TL;DR
Acute myeloid leukemia (AML) is an aggressive neoplasm of the myeloid lineage caused by acquired somatic defects due to either environmental influences e.g. chemicals, drugs, radiation or infections e.g.bacteria, protozoa in early hematopoietic blasts that impede or significantly impair differentiation leading to accumulation of undifferentiated blasts in the marrow, spilling into the peripheral blood and infiltrate other tissues. Leukemic blasts are too immature to be functional and are prone to suppress or supplant the production of normal hematopoiesis causing pancytopenia. Peak incidence at 80 years old.
Predisposing factors ▾
- DNA damaging agents e.g. radiotherapy, chemotherapy
- Inherited DNA repair mutations
- Smoking
- Toxins e.g. benzene
- MPN/MDS
Signs and symptoms ▾
- Fatigue
- Gum bleeding
- Fever
- DIC
- Blasts sludging
Laboratory Investigations ▾
- FBC: Normochromic, normocytic anemia with thrombocytopenia and variable leukopenia
- PBF: Presence of myeloblasts
- BMAT: Hypercellular with ≥ 20% myeloblasts
Treatment and Management ▾
- Induction therapy
- Consolidation therapy
- Stem cell transplantation
- Targeted therapy
*Click ▾ for more information
Introduction
Acute myeloid leukemia (AML) encompasses a group of molecularly distinct entities that share a single morphologic feature, the accumulation of undifferentiated blasts of myeloid origin in the bone marrow. It is an aggressive neoplasm caused by acquired somatic defects due to either environmental influences e.g. chemicals, drugs, radiation or infections in early hematopoietic blast cells that block differentiation leading to accumulation of undifferentiated blasts in the marrow, spilling into the peripheral blood and infiltrate other tissues. Leukemia blasts are too immature to be functional and are prone to suppress or supplant the production of normal hematopoiesis causing pancytopenia.
Associated risk factors
While the exact causes of acute myeloid leukemia (AML) remain unclear, certain factors can increase an individual’s likelihood of developing the disease.
Age: Acute myeloid leukemia (AML) incidence increases significantly with age, particularly in adults over the age of 60. This is likely due to age-related changes in the immune system and the accumulation of genetic mutations over time. Acute myeloid leukemia (AML) is the most common acute leukemia in adults which peaks in those over 80 years of age.
Smoking: Smoking cigarettes is a well-established risk factor for acute myeloid leukemia (AML). The chemicals in tobacco smoke can damage DNA and increase the risk of genetic mutations that can lead to acute myeloid leukemia (AML).
Exposure to Radiation: Exposure to high levels of ionizing radiation, such as from X-rays or nuclear accidents, can increase the risk of acute myeloid leukemia (AML). Radiation can damage DNA and increase the risk of genetic mutations that can lead to acute myeloid leukemia (AML).
Previous Cancer Treatment: Individuals who have been treated for other types of cancer, particularly with certain chemotherapy drugs, have an increased risk of developing acute myeloid leukemia (AML). These chemotherapy drugs can damage DNA and increase the risk of genetic mutations that can lead to acute myeloid leukemia (AML).
Chemical Exposure: Exposure to certain chemicals, such as benzene, a component of gasoline and industrial solvents, has been linked to an increased risk of acute myeloid leukemia (AML). Benzene can damage DNA and increase the risk of genetic mutations that can lead to acute myeloid leukemia (AML).
Genetic Predisposition: Individuals with certain genetic conditions, such as Down syndrome and Fanconi anemia, have an increased risk of developing acute myeloid leukemia (AML). These genetic conditions make individuals more susceptible to genetic mutations that can lead to acute myeloid leukemia (AML).
Family History: Individuals with a family history of acute myeloid leukemia (AML) have a slightly increased risk of developing the disease. This suggests that there may be a genetic component to acute myeloid leukemia (AML) susceptibility.
Pre-existing conditions: Individuals with a pre-existing myeloproliferative neoplasm or a myelodysplastic syndrome are at high risk for progression to acute myeloid leukemia (AML).
Acute myeloid leukemia (AML) symptoms
The presentation of acute myeloid leukemia (AML) is similar to ALL with some subtle differences. Most acute myeloid leukemia (AML) symptoms and signs are related to anemia, thrombocytopenia and neutropenia. Acute myeloid leukemia (AML) is more likely to infiltrate soft tissues such as the gums. Myeloid blasts are also larger and stickier than lymphoblasts thus patients with very high blast counts sometimes present with pulmonary or central nervous symptoms due to sludging of blasts in capillary beds. Some patients also present with disseminated intravascular coagulation of variable degree. Bone pain is a frequent acute myeloid leukemia (AML) symptom, caused by the infiltration of leukemic cells into the bone marrow. This pain can be severe and may interfere with daily activities.
What are the laboratory investigations for AML?
Accurate diagnosis and classification of acute myeloid leukemia (AML) are crucial for guiding treatment decisions and prognostication. Laboratory investigations play a pivotal role in achieving these objectives, providing valuable information about the presence, extent, and characteristics of acute myeloid leukemia (AML).
Complete Blood Count (CBC)
A complete blood count (CBC) is a routine blood test that assesses the levels and morphology of various blood cells. In acute myeloid leukemia (AML), the CBC typically reveals:
- Anemia: Decreased red blood cell count (RBC) and hemoglobin (Hb) levels due to the suppression of erythropoiesis in the bone marrow.
