TL;DR
Hereditary hemochromatosis is a genetic disorder affecting iron metabolism, leading to excessive iron absorption from the diet. It is the most common genetic disease in populations of Northern European descent.
Causes ▾
- Primarily caused by mutations in the HFE gene, particularly C282Y and H63D mutations, disrupting iron regulation.
- Inherited in an autosomal recessive pattern.
Symptoms ▾
- Early symptoms are often non-specific (fatigue, weakness, joint pain).
- Later stages can involve organ damage and specific symptoms like:
- Liver problems (cirrhosis)
- Heart issues (cardiomyopathy)
- Diabetes
- Skin changes (bronze pigmentation)
- Joint dysfunction
- Sexual dysfunction
Diagnosis ▾
- Iron studies
- Genetic testing and, in some cases, liver biopsy can confirm the diagnosis
Treatment and Management ▾
- Therapeutic phlebotomy: Regular blood removal to deplete iron stores.
- Early diagnosis is crucial to prevent complications.
- Lifelong management with monitoring and phlebotomy is necessary.
- Diet: Iron restriction is generally not needed but avoiding iron-fortified foods and supplements is recommended.
Prognosis ▾
- Positive with early diagnosis and consistent treatment.
*Click ▾ for more information
Introduction
Hereditary hemochromatosis is a genetic disorder that affects the body’s ability to regulate iron metabolism. This leads to excessive iron absorption from the diet, resulting in a buildup of iron in the body’s tissues and organs. Iron is a mineral that is essential for many bodily functions, but too much iron can be toxic. In people with hereditary hemochromatosis, the excess iron builds up in the body’s tissues and organs over time, which can lead to serious health problems.
Prevalence
Hereditary hemochromatosis is most prevalent in Europe, Australia, and other Western countries with a high concentration of people with Celtic ancestry. The prevalence of hereditary hemochromatosis varies depending on ethnicity:
- Most common: It’s considered the most common genetic disorder in people of Northern European descent, affecting roughly 1 in 300 to 500 non-Hispanic White people.
- Less common: The prevalence is significantly lower in other ethnicities, including Hispanic, Asian American, Pacific Islander, and Black populations.
Carrier frequency: Even within high-risk populations, only a small percentage (around 10%) actually develop the disease. Many people carry one copy of the mutated gene (carriers) without experiencing symptoms.
Causes
Hereditary hemochromatosis is primarily caused by mutations in the HFE gene. The HFE gene provides instructions for making a protein involved in regulating iron absorption in the intestines. When mutated, the HFE protein malfunctions, leading to excessive iron absorption from food.
HFE gene
Characteristics
- Chromosomal location: 6p21.3
Function
- The HFE protein functions as a regulatory protein within the iron absorption pathway. It’s primarily expressed in the following tissues:
- Liver: Plays a major role in iron storage and regulation.
- Small intestine: Where dietary iron gets absorbed.
- Macrophages: Immune cells that recycle iron from worn-out red blood cells.
- HFE protein interacts with other proteins like beta-2 microglobulin (β2M) and the transferrin receptor 1 (TFR1) to regulate iron uptake.
- β2M binding: HFE normally binds to β2M, which is essential for its proper function and stability.
- TFR1 interaction: When iron levels are sufficient, the HFE-β2M complex binds to TFR1 on the surface of intestinal cells. This interaction prevents TFR1 from binding to transferrin, the iron transport protein in the blood, thereby limiting iron absorption from the diet.
- Iron deficiency: During iron deficiency, the HFE-β2M complex dissociates from TFR1, allowing TFR1 to bind transferrin and absorb iron.
Mutations and Consequences
C282Y and H63D mutations: These are the most common mutations in the HFE gene associated with hereditary hemochromatosis. They alter the protein structure, hindering its function in iron regulation.
C282Y Mutation
This mutation involves a substitution of a single amino acid building block in the HFE protein. At position 282, a cysteine (C) is replaced by tyrosine (Y). This change disrupts the protein’s structure and function in several ways:
- β2M binding: Cysteine at position 282 is crucial for binding to β2M. With the C282Y mutation, the HFE protein loses its ability to bind β2M effectively, rendering it unstable and dysfunctional.
