TL;DR
T-ALL, or T-cell Acute Lymphoblastic Leukemia, is a type of blood cancer that affects T cells, a type of white blood cell. It is characterized by the uncontrolled growth and proliferation of immature T cells, leading to the replacement of healthy blood cells in the bone marrow.
Symptoms of T-ALL ▾
- Fatigue
- Pallor
- Frequent infections
- Bone pain
- Swollen lymph nodes
- Enlarged liver or spleen
- Shortness of breath (if the leukemia affects the mediastinum)
Causes of T-ALL
The exact cause of T-ALL is unknown, but it is believed to be due to genetic mutations that disrupt the normal development and function of T cells.
Laboratory Investigations for T-ALL ▾
Laboratory investigations play a crucial role in diagnosing T-ALL and assessing its extent. Common tests include:
- Complete Blood Count (CBC): In T-ALL, there may be a decreased number of red blood cells (anemia), a decreased number of platelets (thrombocytopenia), and an increased number of white blood cells, mainly immature T cells.
- Peripheral Blood Smear: Presence of blast cells (immature T cells) in T-ALL.
- Flow Cytometry: CD3+, CD5+ and negative for CD19
- Bone Marrow Aspiration and Biopsy: Presence of blast cells
- Cytogenetics and Molecular Biology: Positive for genetic abnormalities associated with T-ALL.
Treatment of T-ALL ▾
Treatment for T-ALL typically involves a combination of chemotherapy, radiation therapy, and sometimes a stem cell transplant.
*Click ▾ for more information
Acute Lymphoblastic Leukemia (ALL)
Acute lymphoblastic leukemia (ALL) which can be divided into B-cell acute lymphoblastic leukemia (B-ALL) and T-cell acute lymphoblastic leukemia (T-ALL), is a type of blood cancer that begins in the bone marrow. The bone marrow is the soft tissue inside the bones where blood cells are made. In ALL, the bone marrow makes too many immature white blood cells called lymphoblasts. Lymphoblasts are a type of lymphocyte, which is a type of white blood cell.
ALL is defined as an aggressive neoplasm of the lymphoid lineage caused by acquired somatic defects due to either inherited factors or infections e.g. viruses in early haematopoietic blasts that impede or significantly impair differentiation leading to accumulation of undifferentiated blasts in the marrow, spilling into the peripheral blood and infiltrate other tissues. Leukemic blasts are too immature to be functional and are prone to suppress or supplant the production of normal haematopoiesis causing pancytopenia.
ALL is the most common type of cancer in children, but it can also occur in adults. It is most common in children under the age of 5 and in adults over the age of 65.
T-cell Acute Lymphoblastic Leukemia (T-ALL)
T-cell acute lymphoblastic leukemia (T-ALL) is a subtype of acute lymphoblastic leukemia (ALL), which is the most common type of cancer in children. T-cell acute lymphoblastic leukemia (T-ALL) is less common than B-cell ALL, but it is still the second most common type of ALL in children. T-cell acute lymphoblastic leukemia (T-ALL) makes up roughly 15% of ALLs.
T-cell acute lymphoblastic leukemia (T-ALL) is caused by the uncontrolled growth of immature T cells. T cells are a type of white blood cell that helps the body fight infection. When T cells become cancerous, they can crowd out healthy blood cells in the bone marrow and bloodstream. This can lead to a number of problems, including fatigue, weakness, and an increased risk of infection.
Risk factors for T-cell acute lymphoblastic leukemia (T-ALL)
The exact cause of T-cell acute lymphoblastic leukemia (T-ALL) is unknown, but there are a number of risk factors that may increase the risk of developing the disease. These risk factors include:
- Age: T-cell acute lymphoblastic leukemia (T-ALL) is most common in children under the age of 5 and in adults over the age of 65. The peak incidence is at about 15 years old.
- Gender: There is a 2:1 male predominance.
- Family history: People with a family history of T-cell acute lymphoblastic leukemia (T-ALL) are at an increased risk of developing the disease.
- Certain genetic conditions: People with certain genetic conditions, such as Down syndrome and Noonan syndrome, are at an increased risk of developing T-cell acute lymphoblastic leukemia (T-ALL) .