- Neutropenia: Decreased neutrophil count, the primary type of white blood cells that fight bacterial infections due to suppression leukopoiesis in the bone marrow
- Thrombocytopenia: Decreased platelet count, responsible for blood clotting, leading to easy bruising and bleeding due to suppression of thrombopoiesis in the bone marrow.
Peripheral Blood Smear
In acute myeloid leukemia (AML), the peripheral blood smear may reveal:
- Blast cells: Immature myeloid cells that are the hallmark of acute myeloid leukemia (AML). The percentage of blast cells in the blood is an important prognostic factor.
- Auer rods: Needle-like structures found in the cytoplasm of some myeloid cells, which can be associated with acute myeloid leukemia (AML).
- Abnormal cell morphology: The presence of abnormal features in mature myeloid cells, such as dysplastic changes, can also suggest acute myeloid leukemia (AML).
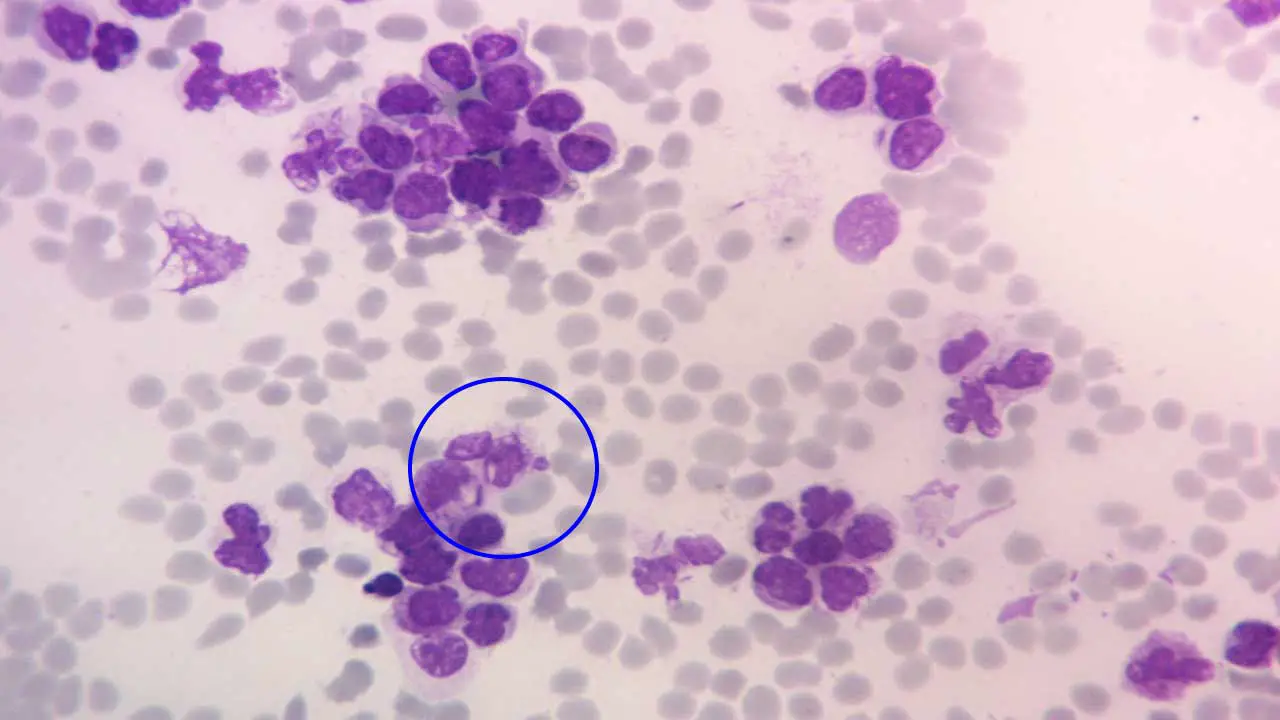
Bone Marrow Aspiration and Biopsy
Bone marrow aspiration involves withdrawing a sample of liquid bone marrow from the posterior iliac crest, while bone marrow biopsy involves removing a small core of bone marrow tissue. These procedures are considered the gold standard for diagnosing and classifying acute myeloid leukemia (AML).
The bone marrow is usually hypercellular, and marrow blasts are typically more than 20% of cells although if certain genetic abnormalities are present, the 20% blast threshold is not necessary for the diagnosis.
Biochemical tests
Other common abnormalities in laboratory tests include hyperuricemia caused by increased cellular turnover, hyperphosphatemia due to cell lysis and hypocalcemia due to progressive bone destruction.
Flow Cytometry
Flow cytometry is a technique that utilizes antibodies to identify and characterize different types of blood cells based on their surface antigens. In acute myeloid leukemia (AML), flow cytometry is used to determine the immunophenotype of the blast cells, which provides important information for acute myeloid leukemia (AML) classification and treatment planning.
Cytogenetic Analysis
Cytogenetic analysis involves examining the chromosomes in bone marrow cells to identify chromosomal abnormalities associated with acute myeloid leukemia (AML). These abnormalities can provide prognostic information and influence treatment decisions.
Fluorescence In Situ Hybridization (FISH)
FISH is a technique that utilizes fluorescent probes to detect specific genetic abnormalities in acute myeloid leukemia (AML) cells. FISH can provide more precise information about certain chromosomal abnormalities than traditional cytogenetic analysis.
