Introduction
A Coagulation Screening Panel (Coagulation Panel) is a group of blood tests commonly ordered together to assess a person’s blood clotting ability. Its primary purpose is to provide a broad overview of the hemostatic system, which is the complex process that stops bleeding and prevents excessive blood loss.
Brief Overview of Hemostasis & Coagulation
Definition of Hemostasis
Hemostasis is the physiological process that stops bleeding (hemorrhage) at the site of vascular injury while maintaining blood fluidity within the intact circulatory system. It is a tightly regulated process involving a complex interplay of blood vessels, platelets, and coagulation factors. The ultimate goal of hemostasis is to form a stable clot to seal the damaged vessel, followed by the orderly removal of the clot once the vessel wall has healed. This dynamic process is essential for life, protecting the body from both excessive blood loss and unwanted clot formation.
Overview of the Coagulation Cascade
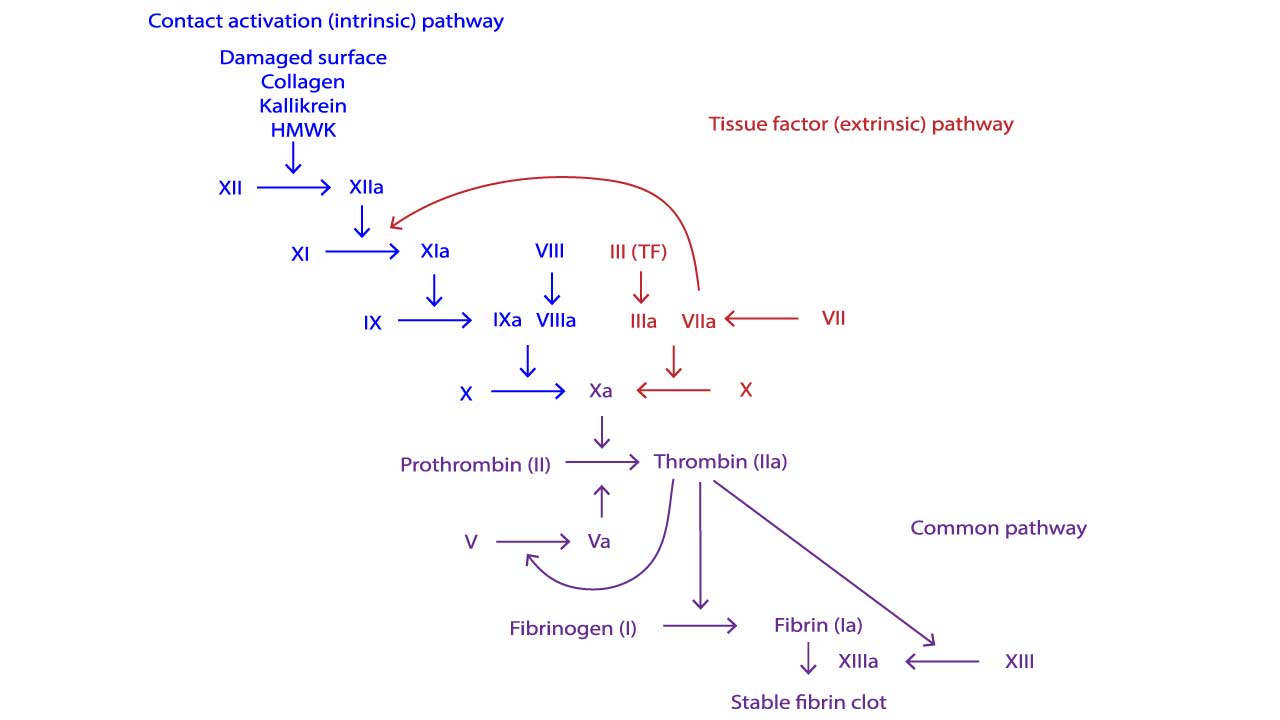
The coagulation cascade leads to the formation of a stable fibrin clot. It is a sequential series of enzymatic reactions involving various coagulation factors. Traditionally, the cascade is divided into three main pathways:
- Extrinsic Pathway: This pathway is initiated rapidly upon vascular injury when blood comes into contact with Tissue Factor (TF), a protein expressed by cells outside the vasculature (e.g., fibroblasts in the vessel wall). TF binds to Factor VIIa, forming a complex that directly activates Factor X and Factor IX. This pathway is considered the primary physiological initiator of coagulation in vivo.