- Exposure to radiation: Exposure to high levels of radiation, such as from X-rays or nuclear fallout, can increase the risk of developing T-cell acute lymphoblastic leukemia (T-ALL) .
- Exposure to certain chemicals: Exposure to certain chemicals, such as benzene, has been linked to an increased risk of developing T-cell acute lymphoblastic leukemia (T-ALL) .
Clinical Features of T-ALL
About ⅔ of T-cell acute lymphoblastic leukemias (T-ALL) present as mediastinal lymphomas associated with large masses in the thymus. The enlarging tumor mass often compresses structures such as major airways and blood vessels, producing cough, shortness of breath and superior vena cava syndrome which is characterized by swelling and redness of the face and upper extremities due to blockage of venous return to the heart. T-cell acute lymphoblastic leukemia (T-ALL) has a greater tendency than B-ALL to produce organomegaly and lymphadenopathy and it often spreads to the central nervous system too.
Laboratory Investigations for T-ALL
Laboratory investigations play an important role in the diagnosis, staging, and monitoring of T-cell acute lymphoblastic leukemia (T-ALL). The following tests are commonly used:
Complete blood count (CBC)
A CBC measures the number of red blood cells, white blood cells, and platelets in the blood. In T-ALL, the white blood cell count is often elevated, and the differential may show a large number of immature T cells (blasts). Full blood count is similar to B-ALL.
Peripheral blood smear
A peripheral blood smear is a blood test that examines the different types of blood cells under a microscope. In T-cell acute lymphoblastic leukemia (T-ALL), the peripheral blood smear may show blasts and other abnormal cells. Peripheral blood smear is similar to B-ALL.
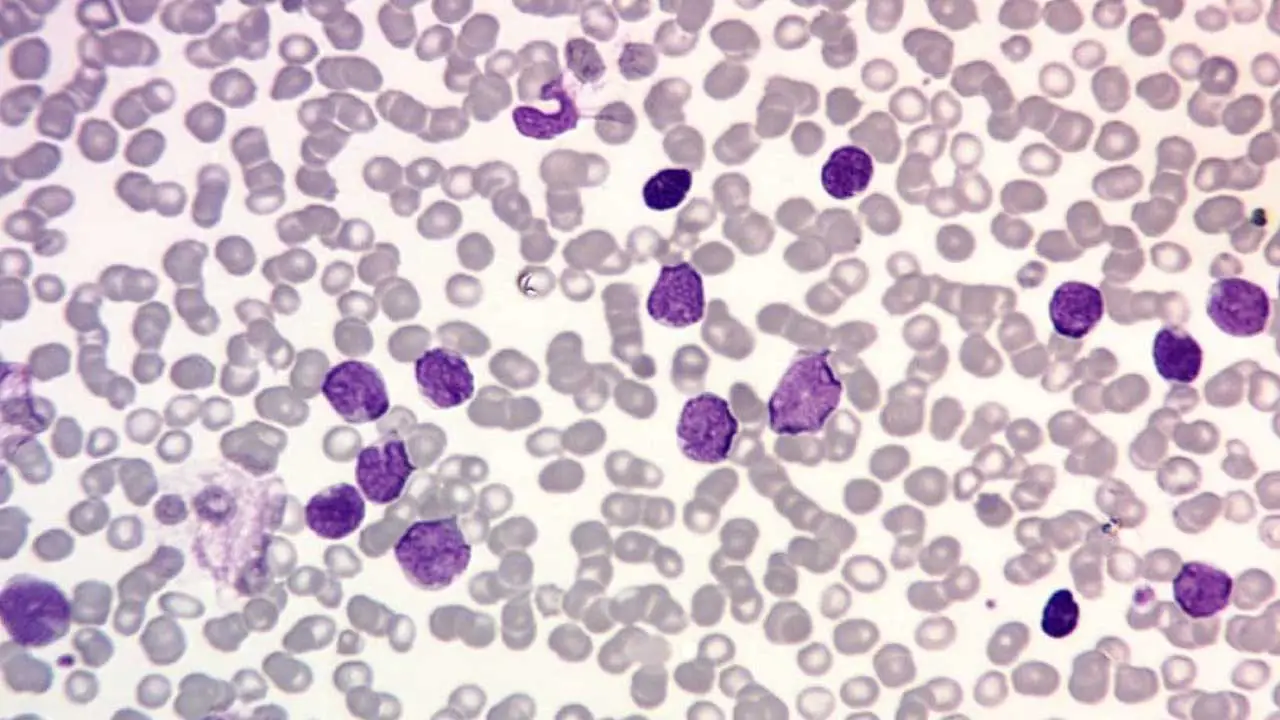
Bone marrow aspiration and biopsy
A bone marrow aspiration and biopsy are procedures that remove a sample of bone marrow for examination under a microscope. Bone marrow is the soft tissue inside the bones where blood cells are made. In T-cell acute lymphoblastic leukemia (T-ALL) , the bone marrow is usually infiltrated with blasts. The diagnosis is based on morphologic demonstration of lymphoblasts replacing tissues such as the thymus and the bone marrow.