Molecular Analysis
Molecular analysis involves detecting specific mutations in genes that play a role in the development of acute myeloid leukemia (AML). These mutations can provide further prognostic information and guide targeted therapy options.
Additional Tests
In some cases, additional tests may be performed to assess the extent of acute myeloid leukemia (AML) and rule out other conditions. These may include:
- Lumbar puncture: To assess the presence of leukemic cells in the cerebrospinal fluid.
- Imaging studies: Chest X-ray, CT scans, or PET scans may be used to detect enlarged lymph nodes, bone lesions, or other manifestations of acute myeloid leukemia (AML).
Cytochemical stains can be used to differentiate ALL and AML
Techniques such as flow cytometry, cytogenetic analysis and molecular testing are now commonly used in the diagnosis of acute leukemias. However, older techniques such as cytochemical stains still retain their importance. An advantage of cytochemical stains is that they are relatively inexpensive and can be performed by many laboratories.
- Myeloperoxidase (MPO) is an enzyme found in the primary granules or granulocytic cells but not found in lymphocytes.
- Sudan black B stains cellular lipids with patterns quite similar to that of MPO to differentiate AML from ALL.
- Esterase reactions are used to differentiate myeloblasts and neutrophilic granulocytes from cells of monocytic origin. Substrate esters commonly used are alpha-naphthyl acetate, alpha-naphthyl butyrate and napthol AS-D chloroacetate.
World Health Organization (WHO) classification
The World Health Organization (WHO) classification of acute myeloid leukemia (AML) is a standardized system for categorizing acute myeloid leukemia (AML) subtypes based on their genetic and morphological characteristics. This classification is essential for guiding treatment decisions, prognostication, and monitoring disease progression.
The Revised WHO Classification of AML
The revised WHO classification of acute myeloid leukemia (AML), published in 2017, introduced significant changes to the previous classification, reflecting advancements in the understanding of acute myeloid leukemia (AML) biology and genetics. The revised classification categorizes acute myeloid leukemia (AML) into six main categories.
AML with recurrent genetic abnormalities
This category encompasses acute myeloid leukemia (AML) subtypes defined by specific chromosomal rearrangements or gene mutations that are associated with distinct clinical features and treatment options.
AML with translocation(8;21)(q22;q22);RUNX1/RUNX1T1 has myeloblasts with dysplastic granular cytoplasm, Auer rods and some maturation, similar to FAB M2 classification. Various anomalies such as pseudo-Pelger Huet cells and hypogranulation can be seen.
AML with inv(16)(p13.1q22) or translocation(16:16)(p13.1;q22);CBFB-MYH11 has myeloblasts, monoblasts and promyelocytes in the peripheral blood and bone marrow. In the bone marrow there may be eosinophilia with dysplastic changes. The incidence of extramedullary disease is higher than most types of acute myeloid leukemia (AML) and the CNS is a common site for relapse.
AML with t(15;17)(q22;q12);PML-RARA is also known as acute promyelocytic leukemia. Characteristic presentations are abnormal hypergranular promyelocytes with some Auer rods. The treatment of APL is significantly different from all other types of acute myeloid leukemia (AML) and therefore it is important to arrive at an accurate diagnosis. Treatment includes all-trans-retinoic acid (ATRA) and arsenic trioxide. ATRA is a vitamin A analogue and induces differentiation of the malignant promyelocytes.
AML with myelodysplasia-related changes (MRC)
This category includes acute myeloid leukemia (AML) subtypes that arise from myelodysplastic syndromes (MDS), a group of disorders characterized by ineffective hematopoiesis and an increased risk of transforming into acute myeloid leukemia (AML).
Acute myeloid leukemia (AML) with myelodysplasia-related changes affects primarily older adults and has a poor prognosis. Significant dysplastic morphology includes pancytopenia with neutrophil hypogranulation or hypergranulation, pseudo-Pelger Huet cells and unusually segmented nuclei. Erythrocyte precursors have vacuoles, karyorrhexis, megaloblastoid features and ringed sideroblasts.
Therapy-related myeloid neoplasms (t-MN)
This category encompasses acute myeloid leukemia (AML) subtypes that are secondary to prior treatment for other cancers, such as chemotherapy or radiation therapy.
AML, not otherwise specified (NOS)
This category includes acute myeloid leukemia (AML) subtypes that do not fit into the other categories due to insufficient information or a lack of distinctive genetic or morphological features but still grouped according to the FAB classification.
Myeloid sarcoma
This category encompasses acute myeloid leukemia (AML) that primarily involves the formation of solid tumors in the skin, bone, or other extramedullary tissues.
Myeloid proliferations related to Down syndrome (DS)
This category includes acute myeloid leukemia (AML) subtypes that are specifically associated with Down syndrome.
French-American-British (FAB) Classification
The French-American-British (FAB) classification of acute myeloid leukemia (AML), developed in the 1970s, was the first widely accepted system for categorizing acute myeloid leukemia (AML) subtypes based on their morphological characteristics. This classification was based on the microscopic appearance of blast cells, the immature myeloid cells that are the hallmark of acute myeloid leukemia (AML). The FAB classification divided acute myeloid leukemia (AML) into eight subtypes, designated as M0 to M7, based on the degree of maturation and differentiation of the blast cells.
