogen solution (for HRP detection) or fluorescent mounting medium
- Hematoxylin and eosin (H&E) staining reagents (optional)
- Coplin jars or staining dishes
- Microscope slides and coverslips
- Forceps and lint-free wipes
- Pipettes and pipette tips
- Wash buffer (PBS or TBS)
- Waste disposal containers
Protocol
Deparaffinization and Rehydration
- Place slides in a Coplin jar containing xylene for 10 minutes at room temperature (repeat 2-3 times).
- Transfer slides to 100% ethanol for 5 minutes (repeat 2 times).
- Descend through graded ethanol solutions (95%, 70%, 50%) for 2 minutes each.
- Rinse slides briefly in deionized water.
Antigen Retrieval (Optional)
This step may be necessary to unmask hidden epitopes, depending on the antibody and tissue fixation. Refer to the antibody datasheet for specific recommendations.
- Heat the antigen retrieval buffer in a pressure cooker or steamer to the desired temperature and time (follow manufacturer’s instructions).
- Place slides in the hot buffer and incubate for the specified time.
- Allow the solution to cool down gradually before removing the slides.
- Rinse slides with deionized water.
Blocking
- Incubate slides in blocking buffer for 15-30 minutes at room temperature to block nonspecific binding sites.
Primary Antibody Incubation
- Apply the diluted primary antibody solution to the tissue sections. Cover with a plastic coverslip to prevent drying.
- Incubate in a humidified chamber at the recommended temperature and time (usually overnight at 4°C).
Washing
- Rinse slides gently with wash buffer 3 times for 5 minutes each.
Secondary Antibody Incubation
- Apply the secondary antibody solution diluted in antibody diluent to the tissue sections.
- Incubate for 30-60 minutes at room temperature in the dark.
Washing
- Repeat step 5.
Detection
HRP detection:
- Incubate slides in DAB chromogen solution for 5-10 minutes, monitoring for brown precipitate development.
- Rinse slides with deionized water to stop the reaction.
Fluorescent detection:
- Rinse slides with wash buffer and deionized water.
- Mount slides with fluorescent mounting medium and coverslips.
Counterstaining and Mounting (Optional)
- Stain slides with hematoxylin and eosin (H&E) for morphological evaluation if desired.
- Dehydrate and clear slides in graded ethanol solutions and xylene.
- Mount slides with permanent mounting medium and coverslips.
Microscopic Examination
- Examine stained slides under a microscope to visualize the specific protein expression patterns revealed by the IHC staining.
This protocol does not employ a hydrogen peroxide (H₂O₂) block step for several reasons:
- ADEC secondary antibodies: ADEC (antibody-dependent enzyme conjugation) secondary antibodies pre-conjugated to HRP (horseradish peroxidase) is used. These lack the Fc region that interacts with endogenous peroxidase, eliminating the need for H₂O₂ blocking to prevent non-specific staining.
- DAB specificity: The chosen chromogen, DAB (3,3′-diaminobenzidine), reacts specifically with HRP in the presence of H₂O₂. Hence, even if some endogenous peroxidase persists, it wouldn’t generate a brown precipitate with DAB, mitigating the need for a block.
- Optimized washing: Thorough washing steps following each antibody incubation effectively remove unbound antibodies and potential endogenous enzymes, further reducing the requirement for an H₂O₂ block.
The decision to exclude H₂O₂ block was based on a combination of factors like antibody type, chromogen choice, and optimized washing steps for the specific tissue and fixation method used.