- Intrinsic Pathway: This pathway is activated when blood comes into contact with negatively charged surfaces, such as exposed collagen at a site of vascular injury. It involves a series of contact activation factors (Factor XII, Factor XI, prekallikrein, high molecular weight kininogen) that ultimately activate Factor IX. The intrinsic pathway then proceeds with Factor IXa activating Factor X (in conjunction with Factor VIIIa).
- Common Pathway: Both the extrinsic and intrinsic pathways converge on the common pathway. This pathway begins with the activation of Factor X (to Factor Xa). Factor Xa, along with its cofactor Factor Va (activated Factor V), forms the prothrombinase complex. This complex converts prothrombin (Factor II) into thrombin (Factor IIa). Thrombin is a pivotal enzyme with multiple roles: (1) it converts fibrinogen (Factor I) into fibrin monomers; (2) activates Factor XIII (which cross-links fibrin to stabilize the clot); and (3) amplifies the cascade by activating Factors V, VIII, and XI. The fibrin monomers spontaneously polymerize to form a soft fibrin clot, which is then stabilized by Factor XIIIa into a strong, cross-linked fibrin meshwork.
Importance of Balanced Hemostasis (Prevention of Bleeding and Thrombosis)
The hemostatic system operates on a delicate balance. Maintaining this balance is critically important for human health.
- Prevention of Bleeding (Hemorrhage): An underactive or deficient hemostatic system leads to excessive bleeding, which can range from minor bruising to life-threatening hemorrhage. Conditions like hemophilia (deficiency in clotting factors) or severe thrombocytopenia (low platelet count) exemplify this side of the imbalance.
- Prevention of Thrombosis (Pathological Clotting): An overactive or dysregulated hemostatic system can lead to the inappropriate formation of blood clots (thrombosis) within intact blood vessels. These clots can block blood flow, causing serious conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (heart attack), or stroke. Conditions like Factor V Leiden mutation or antiphospholipid syndrome predispose individuals to thrombosis.
A healthy hemostatic system ensures that clots form only when and where they are needed (at the site of injury) and are subsequently dissolved once their purpose is served, thereby preventing both excessive bleeding and dangerous clotting.
Rationale for Coagulation Screening Panel (Coagulation Panel)
Given the complexity and critical balance of the hemostatic system, accurately assessing its function is very important. The purpose of a coagulation screening panel (coagulation panel) extends beyond simply measuring clotting times as it is designed to offer a multifaceted assessment of a patient’s hemostatic capabilities. Coagulation screening panels (coagulation panels) serve as essential diagnostic tools for several key reasons.
Initial Assessment of Hemostatic Function and Detection of Bleeding Disorders
The primary and most frequent purpose of the coagulation screening panel (coagulation panel) is to quickly evaluate if a patient has a tendency to bleed abnormally. This could be due to an inherited condition (like hemophilia, which causes a deficiency in specific clotting factors) or an acquired one (such as liver disease, which impairs the production of many clotting factors, or severe vitamin K deficiency).
By assessing the extrinsic (PT/INR) and intrinsic (aPTT) pathways, and evaluating platelet count, the panel helps pinpoint which part of the complex coagulation cascade might be dysfunctional, guiding further, more specific diagnostic tests. For instance, an isolated prolonged aPTT might suggest an intrinsic pathway defect, pointing towards hemophilia A or B.
Pre-operative Risk Assessment
Before any surgical procedure, especially those involving significant blood loss, it’s crucial to know if a patient is at an increased risk of bleeding. The coagulation screening panel (coagulation panels) helps identify individuals who might require prophylactic measures (e.g., administering clotting factors) or whose surgery might need to be delayed until a clotting abnormality is addressed. This is a critical step in ensuring patient safety during invasive procedures.