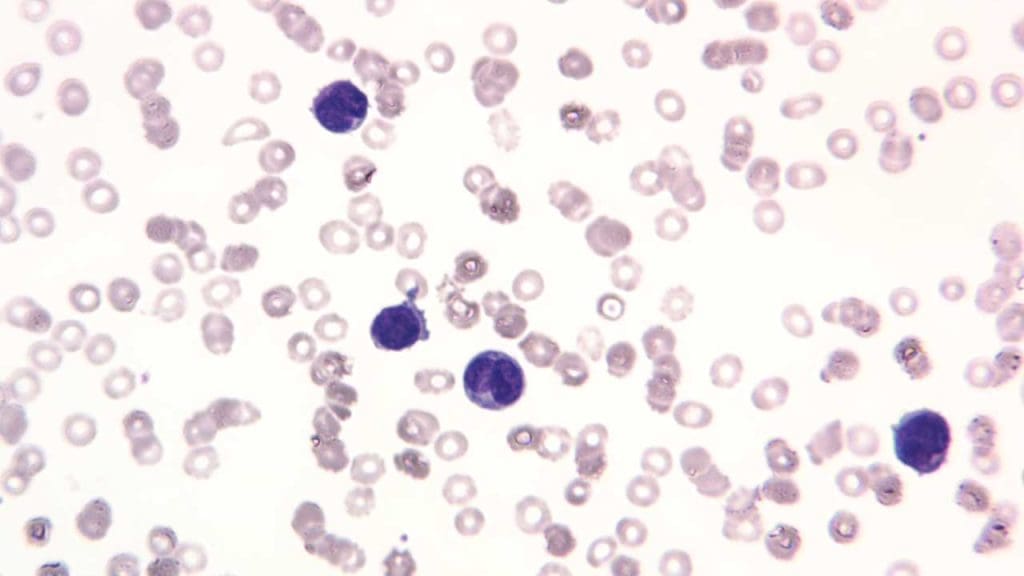
FAB Classification Subtypes
- M0: Minimal differentiation AML. M0 has blasts with CD13+, CD33+, CD11b+, CD11c+, Cd14+ and CD15+. The cells have no clear evidence of cellular maturation and cytochemical stains MPO and Sudan Black B are negative.
- M1: Myeloblastic AML without maturation. M1 is close to M0 in terms of surface markers but the blasts have Auer rods and usually give positive results with MPO and SBB stains and CD13, 33 and CD117 positive.
- M2: Myeloblastic AML with maturation. M2 has greater than 20% blasts, at least 10 % maturing cells of neutrophil lineage and fewer than 20% precursors with monocytic lineage. Auer rods and other aspects of dysplasia are present. MPO and SBB stains positive with CD13 and CD15+.
- M3: Promyelocytic AML (APL). M3 has promyelocytes with abundant and intensely azurophilic granulations. The nucleus is usually monocytic in appearance and immunophenotyping reveals CD13 and CD33+ while HLA-DR and CD34-.
- M4: Myelomonocytic AML. M4 is characterized by a significantly elevated WBC count and the presence of myeloid and monocytoid cells in the peripheral blood and bone marrow. Monocytic cells consisting of monoblasts and promonocytes constitute at least 20% of all marrow cells. The cells are positive for myeloid antigens CD13 and CD33 and the monocytic antigens CD14, CD4, CD11b, CD11c, CD64 and CD36.
- M5: Monocytic AML. Acute monoblastic and monocytic leukaemias are based on the degree of maturity of the monocytic cells present in the marrow and peripheral blood, more than 80% of the marrow cells are of monocytic origin. It has 2 subtypes, the M5a acute monoblastic leukemia and M5b the acute monocytic leukemia. These cells are CD14+, CD4+, CD11b+, CD11c+, CD36+, CD64+ and CD68+. Nonspecific esterase tests are usually positive. This is the most common acute myeloid leukemia (AML) in younger individuals.
- M6: Erythroleukemic AML. Acute erythroid leukemia involves RBC precursors with significant dysplastic features such as multinucleation, megaloblastoid asynchrony and vacuolization. The nucleated RBCs in the peripheral blood may account for more than 50% of the total number of nucleated cells. The immunophenotyping reveals CD13, CD33, CD15, glycophorin A and glycophorin C positive cells.
- M7: Megakaryocytic AML. Patients usually have cytopenias, although some may have thrombocytosis. Dysplastic features are often present in all cell lines. Diagnosis requires the presence of at least 20% blasts of which at least 50% must be of megakaryocytes origin. Megakaryoblasts are identified by immunostaining and employing antibodies specific for cytoplasmic von Willebrand factor or platelet membrane antigens CD41, CD42b or CD61.