Immunophenotyping
Immunophenotyping is a laboratory test that uses antibodies to identify the type of white blood cells present in a sample. In T-cell acute lymphoblastic leukemia (T-ALL), immunophenotyping can be used to identify the specific type of T cell that is involved in the cancer. Immunotyping is essential to confirm the diagnosis and expresses a variable combination of T-lineage antigens such as CD3, CD5 and negative for CD19 which is a B-cell lineage antigen.
Cytogenetic analysis
Cytogenetic analysis is a laboratory test that examines the chromosomes in cells. Chromosomal abnormalities are common in T-cell acute lymphoblastic leukemia (T-ALL). Cytogenetics, however, has not proved helpful in predicting patient outcomes.
Fluorescence in situ hybridization (FISH)
FISH is a laboratory test that uses fluorescent probes to identify specific genes or chromosomal sequences. FISH can be used to detect chromosomal abnormalities and other genetic changes in T-cell acute lymphoblastic leukemia (T-ALL) cells.
Molecular testing
Molecular testing can be used to detect the genetic changes that cause T-cell acute lymphoblastic leukemia (T-ALL). This includes testing for the NOTCH1 gene mutation, which is present in about 60% of adults with T-cell acute lymphoblastic leukemia (T-ALL) . Common activating mutations include mutations in NOTCH1, chromosomal rearrangements or other aberrations that lead to increased expression of several other transcription factors.
Staging T-ALL
The following laboratory tests are used to stage T-cell acute lymphoblastic leukemia (T-ALL):
- Bone marrow blast count: The bone marrow blast count is the percentage of blasts in the bone marrow. A higher bone marrow blast count is associated with a worse prognosis.
- Central nervous system (CNS) involvement: CNS involvement can be detected by examining the cerebrospinal fluid (CSF) under a microscope. The presence of blasts in the CSF is a sign of CNS involvement.
- Minimal residual disease (MRD): MRD is the presence of a small number of cancer cells in the body after treatment. MRD can be detected using molecular testing.
Monitoring T-ALL
The following laboratory tests are used to monitor T-cell acute lymphoblastic leukemia (T-ALL):
- Complete blood count (CBC): The CBC is used to monitor the response to treatment and to detect early signs of relapse.
- Peripheral blood smear: The peripheral blood smear is used to monitor the response to treatment and to detect early signs of relapse.
- Bone marrow aspiration and biopsy: Bone marrow aspiration and biopsy are used to monitor the response to treatment and to detect early signs of relapse.
- Molecular testing: Molecular testing can be used to detect MRD and to monitor the response to treatment.