Monitoring Anticoagulant Therapy
Many patients take anticoagulant medications (often referred to as “blood thinners”) to prevent dangerous blood clots. The coagulation screening panels (coagulation panels) are essential for monitoring the effectiveness and safety of these drugs.
- Warfarin (an oral anticoagulant): The PT/INR is the standard test for monitoring warfarin therapy. It helps ensure that the patient’s blood is thin enough to prevent clots but not so thin that it causes excessive bleeding. Dosing adjustments are made based on INR results.
- Unfractionated Heparin (an intravenous anticoagulant): The aPTT is commonly used to monitor unfractionated heparin. It ensures that the patient is receiving a therapeutic dose to prevent clot formation without increasing the risk of hemorrhage.
While the standard coagulation screening panel (coagulation panel) may not monitor newer anticoagulants directly, understanding its basics is foundational before moving to specific assays for those.
Evaluation of Liver Disease
The liver is the primary site of synthesis for most coagulation factors (including Factors I, II, V, VII, IX, X, XI, XII, and XIII). Therefore, significant liver dysfunction or failure can lead to impaired production of these factors. This results in a prolonged PT/INR and often aPTT.
Abnormal results on a coagulation screening panel (coagulation panel) can thus serve as indicators of the severity of liver disease and its impact on hemostasis.
Diagnosis and Management of Disseminated Intravascular Coagulation (DIC)
DIC is a life-threatening condition characterized by widespread, uncontrolled activation of the coagulation system, leading to widespread microvascular clotting. This is followed by the consumption of clotting factors and platelets, ultimately resulting in paradoxical bleeding.
A coagulation screening panel (along with D-dimer and fibrinogen) is crucial for the diagnosis and monitoring of DIC, as it typically shows prolongation of both PT/INR and aPTT, and a decrease in platelet count.
Investigation of Unexplained Bleeding or Thrombotic Events
When a patient presents with symptoms such as easy bruising, frequent nosebleeds, prolonged bleeding from minor cuts, or, conversely, unexplained blood clots (like deep vein thrombosis or pulmonary embolism), a coagulation screening panel (coagulation panel) is often among the first tests ordered. While it’s a “screening” tool, its results help narrow down the diagnostic possibilities and direct further, more specialized investigations (e.g., specific factor assays, platelet function tests, thrombophilia screens).
Components of a Standard Coagulation Screening Panel (Coagulation Panel) and Their Uses
Prothrombin Time (PT) and International Normalized Ratio (INR)
Definition and Principle
The Prothrombin Time (PT) is a laboratory test that measures the time it takes for a clot to form in a sample of blood plasma after the addition of an exogenous activator, tissue factor (thromboplastin), and calcium. The PT specifically assesses the integrity and function of the coagulation factors involved in the extrinsic pathway (Factor VII) and the common pathway (Factors X, V, II [prothrombin], and I [fibrinogen]).
A blood sample is collected into a tube containing sodium citrate, which acts as an anticoagulant by chelating calcium ions. The plasma is then separated from the blood cells. In the laboratory, an excess of tissue factor (thromboplastin) and calcium chloride are added to the plasma. The tissue factor bypasses the intrinsic pathway and directly activates Factor VII (extrinsic pathway). Calcium is essential for the coagulation cascade to proceed. The time taken for the first visible fibrin clot to form is measured, typically in seconds.
The International Normalized Ratio (INR) was developed to standardize PT results across different laboratories, as variations in the sensitivity of tissue factor reagents can lead to different PT values for the same plasma sample. The INR is a mathematical calculation derived from the PT.
INR=(Patient’s PT/Mean Normal PT)ISI
Where:
- Patient’s PT is the measured prothrombin time of the patient’s plasma.
- Mean Normal PT is the geometric mean of PTs from a reference population of healthy individuals, typically established by each laboratory.
- ISI (International Sensitivity Index) is a value assigned to each batch of tissue factor reagent by its manufacturer, indicating its sensitivity compared to an international reference thromboplastin.
The INR allows for consistent interpretation of PT results regardless of the specific reagent or instrument used, which is particularly critical for monitoring anticoagulant therapy.
Clinical Uses
The PT and INR are part of the coagulation screening panel (coagulation panel) as they are invaluable diagnostic tools with several important clinical applications.