Interpretation
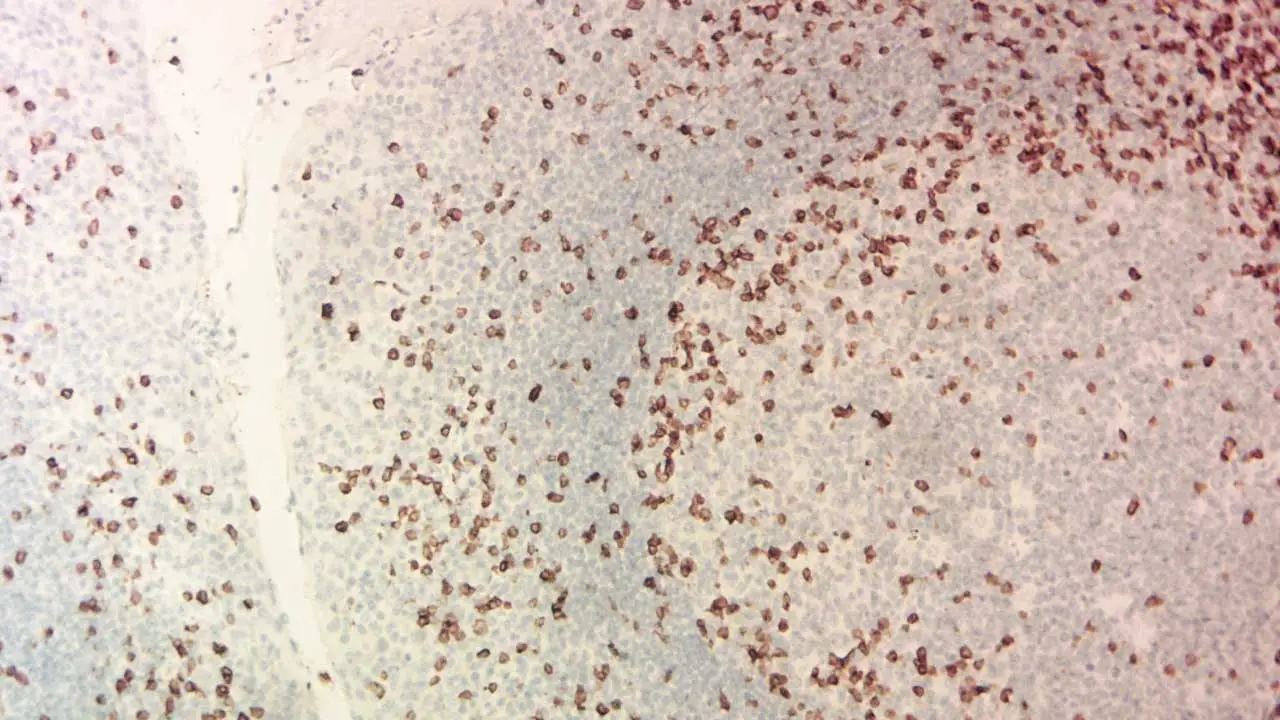
Interpreting IHC stains requires a keen eye and a thorough understanding of the specific antibody, target antigen, and tissue context. Here’s a breakdown of what to expect and how to decode the visual clues:
Staining Patterns
The pattern of antibody binding in IHC staining reveals valuable information about protein expression and cellular distribution. Some common patterns include:
- Membranous: Staining concentrated on the cell membrane. (Think of a fence marking the cell boundaries)
- Cytoplasmic: Staining filling the cytoplasm within the cell. (Imagine confetti scattered inside the cell)
- Nuclear: Staining focused within the cell nucleus. (Think of a highlighter marking important text in the DNA library)
- Focal: Staining scattered in specific areas or cell clusters. (Like spotlights highlighting certain regions)
- Negative: No detectable staining, indicating the target antigen is absent or below the detection threshold.
