What is acute lymphoblastic leukemia (ALL)?
ALL can be divided into B-cell ALL (B-ALL) and T-cell ALL (T-ALL). Acute lymphoblastic leukemia (ALL) is a type of blood cancer that begins in the bone marrow. The bone marrow is the soft tissue inside the bones where blood cells are made. In ALL, the bone marrow makes too many immature white blood cells called lymphoblasts. Lymphoblasts are a type of lymphocyte, which is a type of white blood cell.
ALL is defined as an aggressive neoplasm of the lymphoid lineage caused by acquired somatic defects due to either inherited factors or infections e.g. viruses in early haematopoietic blasts that impede or significantly impair differentiation leading to accumulation of undifferentiated blasts in the marrow, spilling into the peripheral blood and infiltrate other tissues. Leukemic blasts are too immature to be functional and are prone to suppress or supplant the production of normal haematopoiesis causing pancytopenia.
ALL is the most common type of cancer in children, but it can also occur in adults. It is most common in children under the age of 5 and in adults over the age of 65.
B-ALL
B-cell acute lymphoblastic leukemia (B-ALL) is the most common type of ALL in children and adults, accounting for about 75-85% of all cases.
B-ALL is caused by the uncontrolled growth of immature B cells. B cells are a type of white blood cell that helps the body fight infection. When B cells become cancerous, they can crowd out healthy blood cells in the bone marrow and bloodstream. This can lead to a number of problems, including fatigue, weakness, and an increased risk of infection.
Risk factors for B-ALL
The exact cause of B-ALL is unknown, but there are a number of risk factors that may increase the risk of developing the disease. These risk factors include:
- Age: B-ALL is most common in children under the age of 5 and in adults over the age of 65.
- Gender: Males are slightly more likely to develop B-ALL than females.
- Family history: People with a family history of B-ALL are at an increased risk of developing the disease.
- Certain genetic conditions: People with certain genetic conditions, such as Down syndrome, Klinefelter syndrome, and Noonan syndrome, are at an increased risk of developing B-ALL.
- Exposure to radiation: Exposure to high levels of radiation, such as from X-rays or nuclear fallout, can increase the risk of developing B-ALL.
- Exposure to certain chemicals: Exposure to certain chemicals, such as benzene, has been linked to an increased risk of developing B-ALL.
Clinical Features of B-ALL
The clinical features of B-ALL can vary depending on the stage of the disease and the individual patient. However, some of the most common clinical features include:
- Fatigue: Fatigue is a common symptom of many cancers, including B-ALL. It is caused by a decrease in the number of red blood cells, which carry oxygen to the tissues.
- Weakness: Weakness is another common symptom of B-ALL. It is caused by a decrease in the number of healthy blood cells, including red blood cells, white blood cells, and platelets.
- Pale skin: Pale skin is a sign of anemia, which is a condition in which the body does not have enough healthy red blood cells. Anemia is a common complication of B-ALL.
- Easy bleeding or bruising: Easy bleeding or bruising is a sign of thrombocytopenia, which is a condition in which the body does not have enough platelets. Platelets are blood cells that help the blood to clot. Thrombocytopenia is a common complication of B-ALL.
- Fever: Fever is a sign of infection. Infections are common in patients with B-ALL because they have a decreased number of white blood cells, which fight infection.
- Pain in the bones or joints: Pain in the bones or joints can be a sign of anemia, thrombocytopenia, or bone marrow infiltration. Bone marrow infiltration is a condition in which cancer cells invade the bone marrow.
- Loss of appetite: Loss of appetite is a common symptom of many cancers, including B-ALL. It is caused by a number of factors, including the cancer itself and the side effects of treatment.
- Weight loss: Weight loss is another common symptom of many cancers, including B-ALL. It is caused by a number of factors, including the cancer itself, the side effects of treatment, and loss of appetite.