- TFR1 interaction: Without proper β2M binding, the HFE protein cannot effectively interact with TFR1 on intestinal cells. This disrupts the normal iron regulation mechanism.
- Constant iron absorption: Even when iron stores are sufficient, the lack of functional HFE-β2M complex allows TFR1 to continuously bind transferrin and absorb iron from the diet, leading to iron overload.
H63D Mutation
This mutation also involves a single amino acid substitution in the HFE protein. At position 63, a histidine (H) is replaced by aspartic acid (D). The effect of H63D is somewhat less severe than C282Y.
- Partial β2M binding: The HFE protein with the H63D mutation may still retain some ability to bind β2M, although it might be weaker than normal.
- Partially functional TFR1 interaction: Some degree of interaction with TFR1 might still occur, but it might be less efficient compared to a healthy HFE protein.
- Increased iron absorption: While not as pronounced as with C282Y, the H63D mutation can still lead to increased iron absorption over time, potentially contributing to iron overload.
Combined Effects
- Compound heterozygotes: Individuals who inherit both C282Y and H63D mutations from each parent (compound heterozygotes) are at a higher risk of developing iron overload compared to carrying just one mutation.
- Severity spectrum: The severity of iron overload can vary depending on the specific HFE gene mutations and other factors like dietary iron intake.
Inheritance pattern: Hereditary hemochromatosis follows an autosomal recessive inheritance pattern. This means an individual needs to inherit two copies of the mutated HFE gene (one from each parent) to develop the disease. People with only one copy of the mutated gene are carriers and typically don’t experience symptoms but can pass the gene on to their children.
Other genes and types: While HFE mutations are the most frequent cause, there are rarer types of hereditary hemochromatosis linked to mutations in different genes.
- Type 2 (juvenile hemochromatosis): Caused by mutations in the HJV or HAMP gene, affecting iron regulation differently.
- Type 3: Mutations in the TFR2 gene disrupt iron transport within the body.
- Type 4: Mutations in the SLC40A1 gene cause a different form of iron overload.
Hereditary Hemochromatosis Symptoms
Hereditary hemochromatosis can be a tricky disease to diagnose early on because the initial hereditary hemochromatosis symptoms are often vague and non-specific.
Challenge of Early Diagnosis
Non-specific symptoms: Early signs of hereditary hemochromatosis can easily mimic other more common conditions, making early detection difficult. These hereditary hemochromatosis symptoms include:
- Fatigue: A general feeling of tiredness and lack of energy is a frequent complaint.
- Weakness: Reduced muscle strength and stamina can be present.
- Joint pain: Pain and stiffness, particularly in the small joints like fingers, can occur.
- Abdominal pain: Discomfort or dull ache in the upper right abdomen can be experienced.
- Weight loss: Unexplained weight loss might be noticed.
- Brain fog: Difficulties with concentration, memory lapses, and decreased mental clarity might be present.
- Mood swings: Changes in mood like depression, irritability, or anxiety can occur.
Manifestations in Later Stages
If left undiagnosed and untreated, iron overload can progressively damage various organs, leading to more specific and severe symptoms.
- Liver: Cirrhosis, a condition with significant scarring of the liver, can develop. This can manifest as jaundice (yellowing of the skin and eyes), fluid buildup in the abdomen (ascites), and impaired liver function.
- Heart: Cardiomyopathy (weakening of the heart muscle) and arrhythmias (irregular heartbeats) can occur. These can lead to chest pain, shortness of breath, and fatigue.
- Pancreas: Iron overload can disrupt insulin production, leading to diabetes mellitus. Symptoms include excessive thirst, frequent urination, and weight loss.
- Joints: Severe arthritis with pain, stiffness, and joint damage can develop, particularly in the hands, hips, and knees.
- Skin changes: Bronze pigmentation of the skin, particularly on the face, hands, and forearms, can be a characteristic sign. However, this is more noticeable in lighter skin tones.