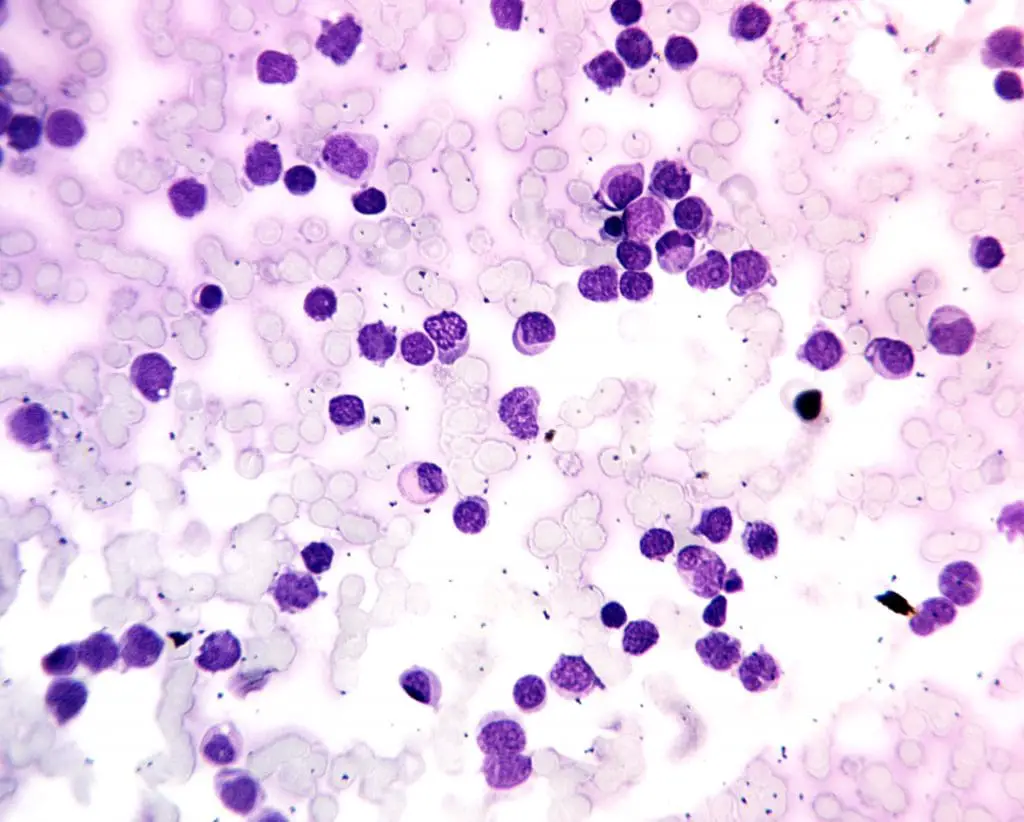
Limitations of the FAB Classification
While the FAB classification provided a valuable framework for understanding acute myeloid leukemia (AML) subtypes, it had several limitations:
- It relied solely on morphological criteria, which can be subjective and vary among different observers.
- It did not account for genetic abnormalities, which play a crucial role in acute myeloid leukemia (AML) classification and treatment planning.
- It did not adequately distinguish between acute myeloid leukemia (AML) subtypes with different clinical outcomes.
Replacement of the FAB Classification
The FAB classification was largely replaced by the World Health Organization (WHO) classification of acute myeloid leukemia (AML) in 2001. The WHO classification incorporated genetic and molecular information alongside morphological criteria, providing a more comprehensive and clinically relevant system for acute myeloid leukemia (AML) classification. However, the FAB classification remains a historical landmark in the development of acute myeloid leukemia (AML) classification and continues to be used in some settings.
Classifications Summary
| WHO | FAB subtype (if any) |
| AML with recurrent genetic abnormalities t(8;21)(q22;q22) inv(16)(p13q22) or t(16;16)(p13;q22) t(15;17)(q22;q12), (PML/RARɑ) and variants 11q23 (MLL) abrnomalities | M2 M4eo M3 |
| AML with multilineage dysplasia Following MDS or MDS/MPD Without a history of MDS or MPD, with dysplasia in at least 50% of cells in 2 or more myeloid lineages | |
| AML and MDS, therapy-related Alkylating agents/radiation-related Topoisomerase-2 inhibitor-related Others | |
| AML, NOS Minimally differentiated Without maturation With maturation Acute myelomonocytic leukemia Acute monoblastic/monocytic leukemia Acute erythroid leukemia Acute megakaryoblastic leukemia Acute basophilic leukemia Acute panmyelosis with myelofibrosis | M0 M1 M4 M5 M6 M7 |
| Myeloid sarcoma | |
| Myeloid proliferations associated with Down syndromeTransient abnormal myelopoiesis (TAM) associated with Down syndromeMyeloid leukemia associated with Down syndrome |
*MDS myelodysplasia syndrome, MPD myeloproliferative disorders
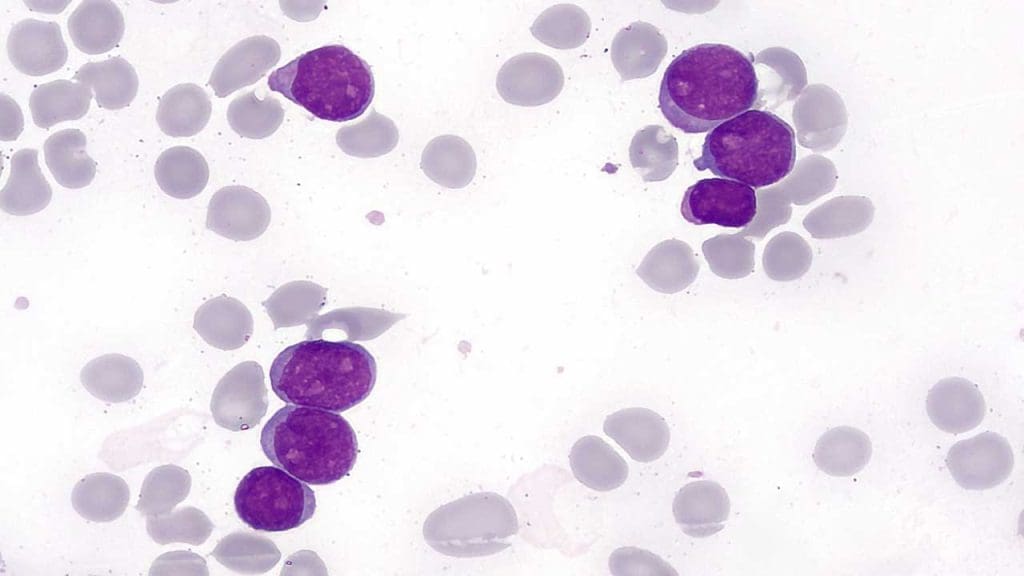
| FAB subtypes | Morphology | Immunopheno-typing | |
| M0 | Minimally differentiated AML | Medium-sized blasts with rounded nucleus and prominent nucleolus. Basophilic agranular cytoplasm | +: 13, 33, 11b, 11c, 14, 15 |
| M1 | AML without maturation | Medium-sized blasts with rounded nucleus and prominent nucleolus. Fine azurophilic granulation or Auer rods in cytoplasm. | +: MPO, 13, 33, 117 +/-: CD34 |
| M2 | AML with maturation | Small to medium-sized blasts with rounded nucleolus. Primary azurophilic granulation or Auer rods in basophilic cytoplasm. | +: MPO, Sudan Black, 13, 15 +/-: CD34, HLA-DR, CD117 |
| M3 | Promyelocytic leukemia | Irregular or bilobed with a deep cleft monocytic-like nucleus. Abundance of deep-staining azurophilic granulation. | +: CD13, CD33 -: CD34, HLA-DR |
| M4 | Acute myelomonocytic leukemia | Large blasts with rounded, kidney-shaped or irregular nucleus and prominent nucleoli. There is variable basophilia. | +: CD13, CD15, CD33, CD11b, CD11c, CD14, CD64, CD4 |