In addition to the clinical features listed above, B-ALL can also cause other problems, such as:
- Central nervous system (CNS) involvement: CNS involvement occurs when cancer cells invade the brain and spinal cord. CNS involvement can cause a variety of symptoms, including headache, seizures, and paralysis.
- Organomegaly: Organomegaly is a condition in which the organs are enlarged. The liver and spleen are the most commonly affected organs in B-ALL.
- Disseminated intravascular coagulation (DIC): DIC is a condition in which blood clots form throughout the body. DIC can cause a variety of problems, including bleeding, organ damage, and death.
Prognosis of B-ALL
The prognosis of B-cell acute lymphoblastic leukemia (B-ALL) has improved significantly in recent years, due to advances in chemotherapy and other treatments. Today, more than 90% of children with B-ALL are cured, and the overall 5-year survival rate for adults with B-ALL is about 70%.
However, the prognosis for B-ALL can vary depending on a number of factors, including:
- Age: Children with B-ALL have a better prognosis than adults.
- White blood cell count at diagnosis: Patients with a lower white blood cell count at diagnosis have a better prognosis.
- Central nervous system (CNS) involvement: Patients with CNS involvement have a worse prognosis.
- Minimal residual disease (MRD): Patients with MRD after treatment have a worse prognosis.
- Genetic factors: Some genetic abnormalities, such as the BCR-ABL gene fusion, are associated with a worse prognosis.
Prognosis for children with B-ALL
The prognosis for children with B-ALL is excellent. Today, more than 90% of children with B-ALL are cured. The best outcomes are seen in children under the age of 5.
Prognosis for adults with B-ALL
The prognosis for adults with B-ALL is good, but it is not as good as it is for children. The overall 5-year survival rate for adults with B-ALL is about 70%. The best outcomes are seen in younger adults and adults with less aggressive forms of B-ALL.
Factors that can improve the prognosis for B-ALL
There are a number of factors that can improve the prognosis for B-ALL, including:
- Early diagnosis and treatment: Early diagnosis and treatment are essential for a good prognosis.
- Aggressive chemotherapy: Aggressive chemotherapy is the most effective treatment for B-ALL.
- Stem cell transplant: A stem cell transplant can be a curative treatment for B-ALL in some patients.
- Targeted therapy: Targeted therapies are drugs that target specific molecules that are involved in the growth and development of cancer cells. Targeted therapies can be used to treat B-ALL in patients who are resistant to chemotherapy or who have relapsed after chemotherapy.
Laboratory Investigations for B-ALL