- Sexual dysfunction: Loss of libido and erectile dysfunction in men and irregular periods in women can occur due to hormonal imbalances.
- Pituitary gland: Iron overload can affect the pituitary gland, leading to hypopituitarism, a condition where the gland doesn’t produce enough hormones. This can cause various symptoms depending on the affected hormone(s).
Laboratory Investigations
Full blood count (CBC): While not definitive for hereditary hemochromatosis, a CBC might reveal microcytic anemia.
Liver function tests (LFTs): These can show abnormalities, particularly in later stages, suggesting potential liver damage.
- Elevated liver enzymes: Enzymes like AST (SGOT) and ALT (SGPT) might be elevated if the liver is inflamed or damaged due to iron overload.
- Increased bilirubin: This can indicate impaired bile flow, potentially related to liver damage.
- Transferrin saturation (TSAT): This test measures the percentage of transferrin (iron-carrying protein) that is bound to iron. In hemochromatosis, TSAT levels are typically elevated (>45%) due to iron overload exceeding transferrin’s binding capacity.
- Serum ferritin: This test reflects the amount of iron stored in the body, primarily in the liver. Elevated ferritin levels (>200 ng/mL in men and >120 ng/mL in women) are indicative of iron overload, but can also be caused by other conditions.
Confirmation of Diagnosis
- Genetic testing: Identifying mutations in the HFE gene, particularly C282Y and H63D mutations, is a strong indicator of hereditary hemochromatosis. However, genetic testing alone doesn’t definitively diagnose the disease, as some individuals with these mutations might not experience iron overload.
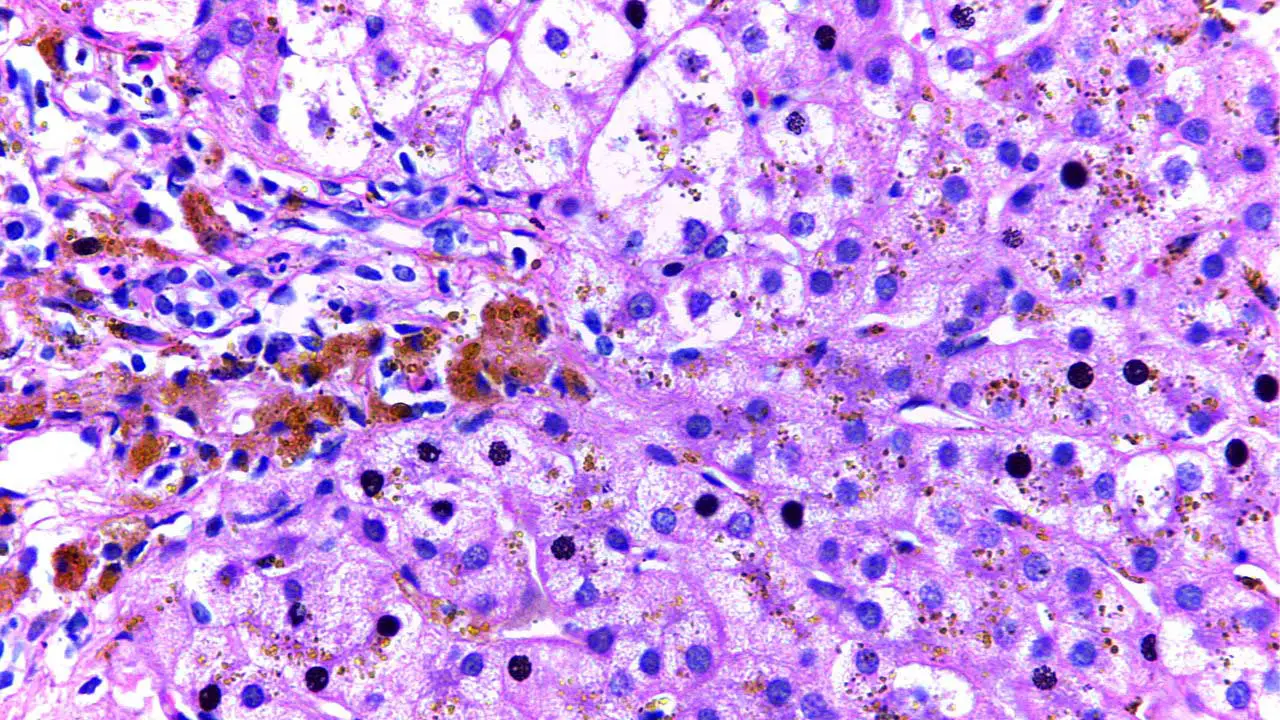
- Liver biopsy: In some cases, a liver biopsy might be necessary to assess the extent of iron overload and rule out other liver diseases. A small tissue sample is extracted from the liver and examined under a microscope for signs of iron deposition and liver damage.