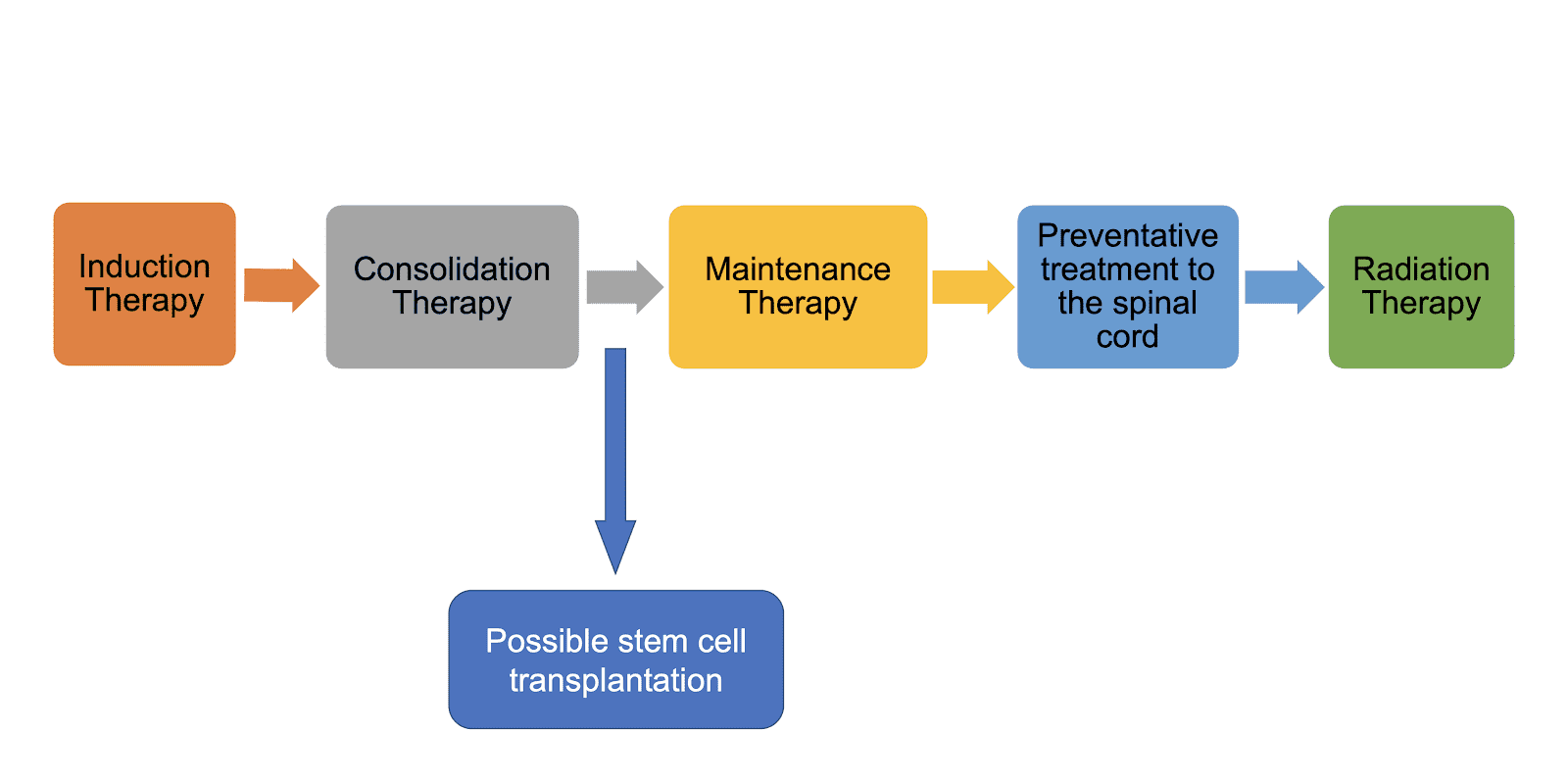
Treatment and Management of T-ALL
The treatment of T-cell acute lymphoblastic leukemia (T-ALL) depends on a number of factors, including the stage of the disease, the patient’s age, and their overall health. The goal of treatment is to achieve complete remission, which is the absence of detectable leukemia cells in the body.
Chemotherapy
The most common treatment for T-cell acute lymphoblastic leukemia (T-ALL) is chemotherapy. Chemotherapy is a type of medication that kills cancer cells. Chemotherapy can be given through an IV or by mouth.
Chemotherapy drugs used to treat T-cell acute lymphoblastic leukemia (T-ALL) include:
- Vincristine
- Prednisone
- L-asparaginase
- Doxorubicin
- Cyclophosphamide
- Methotrexate
- Nelarabine
Chemotherapy is typically given in cycles, with each cycle lasting for several weeks. The patient will have a break between cycles to allow their body to recover.