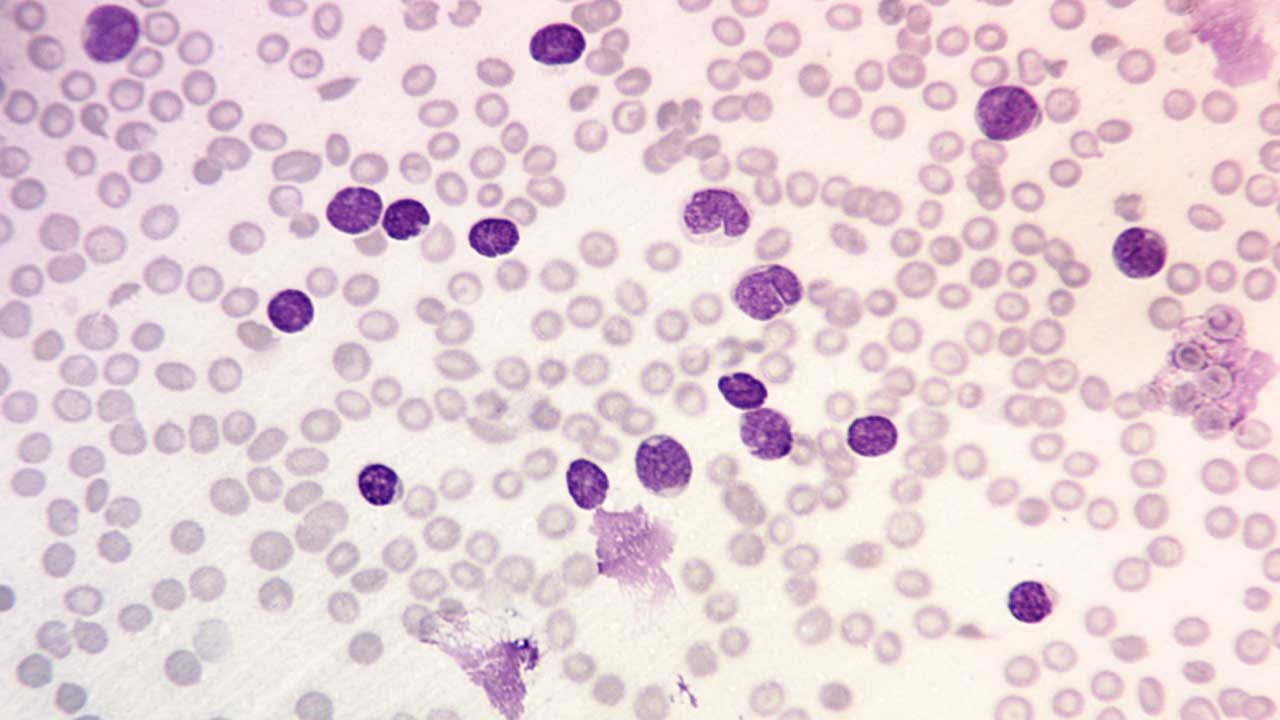
Complete blood count (CBC) with differential: A CBC measures the number of red blood cells, white blood cells, and platelets in the blood. A differential is a type of CBC that counts the different types of white blood cells. In B-ALL, the white blood cell count is often elevated, and the differential may show a large number of immature lymphocytes (blasts).
Peripheral blood smear: A peripheral blood smear is a blood test that examines the different types of blood cells under a microscope. In B-ALL, the peripheral blood smear may show lymphoblasts and other abnormal cells. Normochromic normocytic anemia with thrombocytopenia is seen.
Bone marrow aspiration and biopsy: A bone marrow aspiration and biopsy are procedures that remove a sample of bone marrow for examination under a microscope. Bone marrow is the soft tissue inside the bones where blood cells are made. In B-ALL, the bone marrow is usually infiltrated with blasts. By definition, the diagnosis in those with leukemic presentations requires that lymphoblasts comprise at least 20% of the marrow cellularity.
Immunophenotyping: Immunophenotyping is a laboratory technique used to identify and characterize cells based on the presence or absence of specific surface proteins, called antigens. Immunophenotyping is typically performed using a technique called flow cytometry. Flow cytometry is a method of analyzing single cells as they flow past a laser beam. The laser beam excites the fluorescently labeled antibodies attached to the cells, and the resulting signal is detected by a photodetector. The data is then analyzed using computer software to identify and characterize the cells.The tumours are positive for terminal deoxynucleotidyl transferase (TdT) which is a marker expression by precursor B and T cells as well as CD19 and CD 10 for B-lineage proteins and negative for surface immunoglobulin which appears only on mature B cells. Definitive diagnosis of B-ALL requires immunophenotyping.
Cytogenetic analysis: Cytogenetic analysis is a laboratory test that examines the chromosomes in cells. Chromosomal abnormalities are common in B-ALL. Subtyping of B-ALL is based on cytogenetics, and particular cytogenetics are strongly predictive of clinical outcome. For example, B-ALLs associated with BCR-ABL fusion genes, rearrangements of the MLL gene, or hypodiploidy are associated with poor outcomes with conventional therapies.
Treatment and Management of B-ALL
The treatment and management of B-ALL is complex and depends on a number of factors, including the patient’s age, overall health, and the stage of the disease. The goal of treatment is to achieve complete remission, which is the absence of detectable leukemia cells in the body.