Staining Intensity
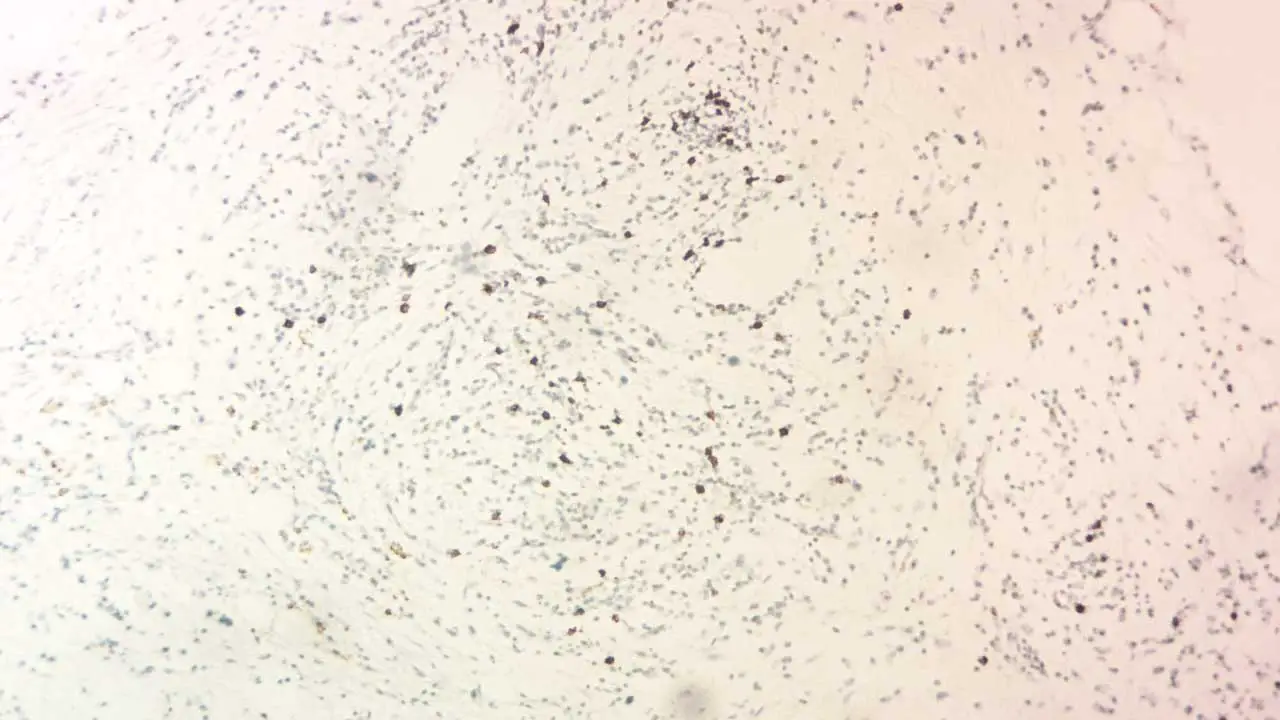
The intensity of the IHC stain reflects the relative abundance of the target antigen. Different shades can be categorized as:
- Negative: No visible staining.
- Weak: Faint brown or fluorescent signal.
- Moderate: Clear brown or brighter fluorescent signal.
- Strong: Intense brown or highly fluorescent signal.
By combining the staining pattern and intensity, you can formulate a preliminary interpretation of the IHC results. However, remember that definitive diagnosis and conclusions often require correlation with other clinical findings and pathological examinations.
Positive vs. Negative Controls in IHC staining
Every IHC staining run should include positive and negative controls to ensure proper staining and interpretation.
- Positive control: Tissue sections known to express the target antigen, confirming the antibody’s functionality and staining effectiveness.
- Negative control: Tissue sections devoid of the target antigen or treated with non-immune serum instead of the primary antibody, revealing background staining levels and non-specific binding.
Tissue Specificity
It’s crucial to consider the tissue type and background staining patterns, as some antigens might show inherent expression in specific cell types within the tissue. Analyzing IHC staining patterns in relation to cell morphology and known tissue architecture helps distinguish specific antibody binding from background noise.
Technical Considerations
Remember that IHC staining intensity and quality can be influenced by technical factors like fixation, tissue processing, and staining protocol variations. Consistent optimization and standardization of procedures are essential for reliable and reproducible results.