In addition to chemotherapy, other treatments may also be used, such as:
- Radiation therapy: Radiation therapy uses high-energy rays to kill cancer cells. Radiation therapy may be used to treat T-cell acute lymphoblastic leukemia (T-ALL) that has spread to the brain or spinal cord.
- Stem cell transplant: A stem cell transplant is a procedure in which the patient’s own stem cells are harvested and then reinfused into the body after high-dose chemotherapy. Stem cell transplants can be used to treat T-cell acute lymphoblastic leukemia (T-ALL) that is resistant to chemotherapy or that has relapsed after chemotherapy.
- Targeted therapy: Targeted therapy is a type of medication that targets specific molecules that are involved in the growth and development of cancer cells. Targeted therapy drugs are becoming increasingly important in the treatment of T-cell acute lymphoblastic leukemia (T-ALL).
Some examples of targeted therapy drugs used to treat T-cell acute lymphoblastic leukemia (T-ALL) include:
- Imatinib (Gleevec)
- Dasatinib (Sprycel)
- Nilotinib (Tasigna)
- Ponatinib (Iclusig)
Targeted therapy may be used alone or in combination with chemotherapy.
Immunotherapy
Immunotherapy is a type of treatment that boosts the body’s own immune system to fight cancer. Immunotherapy is still under investigation for the treatment of T-cell acute lymphoblastic leukemia (T-ALL) , but it has shown promising results in some clinical trials.
One example of immunotherapy that is being used to treat T-cell acute lymphoblastic leukemia (T-ALL) is chimeric antigen receptor (CAR) T-cell therapy. CAR T-cell therapy is a type of immunotherapy that involves engineering the patient’s own T cells to recognize and kill cancer cells.
CAR T-cell therapy has been shown to be very effective in treating T-cell acute lymphoblastic leukemia (T-ALL) in children and young adults. However, it is a complex and expensive treatment, and it is not yet widely available.
Summary of B-ALL vs T-ALL
| Acute Lymphoblastic Leukemia | ||
| B-ALL | T-ALL | |
| Incidence | Most common Peak at 3 y.o. Whites, Hispanics, Down syndrome | Peak at 15 y.o. Male:female 2:1 |
| Signs and Symptoms | Fatigue Easy bruising Fever Spread through meninges to CNS | Mediastinal lymphoma (thymus) Cough Shortness of breath Superior vena cava syndrome e.g. swelling of the face |
| Subtypes | t(12;21)(p13;q22)ETV6/RUNX1 Hyperdiploidy Hypodiploidy t(9;22)(q34;q11)BCR/ABL1 t(v;11q23) | |
| Laboratory investigations | FBC: Normochromic, normocytic anemia with thrombocytopenia PBF: Presence of lymphoblasts BMAT: ≥ 20% lymphoblasts Immunophenotyping: + CD19, CD10- CD45, CD20, CD13, surface light chains | Biopsy: Replacement of thymus or bone marrow tissues with lymphoblasts Immunophenotyping: + CD3, CD5, CD7, HLA-DR – CD34, CD8, CD4, CD19 |
| Prognosis | Excellent: Children Less favorable: Adults | Similar to B-ALL |
Frequently Asked Questions (FAQs)
Is T-cell acute lymphoblastic leukemia (T-ALL) leukemia curable?
Yes, T-ALL (T-cell acute lymphoblastic leukemia) is curable, especially in children.
While it’s a serious condition, advancements in treatment have significantly improved survival rates. Factors like age, overall health, and specific treatment plan influence the outcome.
- Children: Have a higher chance of cure, with survival rates often exceeding 85%.
- Adults: The outlook is less optimistic, with survival rates generally below 50%. However, ongoing research and improved treatments are improving outcomes for adults as well.
It’s important to remember that treatment involves a combination of chemotherapy, sometimes radiation therapy, and potentially bone marrow transplantation. Early detection and aggressive treatment are key factors in achieving a positive outcome.