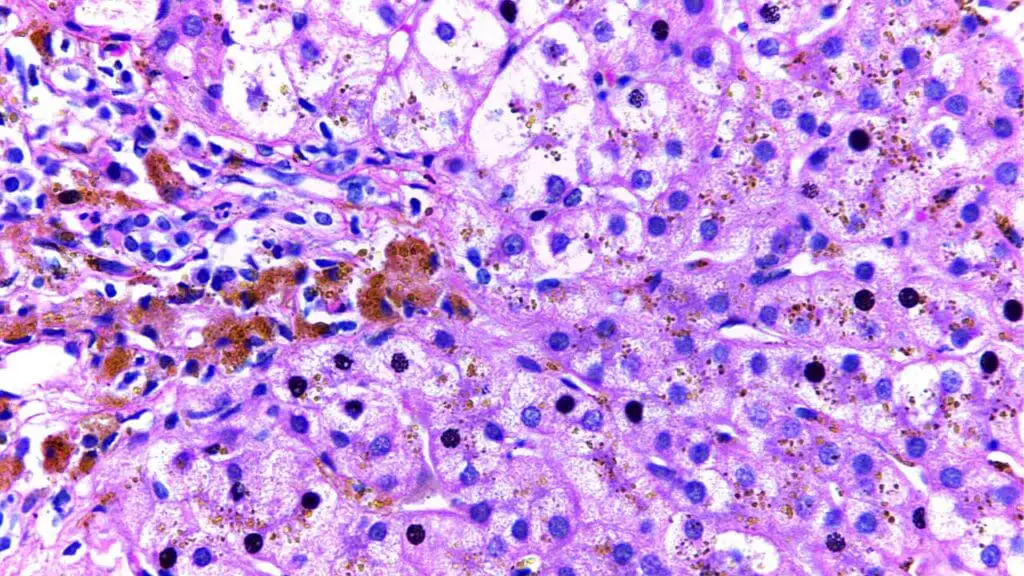
Treatment and Management
Hereditary hemochromatosis is a treatable condition, but early diagnosis is crucial to prevent complications from iron overload.
Therapeutic Phlebotomy
- This is the cornerstone of treatment and involves the regular removal of iron-rich blood through a process similar to blood donation.
- Frequency of phlebotomy sessions depends on individual factors like iron levels and severity of iron overload. Initially, sessions might be frequent (weekly or bi-weekly) until iron stores are depleted. As iron levels normalize, the frequency can be reduced to maintain them within the healthy range.
- Benefits:
- Reduces iron overload in the body.
- Prevents or delays organ damage.
Chelation Therapy
- In some cases, chelation therapy might be used in conjunction with phlebotomy or as an alternative if phlebotomy is not tolerated.
- Chelation drugs bind to iron in the bloodstream, forming a complex that is then excreted through the urine.
- This approach is typically reserved for situations where phlebotomy is not feasible due to underlying health conditions or for patients with severe iron overload refractory to phlebotomy alone.
- Potential side effects of chelation therapy include low blood pressure, kidney problems, and gastrointestinal disturbances.