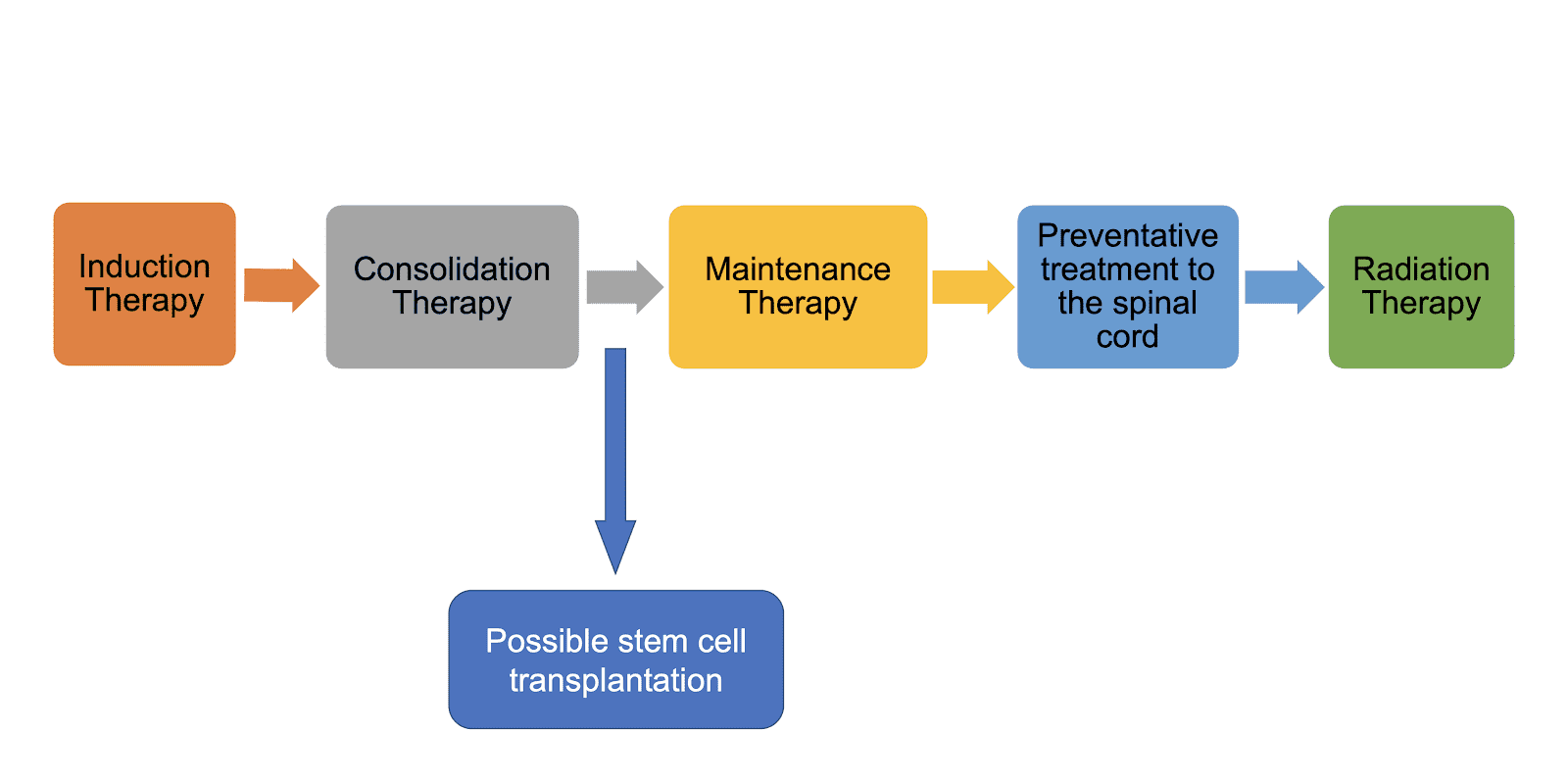
Induction therapy
Induction therapy is the initial phase of treatment for B-ALL. The goal of induction therapy is to bring the leukemia into remission as quickly as possible. Induction therapy typically involves a combination of chemotherapy drugs that are given intravenously (IV).