| M5a | Acute monoblastic leukemia | Large blasts with rounded nucleus and 1-3 nucleoli. Moderately large and deep basophilic cytoplasm with occasional Auer rods. | +: CD14, CD4, CD64, CD68, CD11c, HLA-DR |
| M5b | Acute monocytic leukemia | Promonocytes with a rounded or kidney-shaped nucleus. Slightly basophilic granulated cytoplasm with some vacuoles. | |
| M6a | Acute erythroid leukemia with proliferation of mixed blasts | >50% erythroid precursors and ~30% myeloblasts with dyserythropoiesis. | +: CD13, CD33, CD15, Glycophorin A, Glycophorin C |
| M6b | Pure erythroid leukemia | ~80% erythroid precursors and <3% myeloid cells with dyserythropoiesis. | |
| M7 | Acute megakaryocytic leukemia | Variably sized megakaryoblasts with round or indented nucleus and 1 – 3 prominent nucleoli. Agranular, basophilic cytoplasm with pseudopod formation. Platelet dysplasia seen in peripheral blood. | +: CD41, CD61, CD42, CD13, CD33, CD34 |
What is the treatment and management of AML?
Acute myeloid leukemia (AML), a rapidly progressing and aggressive form of blood cancer, requires prompt and intensive treatment to eradicate leukemic cells and restore normal blood cell production. The primary treatment approach for acute myeloid leukemia (AML) is chemotherapy, which aims to kill leukemic cells throughout the body.
Induction Chemotherapy
Induction chemotherapy is the initial phase of treatment, typically administered intravenously for several days or weeks. The goal of induction chemotherapy is to achieve a complete remission (CR), defined as the absence of detectable leukemic cells in the blood and bone marrow. Commonly used induction chemotherapy regimens include:
- Anthracycline-based regimens: These regimens typically combine an anthracycline, such as daunorubicin or idarubicin, with a cytarabine-based drug.
- High-dose cytarabine-based regimens: These regimens utilize high doses of cytarabine, alone or in combination with other drugs.
Consolidation Therapy
For patients who achieve a CR after induction chemotherapy, consolidation therapy is administered to eliminate any remaining leukemic cells and prevent disease relapse. Consolidation therapy typically involves additional cycles of chemotherapy with high-dose cytarabine (ara-C) (sometimes known as HiDAC), often using different combinations of drugs than induction therapy.
Allogeneic Stem Cell Transplantation (ASCT)
Allogeneic stem cell transplantation (ASCT) is a potentially curative treatment for AML patients who are eligible and have a suitable donor. ASCT involves replacing the patient’s diseased bone marrow with healthy stem cells from a donor, usually a sibling or matched unrelated donor. The donor stem cells engraft in the patient’s bone marrow, restoring normal blood cell production and potentially eradicating any remaining leukemic cells.
Targeted Therapy
Targeted therapy utilizes drugs that specifically target certain molecular abnormalities in AML cells. These drugs can be used in combination with chemotherapy or alone in patients with specific genetic markers. Examples of targeted therapies include:
- FLT3 inhibitors: These drugs target the FLT3 gene, which is mutated in approximately 30% of AML patients. This gene helps the cells make a protein (also called FLT3) that helps the cells grow. Drugs in this categories are like Midostaurin (Rydapt) and quizartinib (Vanflyta).