Frequently Asked Questions (FAQs)
What are the two types of IHC?
he two main types of Immunohistochemistry (IHC) are:
- Chromogenic IHC: This method uses a labeled antibody that, when bound to its target antigen, produces a visible color change. This allows for direct observation of the antigen’s location under a light microscope.
- Fluorescent IHC: In this technique, the antibody is labeled with a fluorescent marker. When the antibody binds to its target, it emits a fluorescent signal that can be detected using a fluorescence microscope. This method is particularly useful for visualizing multiple antigens simultaneously.
What are the most common IHC stains?
Here are some of the most common IHC stains:
- DAB (Diaminobenzidine): A chromogen that produces a brown color when reacted with hydrogen peroxide, often used in conjunction with peroxidase-labeled antibodies.
- AEC (3-amino-9-ethylcarbazole): A chromogen that produces a red color, often used for double staining with DAB.
- Alkaline phosphatase-conjugated antibodies: These antibodies can be detected using substrates like BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium), which produce a blue or purple color.
- Fluorescent dyes: Examples include FITC (fluorescein isothiocyanate), rhodamine, and Cy3/Cy5. These dyes emit light of different colors when excited by specific wavelengths of light.
Some commonly stained antigens include:
- Ki-67: A marker for cell proliferation.
- CD3: A marker for T lymphocytes.
- CD20: A marker for B lymphocytes.
- ER (estrogen receptor): A marker for breast cancer.
- PR (progesterone receptor): A marker for breast cancer.
- HER2: A marker for breast cancer.
- CK (cytokeratin): A marker for epithelial cells.
- Vimentin: A marker for mesenchymal cells.
- S-100: A marker for neural cells.
These are just a few examples, and the choice of stain depends on the specific research question or clinical application.
What are the three most common problem areas in IHC staining?
Three common problem areas in IHC staining are:
- Background staining: This occurs when the antibody binds nonspecifically to other proteins or components in the tissue. It can interfere with the visualization of the target antigen and make it difficult to interpret the results.
- Weak or inconsistent staining: This can be caused by various factors, such as low antibody concentration, inadequate antigen retrieval, or suboptimal staining conditions. Weak staining may make it difficult to detect the target antigen, while inconsistent staining can lead to unreliable results.
- Endogenous peroxidase activity: In chromogenic IHC using peroxidase-labeled antibodies, endogenous peroxidase activity in the tissue can interfere with the detection of the target antigen. This can lead to false positive staining.
To address these problems, researchers can take several steps, including:
- Optimizing antigen retrieval: This process involves treating the tissue with heat or chemicals to expose the target antigen.
- Using appropriate blocking buffers: Blocking buffers can help to reduce nonspecific binding of the antibody to background proteins.
- Adjusting antibody concentrations: The concentration of the primary and secondary antibodies can be adjusted to optimize staining intensity and reduce background noise.
- Using appropriate controls: Positive and negative controls can help to validate the staining and identify potential problems.
- Considering the tissue type and fixation method: The choice of tissue type and fixation method can influence the staining quality.
- Using endogenous peroxidase blocking reagents: These reagents can inhibit endogenous peroxidase activity and prevent false positive staining.
By carefully addressing these problem areas, researchers can improve the quality and reliability of their IHC staining results.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Kim SW, Roh J, Park CS. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J Pathol Transl Med. 2016 Nov;50(6):411-418. doi: 10.4132/jptm.2016.08.08. Epub 2016 Oct 13. PMID: 27809448; PMCID: PMC5122731.
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- Steele J. Histology, Immunohistochemistry and In Situ Hybridisation, Lab Protocols. 2017.
- Nguyen T. Immunohistochemistry: A Technical Guide to Current Practices (Cambridge University Press). 2022.
- Renshaw S. Immunohistochemistry and Immunocytochemistry: Essential Methods (Wiley-Blackwell). 2017.
- Buchwalow IB, Böcker W. Immunohistochemistry: Basics and Methods (Springer). 2010.