The most common chemotherapy drugs used for B-ALL include:
- Vincristine
- Prednisone
- L-asparaginase
- Doxorubicin
- Cyclophosphamide
- Methotrexate
Induction therapy is typically given for 4-6 weeks. During this time, the patient will be closely monitored for side effects of chemotherapy, such as nausea, vomiting, hair loss, and bone marrow suppression.
Consolidation therapy
Consolidation therapy is the second phase of treatment for B-ALL. The goal of consolidation therapy is to eliminate any remaining leukemia cells after induction therapy. Consolidation therapy typically involves a more intensive course of chemotherapy, which may be given in combination with radiation therapy.
Consolidation therapy may last for several months. During this time, the patient will continue to be monitored closely for side effects of treatment.
Maintenance therapy
Maintenance therapy is the third and final phase of treatment for B-ALL. The goal of maintenance therapy is to prevent leukemia from coming back. Maintenance therapy typically involves a less intensive course of chemotherapy, which may be given in combination with other drugs, such as tyrosine kinase inhibitors (TKIs).
Maintenance therapy may last for 2-3 years. During this time, the patient will continue to be monitored for signs of relapse.
Stem cell transplantation
Stem cell transplantation is a procedure that can be used to treat B-ALL that is resistant to chemotherapy or that has relapsed after chemotherapy. Stem cell transplantation involves replacing the patient’s bone marrow with healthy stem cells from a donor.
Stem cell transplantation is a high-risk procedure, but it can be curative for some patients with B-ALL.
Targeted therapy
Targeted therapy is a type of treatment that targets specific molecules that are involved in the growth and development of cancer cells. Targeted therapy is becoming increasingly important in the treatment of B-ALL especially in adults.
Some examples of targeted therapies that are used for B-ALL include:
- Imatinib (Gleevec)
- Dasatinib (Sprycel)
- Nilotinib (Tasigna)
- Ponatinib (Iclusig)
- Asciminib (Scemblix)
Targeted therapy may be used as a first-line treatment for B-ALL, or it may be used in combination with chemotherapy or stem cell transplantation.
Immunotherapy
Immunotherapy is a type of treatment that boosts the body’s own immune system to fight cancer. Immunotherapy is still under investigation for the treatment of B-ALL, but it has shown promising results in some clinical trials.
Management of B-ALL
In addition to treatment, patients with B-ALL also need to be managed for the side effects of treatment and other complications of the disease. Some common complications of B-ALL include:
- Infection
- Bleeding
- Anemia
- Fatigue
- Nausea and vomiting
- Hair loss
- Skin problems
- Nerve problems
The management of B-ALL requires a team approach, including doctors, nurses, social workers, and other healthcare professionals. The team will work together to develop a personalized treatment and management plan for each patient.
Key Points of B-ALL
Definition
An aggressive neoplasm of the lymphoid lineage caused by acquired somatic defects due to either inherited factors or infections e.g. viruses in early hematopoietic blasts that impede or significantly impair differentiation leading to accumulation of undifferentiated blasts in the marrow, spilling into the peripheral blood and infiltrate other tissues. Leukemic blasts are too immature to be functional and are prone to suppress or supplant the production of normal hematopoiesis causing pancytopenia.