- IDH inhibitors: These drugs target the IDH1 or 2 genes, which are mutated in approximately 20% of AML patients. Mutations in one of these genes can stop blood cells from maturing the way they normally would. Examples of these drugs are Olutasidenib (Rezlidhia) and Enasidenib (Idhifa).
- BCL-2 inhibitors: These drugs target the BCL-2 protein, which helps cancer cells evade apoptosis (programmed cell death). Venetoclax (Venclexta) is an example of a BCL-2 inhibitor.
- Hedgehog pathway inhibitors: The hedgehog pathway is crucial for the development of the embryo and fetus and is important in some adult cells, but it can be overactive in leukemia cells. Glasdegib (Daurismo) is a drug that targets a protein in this pathway.
Challenges and Future Directions
Despite significant advancements in acute myeloid leukemia (AML) treatment, challenges remain in improving long-term survival rates and reducing the risk of relapse. Ongoing research focuses on developing novel therapies that are more effective, less toxic, and tailored to individual patients based on their genetic and molecular characteristics. Immunotherapy, gene therapy, and other innovative approaches are being explored to improve treatment outcomes and patient survival.
Frequently Asked Questions (FAQs)
What is the life expectancy of someone with acute myeloid leukemia?
According to the National Cancer Institute, the 5-year relative survival rate for acute myeloid leukemia (AML) is 29.3%.
Is acute myeloid leukemia curable?
Around two-thirds (60-70%) of adults with AML can achieve complete remission (CR) after initial treatment (induction therapy). Among those who achieve CR, over one-quarter (more than 25%) have a good chance of surviving for at least 3 years, potentially achieving a cure. However, a relapse is a possibility.
What causes acute myeloid leukemia?
The exact cause of acute myeloid leukemia (AML) is not fully understood, but it is known to involve genetic mutations in bone marrow cells. These mutations cause the cells to grow and divide uncontrollably, eventually crowding out healthy blood cells. There are some risk factors that can increase the chances of developing AML that have been discussed in the article above.
What are warning signs of leukemia?
While there is no single definitive warning sign, some common symptoms that might indicate leukemia include:
- Fatigue: This is the most common symptom and can be persistent, not improving with rest.
- Frequent infections: Due to abnormal white blood cells, individuals with leukemia are more prone to infections that are frequent, severe, or last longer than usual.
- Easy bruising and bleeding: This occurs because abnormal blood cells may not function properly in forming clots, leading to easy bruising and bleeding.
- Fever or chills: Unexplained fever or chills can be a sign of infection or underlying conditions like leukemia.
- Unexplained weight loss: Weight loss without trying can be a warning sign of various conditions, including leukemia.
- Shortness of breath: This can occur due to anemia, a common complication of leukemia where the blood lacks enough red blood cells to carry oxygen effectively.
- Pale skin: This can be a sign of anemia, as the body doesn’t have enough red blood cells to deliver oxygen throughout the body.
- Swollen lymph nodes: Lymph nodes are part of the immune system and may swell in response to infection or due to leukemia itself.
- Enlarged spleen or liver: These organs may become enlarged in response to leukemia.
- Bone or joint pain: This can be caused by infiltration of leukemia cells in the bones or by low blood cell counts.
It’s important to understand that these symptoms can also be caused by other medical conditions.
What are the 5 stages of acute myeloid leukemia?
Acute myeloid leukemia (AML) doesn’t have a traditional staging system like some other cancers. This is because AML doesn’t typically form tumors, and staging is often based on tumor size and spread.
Instead of stages, doctors use various terms to describe AML and its progression, including:
- Untreated: This refers to newly diagnosed AML before any treatment begins.
- Active disease: This indicates the presence of active leukemia cells, typically defined as having more than 5% blasts (immature blood cells) in the bone marrow.
- Remission: This signifies the absence of detectable leukemia cells after treatment. There are different levels of remission, such as complete remission (CR) where no blasts are found in the blood or bone marrow, and partial remission where some blasts may be present but significantly reduced.
- Measurable residual disease (MRD): This refers to the presence of minimal amounts of leukemia cells even in remission, detectable through very sensitive tests.
- Relapsed: This occurs when leukemia returns after a period of remission.
Can lifestyle cause leukemia?
Lifestyle choices do not directly cause acute myeloid leukemia (AML). However, certain lifestyle factors can increase the risk of developing AML.
- Smoking: Smokers have a higher risk of AML compared to non-smokers. The specific chemicals in cigarettes are thought to damage the DNA in bone marrow cells, potentially leading to the mutations that cause AML.
- Exposure to certain chemicals: Long-term exposure to benzene (found in gasoline, some dyes, and certain plastics) and other specific chemicals like herbicides and benzene derivatives has been linked to an increased risk of AML. It’s crucial to follow safety guidelines and use appropriate protection when handling such chemicals.
- Radiation exposure: High doses of radiation exposure, such as from atomic bombs or certain medical treatments, can increase the risk of AML.
It’s important to remember that even with these risk factors, most people will not develop AML. Additionally, many individuals with AML have no established risk factors, highlighting the complex and not fully understood nature of the disease.
Can leukemia come on suddenly?