- Assessment of the Extrinsic and Common Pathways: The PT is the primary screening test for deficiencies or dysfunctions in Factor VII (extrinsic pathway) and Factors X, V, II, and I (common pathway). A prolonged PT suggests an issue in one or more of these factors. This can be due to inherited deficiencies (e.g., rare Factor VII deficiency) or, more commonly, acquired conditions.
- Monitoring Warfarin Therapy: This is arguably the most crucial and widespread use of the PT/INR. Warfarin is an oral anticoagulant that inhibits the synthesis of vitamin K-dependent clotting factors (Factors II, VII, IX, and X). Since Factor VII has the shortest half-life among these factors, PT/INR is particularly sensitive to the early effects of warfarin. Regular INR monitoring is essential to:
- Ensure the patient is within the therapeutic range (e.g., INR 2.0-3.0 for most indications) to prevent clot formation (e.g., in atrial fibrillation, prosthetic heart valves, DVT/PE).
- Avoid excessive anticoagulation, which could lead to severe bleeding complications.
- Evaluation of Liver Disease: The liver synthesizes many coagulation factors, including all those measured by the PT (Factors II, V, VII, X, I). Therefore, a prolonged PT/INR is a sensitive indicator of significant hepatocellular dysfunction or liver failure. As liver disease progresses, the liver’s ability to produce these factors diminishes, leading to an increased PT/INR. This makes the PT/INR a valuable prognostic marker and a tool for assessing the severity of liver disease.
- Vitamin K Deficiency: Vitamin K is essential for the gamma-carboxylation of Factors II, VII, IX, and X, a process critical for their functional activity. Deficiency of vitamin K (e.g., due to malnutrition, malabsorption, prolonged antibiotic use, or obstructive jaundice) leads to the production of inactive clotting factors, resulting in a prolonged PT/INR. PT is more sensitive to vitamin K deficiency than aPTT because Factor VII (part of the extrinsic pathway) has the shortest half-life among the vitamin K-dependent factors.
- Disseminated Intravascular Coagulation (DIC): DIC is a life-threatening condition characterized by widespread activation of the coagulation system, leading to the consumption of clotting factors and platelets. As clotting factors in both the extrinsic and common pathways are consumed, the PT/INR typically becomes prolonged in DIC. It is often prolonged along with aPTT and associated with thrombocytopenia and elevated D-dimer.
Interpretation of Results (Prolonged PT/INR)
A prolonged PT/INR (a value higher than the established reference range, or above the therapeutic target for warfarin) indicates that the blood is taking longer than normal to clot via the extrinsic and common pathways. This can be due to:
- Deficiency or dysfunction of one or more coagulation factors in the extrinsic or common pathway:
- Factor VII deficiency (congenital or acquired)
- Deficiency of Factors X, V, II (prothrombin), or I (fibrinogen)
- Warfarin therapy: INR values are deliberately kept elevated in therapeutic ranges to prevent clotting.
- Liver disease: Impaired synthesis of clotting factors.
- Vitamin K deficiency: Inability to produce functional vitamin K-dependent factors.
- Disseminated Intravascular Coagulation (DIC): Consumption of clotting factors.
- Presence of inhibitors: Circulating anticoagulants (e.g., specific factor inhibitors or lupus anticoagulant, though lupus anticoagulant predominantly affects aPTT, it can also weakly prolong PT in some cases).
- Analytical errors: Improper sample collection (e.g., underfilled tube leading to excess citrate, which dilutes calcium), sample hemolysis, or delayed processing.
A shortened PT is rare and typically not clinically significant on its own, often due to pre-analytical issues (e.g., clotted sample) or sometimes in the very early stages of a thrombotic process or severe inflammation (due to increased levels of Factor VIII and fibrinogen acting as acute phase reactants, or high levels of procoagulant factors).
Interpreting a prolonged PT/INR always requires considering the patient’s clinical history, current medications, and other laboratory test results (e.g., aPTT, platelet count, liver function tests) to determine the underlying cause and guide appropriate management.