Pathogenesis
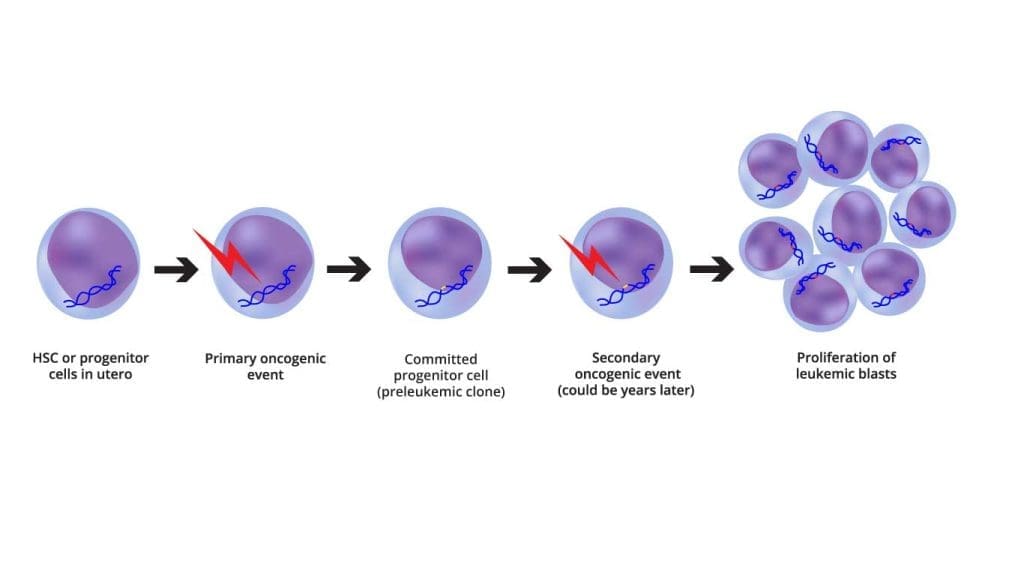
| Acute Lymphoblastic Leukemia | ||
| B-ALL | T-ALL | |
| Incidence | Most common Peak at 3 y.o. Whites, Hispanics, Down syndrome | Peak at 15 y.o. Male:female 2:1 |
| Signs and Symptoms | Fatigue Easy bruising Fever Spread through meninges to CNS | Mediastinal lymphoma (thymus) Cough Shortness of breath Superior vena cava syndrome e.g. swelling of the face |
| Subtypes | t(12;21)(p13;q22)ETV6/RUNX1 Hyperdiploidy Hypodiploidy t(9;22)(q34;q11)BCR/ABL1 t(v;11q23) | |
| Laboratory investigations | FBC: Normochromic, normocytic anemia with thrombocytopenia PBF: Presence of lymphoblasts BMAT: ≥ 20% lymphoblasts Immunophenotyping: + CD19, CD10 – CD45, CD20, CD13, surface light chains | Biopsy: Replacement of thymus or bone marrow tissues with lymphoblasts Immunophenotyping: + CD3, CD5, CD7, HLA-DR – CD34, CD8, CD4, CD19 |
| Prognosis | Excellent: Children Less favorable: Adults | Similar to B-ALL |
Treatment
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022 Jul;36(7):1720-1748. doi: 10.1038/s41375-022-01620-2. Epub 2022 Jun 22. Erratum in: Leukemia. 2023 Sep;37(9):1944-1951. PMID: 35732829; PMCID: PMC9214472.
- Sekeres MA. When Blood Breaks Down: Life Lessons from Leukemia (Mit Press). 2021
- Daniel J. DeAngelo, Elias Jabbour, and Anjali Advani. Recent Advances in Managing Acute Lymphoblastic Leukemia. American Society of Clinical Oncology Educational Book 2020 :40, 330-342.
- Yilmaz M, Kantarjian H, Ravandi-Kashani F, Short NJ, Jabbour E. Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: current treatments and future perspectives. Clin Adv Hematol Oncol. 2018 Mar;16(3):216-223. PMID: 29742077.
- Saleh K, Fernandez A, Pasquier F. Treatment of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia in Adults. Cancers (Basel). 2022 Apr 1;14(7):1805. doi: 10.3390/cancers14071805. PMID: 35406576; PMCID: PMC8997772.