Yes, acute leukemia, including acute myeloid leukemia (AML), can come on suddenly within days or weeks. This is because the abnormal white blood cells in acute leukemia multiply rapidly, causing symptoms to develop quickly. These symptoms can often mimic those of the flu, such as:
- Fatigue
- Fever
- Frequent infections
- Easy bruising and bleeding
Chronic leukemia, on the other hand, typically progresses gradually and may not cause any noticeable symptoms in the early stages. Symptoms like fatigue and weight loss might only become evident as the disease progresses.
Can AML run in families?
AML, or acute myeloid leukemia, can run in families in some rare cases, but it is not generally considered a hereditary disease.
Most cases of AML are not hereditary
- The majority of AML cases (around 90%) are not caused by inherited gene mutations. These cases are thought to arise from spontaneous mutations that occur during a person’s lifetime, potentially due to factors like environmental exposures or random errors during cell division.
However, there are some exceptions
- In rare instances, AML can be linked to inherited genetic mutations or specific genetic syndromes. These mutations or syndromes can increase an individual’s risk of developing AML.
- Examples of such syndromes include Down syndrome, Fanconi anemia, and Li-Fraumeni syndrome.
- Certain inherited gene mutations, such as those in the GATA2, ETV6, CEBPA, and RUNX1 genes, have also been associated with a higher risk of AML.
Family history can be a factor, but not always
- Having a close relative (parent, sibling) with AML can slightly increase your risk of developing the disease. However, this risk is still relatively low, and most people with a family history of AML will not develop it themselves.
- It’s important to remember that family history can also be due to shared environmental factors within the family, not necessarily through inherited genes.
Is AML painful?
Acute myeloid leukemia (AML) itself does not directly cause pain, but some symptoms and complications associated with AML can be painful.
No direct pain from AML
- AML affects the blood and bone marrow, not directly causing pain in tissues or organs.
Painful symptoms and complications
- Bone or joint pain: This can be a common symptom in some AML patients, caused by the abnormal growth of leukemia cells in the bone marrow, putting pressure on nerves or causing inflammation. The pain can be dull or sharp and may worsen at certain times.
- Mouth sores and inflammation: These can cause discomfort and pain, especially during eating or speaking.
- Swollen lymph nodes: While not always painful, enlarged lymph nodes due to AML can sometimes cause discomfort or pressure in the affected area.
- Infections: Individuals with AML are more prone to infections due to compromised immune function. These infections themselves can cause pain in various locations depending on the type and location of the infection.
Does AML spread quickly?
Yes, acute myeloid leukemia (AML) is generally considered to be an aggressive cancer that can spread quickly within the body.
How does acute myeloid leukemia cause death?
Acute myeloid leukemia (AML) can be fatal, and the cause of death depends on various factors like the severity of the disease, response to treatment, and complications that arise. Here are some of the ways AML can lead to death:
- Infections: AML disrupts the production of healthy white blood cells, which are crucial for fighting infections. This weakened immune system makes individuals with AML more susceptible to serious infections. These infections can be bacterial, fungal, or viral and can overwhelm the body, potentially leading to organ failure and death.
- Bleeding complications: AML can affect the production of platelets, which are necessary for blood clotting. This can lead to easy bruising, bleeding, and uncontrolled internal bleeding. Severe bleeding, especially in critical areas like the brain, can be life-threatening.
- Organ failure: The infiltration of abnormal leukemia cells can damage various organs, including the liver, lungs, and kidneys. This damage can eventually lead to organ failure, which can be fatal.
- Progression of the disease: In some cases, despite treatment, AML can progress and become resistant to therapy. This uncontrolled growth of leukemia cells can ultimately lead to death.
It’s important to understand that these are some of the possible causes of death in AML, and the specific course of the disease can vary from person to person. Early diagnosis and aggressive treatment are crucial to improve the chances of successful treatment and minimize the risk of complications that can be fatal.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020 Jul 31;55(S1):S1-S4. doi: 10.5045/br.2020.S001. PMID: 32719169; PMCID: PMC7386892.
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian RP, Larson RA, Levine RL, Miyazaki Y, Niederwieser D, Ossenkoppele G, Röllig C, Sierra J, Stein EM, Tallman MS, Tien HF, Wang J, Wierzbowska A, Löwenberg B. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022 Sep 22;140(12):1345-1377. doi: 10.1182/blood.2022016867. PMID: 35797463.
- Ishii H, Yano S. New Therapeutic Strategies for Adult Acute Myeloid Leukemia. Cancers (Basel). 2022 Jun 5;14(11):2806. doi: 10.3390/cancers14112806. PMID: 35681786; PMCID: PMC9179253.
- Kantarjian, H., Kadia, T., DiNardo, C. et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 11, 41 (2021). https://doi.org/10.1038/s41408-021-00425-3.
- Sekeres MA. When Blood Breaks Down: Life Lessons from Leukemia (Mit Press). 2021
- Bhushan B. AML (Acute Myeloid Leukemia): A survival guide for patients. 2021.
- Greiner J. Immunotherapies for Acute Myeloid Leukemia (MDPI AG). 2020.
- Röllig C, Ossenkoppele GJ.Acute Myeloid Leukemia (Hematologic Malignancies) (Springer). 2021.
- National Cancer Institute. Acute Myeloid Leukemia Treatment (PDQ®)–Health Professional Version