Activated Partial Thromboplastin Time (aPTT)
Definition and Principle
The Activated Partial Thromboplastin Time (aPTT) is a laboratory test that measures the time it takes for a clot to form in blood plasma after the addition of an activator (e.g., silica, kaolin, ellagic acid), a “partial” thromboplastin (phospholipid), and calcium. It is specifically designed to assess the integrity and function of the coagulation factors involved in the intrinsic pathway (Factors XII, XI, IX, VIII, prekallikrein, high molecular weight kininogen) and the common pathway (Factors X, V, II [prothrombin], and I [fibrinogen]).
Similar to the PT, a blood sample is collected into a sodium citrate tube to prevent clotting. The plasma is then separated. In the laboratory, the plasma is incubated with a negatively charged activator (to activate Factor XII and the intrinsic pathway contact factors) and a partial thromboplastin. After a short incubation period, calcium chloride is added. The calcium reverses the citrate’s anticoagulant effect, allowing the coagulation cascade to proceed. The time taken for the first visible fibrin clot to form is measured, typically in seconds. The “partial” thromboplastin distinguishes it from the full thromboplastin used in the PT, which also contains tissue factor.
Clinical Uses
The aPTT is a crucial test with several key clinical applications.
- Assessment of the Intrinsic and Common Pathways: The aPTT is the primary screening test for deficiencies or dysfunctions of coagulation factors in the intrinsic pathway (Factors XII, XI, IX, VIII) and the common pathway (Factors X, V, II, I). A prolonged aPTT indicates an issue in one or more of these factors. This makes it a valuable initial step in investigating a wide range of bleeding disorders.
- Monitoring Heparin Therapy (Unfractionated Heparin): This is one of the most critical uses of the aPTT. Unfractionated heparin (UFH) exerts its anticoagulant effect primarily by binding to and activating antithrombin, which then inactivates thrombin (Factor IIa) and Factor Xa, and to a lesser extent, Factors IXa, XIa, and XIIa. Since UFH inhibits factors in both the intrinsic and common pathways, the aPTT is highly sensitive to its anticoagulant effect. Regular aPTT monitoring is essential to:
- Ensure the patient is receiving a therapeutic dose of UFH to prevent clot extension or new clot formation (e.g., in DVT, PE, acute coronary syndromes).
- Avoid excessive anticoagulation, which could lead to severe bleeding complications.
- Detection of Hemophilia A and B (Factor VIII and IX deficiencies): Hemophilia A (Factor VIII deficiency) and Hemophilia B (Factor IX deficiency) are the most common severe inherited bleeding disorders. Both Factor VIII and Factor IX are crucial components of the intrinsic pathway. Therefore, even moderate deficiencies in these factors will typically lead to a significantly prolonged aPTT. A prolonged aPTT in a patient with a bleeding tendency strongly suggests these conditions, necessitating further specific factor assays for definitive diagnosis.
- Von Willebrand Disease (Severe Forms Affecting Factor VIII): Von Willebrand factor (vWF) is essential for primary hemostasis (platelet adhesion) and also serves as a carrier protein that protects Factor VIII from degradation. In severe forms of von Willebrand disease (particularly types 2N, 3, and some severe type 1 cases), Factor VIII levels can be significantly reduced due to impaired vWF function. This secondary deficiency of Factor VIII can result in a prolonged aPTT.
- Presence of Lupus Anticoagulant (Antiphospholipid Syndrome): The lupus anticoagulant (LA) is an autoantibody that paradoxically prolongs phospholipid-dependent coagulation tests, most notably the aPTT, in vitro. Despite prolonging the aPTT in the lab, LA is associated with an increased in vivo risk of thrombosis (clotting), not bleeding. When an unexplained prolonged aPTT is observed, especially in a patient with a history of thrombotic events or recurrent miscarriages, the presence of LA should be investigated. Specific tests are then used to confirm LA, as a simple prolonged aPTT in a coagulation screening panel (coagulation panel) is not diagnostic.
- Disseminated Intravascular Coagulation (DIC): Widespread activation of coagulation leading to the consumption of clotting factors from both the intrinsic and common pathways is prominent in DIC. As these factors are consumed, the aPTT will typically become prolonged, often along with a prolonged PT/INR and reduced platelet count.