Dietary Management
- Iron restriction is crucial to prevent further iron buildup from dietary sources. This involves:
- Avoiding iron-fortified foods and supplements
- Limiting red meat consumption
- Avoiding cast iron cookware (iron can leach into food)
- Consuming coffee or tea with meals (can inhibit iron absorption)
Importance of Early Diagnosis
- Early diagnosis and consistent treatment can significantly improve the prognosis and prevent organ damage.
- Conversely, delayed diagnosis and untreated iron overload can lead to serious health problems like cirrhosis, heart failure, diabetes, and even death.
Management Strategies
- Regular monitoring: Regular blood tests to assess iron levels, liver function, and other parameters are essential to monitor treatment effectiveness and identify any potential issues. These tests are typically performed every few months initially, with the frequency adjusted based on individual progress.
- Patient education: Understanding the condition, treatment options, and the importance of adherence to therapy is crucial for patients with hereditary hemochromatosis.
- Psychological support: Living with a chronic condition can be challenging. Some hereditary hemochromatosis patients might benefit from psychological counseling to manage any emotional aspects of the disease.
Prognosis
With early diagnosis and consistent treatment, the prognosis for hereditary hemochromatosis is generally positive. Most patients can experience a significant improvement in symptoms, prevent organ damage, and lead normal and healthy lives.
Frequently Asked Questions (FAQs)
What are the 3 types of hemochromatosis?
Hereditary hemochromatosis (HH) is primarily classified into three types:
- Type 1 hereditary hemochromatosis: This is the most common type, caused by mutations in the HFE gene. It’s associated with iron overload in the body’s tissues and organs.
- Type 2 hereditary hemochromatosis: Also known as juvenile hemochromatosis, this type is caused by mutations in the HJV or HAMP genes. It typically presents at a younger age, often before the age of 30, and can lead to more rapid iron overload.
- Type 3 hereditary hemochromatosis: This type is caused by mutations in the TFR2 gene. It’s less common than Type 1 and Type 2, and often results in a milder form of iron overload.
It’s important to note that there are other, rarer forms of hemochromatosis, but these three types are the most commonly recognized.
What is stage 1 of hemochromatosis?
Stage 1 of hemochromatosis is typically characterized by iron overload without any significant symptoms. This early stage often goes unnoticed because the excess iron is still being stored in the body’s organs without causing noticeable damage.
While there may be no overt symptoms at this stage, blood tests can reveal elevated levels of iron in the blood (transferrin saturation) and iron stored in the body (ferritin). Early detection and intervention at this stage are crucial to prevent the progression of hemochromatosis and its associated complications.
What is the life expectancy of someone with hereditary hemochromatosis?
With early diagnosis and treatment, individuals with hereditary hemochromatosis can have a normal life expectancy. The key is to detect and manage the condition before significant organ damage occurs.
However, if hemochromatosis is left untreated or diagnosed late, the excess iron can damage organs like the liver, heart, pancreas, and joints. These complications can significantly impact life expectancy.
What color is your stool if you have hemochromatosis?
The color of your stool is not a reliable indicator of hemochromatosis. While excessive iron can sometimes cause dark or black stools, this can also be due to other factors such as bleeding in the upper digestive tract.
Can you take vitamin B12 if you have hemochromatosis?
Yes, you can take vitamin B12 if you have hemochromatosis. While excessive iron can interfere with the absorption of certain vitamins, including vitamin B12, it is generally safe for individuals with hemochromatosis to supplement with vitamin B12.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Porter JL, Rawla P. Hemochromatosis. [Updated 2023 Mar 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- Murphree CR, Nguyen NN, Raghunathan V, Olson SR, DeLoughery T, Shatzel JJ. Diagnosis and management of hereditary haemochromatosis. Vox Sang. 2020 May;115(4):255-262. doi: 10.1111/vox.12896. Epub 2020 Feb 20. PMID: 32080859.
- Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996 Apr;110(4):1107-19. doi: 10.1053/gast.1996.v110.pm8613000. PMID: 8613000.
- Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002 Mar 1;65(5):853-60. PMID: 11898957.