What is the survival rate for T-cell acute lymphoblastic leukemia?
Acute Lymphoblastic Leukemia (ALL) is a cancer of the white blood cells. It’s divided into two main types based on the type of white blood cell affected: T-cell ALL and B-cell ALL.
- Most common type of ALL, especially in children.
- Originates from immature B cells, which are white blood cells responsible for antibody production.
T-cell ALL
- Less common than B-cell ALL, and more frequently seen in adults.
- Originates from immature T cells, which are white blood cells responsible for cellular immunity.
- Often considered more aggressive than B-cell ALL.
| Feature | B-cell ALL | T-cell ALL |
|---|---|---|
| Prevalence | More common | Less common |
| Age group | Predominantly children | More common in adults |
| Aggressiveness | Generally less aggressive | Often more aggressive |
While both types of ALL share similar symptoms and treatment approaches, the specific characteristics of the leukemia cells can influence treatment strategies and prognosis.
Why is chemotherapy used for T-cell acute lymphoblastic leukemia?
Chemotherapy is the primary treatment for T-ALL because:
- Rapid cell division: Leukemia cells, including T-ALL cells, divide rapidly to produce new cancer cells. Chemotherapy drugs are designed to target and kill rapidly dividing cells.
- Systemic disease: T-ALL is a systemic disease, meaning it can spread throughout the body. Chemotherapy drugs can reach cancer cells in various locations.
- Induction of remission: The goal of initial chemotherapy (induction phase) is to achieve remission, which means no detectable leukemia cells in the bone marrow.
Key points to remember
- Combination therapy: Multiple chemotherapy drugs are often used together to increase effectiveness and reduce the risk of cancer cells developing resistance.
- Intrathecal chemotherapy: Since T-ALL cells can spread to the central nervous system, chemotherapy is often administered directly into the spinal fluid as a preventive measure.
- Side effects: While chemotherapy is effective, it also affects healthy cells, leading to side effects like hair loss, nausea, and fatigue.
Additional treatments
- Stem cell transplantation: This might be considered for patients who don’t respond to chemotherapy or relapse.
- Targeted therapy: Newer treatments focus on specific molecular targets within leukemia cells.
Is T-cell acute lymphoblastic leukemia aggressive?
Yes, T-cell acute lymphoblastic leukemia (T-ALL) is generally considered more aggressive than B-cell ALL.
This means that T-ALL tends to progress faster and may be more difficult to treat. However, advancements in treatment have significantly improved outcomes for both children and adults with T-ALL.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022 Jul;36(7):1720-1748. doi: 10.1038/s41375-022-01620-2. Epub 2022 Jun 22. Erratum in: Leukemia. 2023 Sep;37(9):1944-1951. PMID: 35732829; PMCID: PMC9214472.
- Sekeres MA. When Blood Breaks Down: Life Lessons from Leukemia (Mit Press). 2021
- Daniel J. DeAngelo, Elias Jabbour, and Anjali Advani. Recent Advances in Managing Acute Lymphoblastic Leukemia. American Society of Clinical Oncology Educational Book 2020 :40, 330-342.
- Hefazi M, Litzow MR. Recent Advances in the Biology and Treatment of T Cell Acute Lymphoblastic Leukemia. Curr Hematol Malig Rep. 2018 Aug;13(4):265-274. doi: 10.1007/s11899-018-0455-9. PMID: 29948644.
- Caracciolo, D., Mancuso, A., Polerà, N. et al. The emerging scenario of immunotherapy for T-cell Acute Lymphoblastic Leukemia: advances, challenges and future perspectives. Exp Hematol Oncol 12, 5 (2023). https://doi.org/10.1186/s40164-022-00368-w
- Fattizzo B, Rosa J, Giannotta JA, Baldini L, Fracchiolla NS. The Physiopathology of T- Cell Acute Lymphoblastic Leukemia: Focus on Molecular Aspects. Front Oncol. 2020 Feb 28;10:273. doi: 10.3389/fonc.2020.00273. PMID: 32185137; PMCID: PMC7059203.
- Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):580-588. doi: 10.1182/asheducation-2016.1.580. PMID: 27913532; PMCID: PMC6142501.