Interpretation of Results (Prolonged aPTT)
A prolonged aPTT (a value higher than the established reference range) indicates that the blood is taking longer than normal to clot via the intrinsic and common pathways. This can be due to:
- Deficiency or dysfunction of one or more coagulation factors in the intrinsic or common pathway:
- Deficiencies in Factors XII, XI, IX, or VIII.
- Deficiencies in Factors X, V, II, or I (fibrinogen). Note that severe deficiencies in common pathway factors will also prolong PT.
- Heparin therapy (unfractionated heparin): The most common cause of a prolonged aPTT in hospitalized patients.
- Presence of an inhibitor:
- Specific factor inhibitors e.g., Factor VIII inhibitor in acquired hemophilia.
- Lupus Anticoagulant (LA): As discussed above, these antibodies prolong phospholipid-dependent tests in vitro but are associated with thrombosis in vivo.
- Severe liver disease: Impaired synthesis of factors in both intrinsic and common pathways.
- Severe Vitamin K deficiency: Though PT is more sensitive, severe vitamin K deficiency can also prolong aPTT (due to deficiency of Factors IX, X, II).
- Disseminated Intravascular Coagulation (DIC): Consumption of factors.
- Analytical errors: Similar to PT, improper sample collection (e.g., underfilled tube, difficult draw, contamination) or processing can affect results.
A shortened aPTT is uncommon and generally not clinically significant. It can sometimes be seen in the early stages of DIC or in patients with acute phase reactant elevation (e.g., Factor VIII is an acute phase reactant).
When a prolonged aPTT is detected, further investigation, often involving a mixing study (or correction study), is performed to differentiate between a factor deficiency (which will usually correct when mixed with normal plasma) and the presence of an inhibitor (which typically will not correct or will correct only partially over time).
Platelet Count
Definition and Principle
The Platelet Count (Plt) in a coagulation screening panel (coagulation panel) is a quantitative measure of the number of platelets (thrombocytes) present in a given volume of whole blood. Platelets are small, anucleated cell fragments derived from megakaryocytes in the bone marrow. They play a pivotal role in primary hemostasis, the initial phase of blood clot formation, by forming a temporary plug at the site of vascular injury.
Blood is typically collected into a tube containing the anticoagulant EDTA. The platelet count is most commonly performed using automated hematology analyzers. The number of platelets is then reported as cells per microliter (μL), per liter (L), or per cubic millimeter (mm3) of blood.
While the platelet count quantifies the number of platelets, it does not directly assess their function. However, an adequate number of functionally normal platelets is essential for effective primary hemostasis.
Clinical Uses
The platelet count in the coagulation screening panel (coagulation panel) is a fundamental component of the complete blood count (CBC) and is crucial for evaluating hemostasis and identifying various hematological conditions.
- Assessment of Primary Hemostasis: Platelets are the first responders to vascular injury. They adhere to the exposed subendothelial collagen, activate, and aggregate to form a loose platelet plug. An insufficient number of platelets (thrombocytopenia) or dysfunctional platelets (thrombocytopathy, not directly assessed by count) impairs this initial plug formation leading to bleeding symptoms particularly mucocutaneous bleeding (e.g., petechiae, purpura, nosebleeds, gum bleeding). Therefore, the platelet count is an essential indicator of the potential for effective primary hemostasis.
- Detection of Thrombocytopenia: Thrombocytopenia refers to a platelet count below the normal reference range (typically <150×109/L). It is a common cause of bleeding and can arise from various mechanisms:
- Decreased production: Bone marrow failure (e.g., aplastic anemia), leukemia, chemotherapy, radiation, nutritional deficiencies (e.g., B12, folate), viral infections (e.g., HIV, parvovirus B19), or alcohol abuse.
- Increased destruction/consumption:
- Immune Thrombocytopenia (ITP): Autoimmune destruction of platelets.
- Thrombotic Thrombocytopenic Purpura (TTP): Microangiopathic hemolytic anemia with severe platelet consumption and organ damage.
- Hemolytic Uremic Syndrome (HUS): Similar to TTP but often triggered by Shiga toxin-producing E. coli.
- Drug-induced thrombocytopenia: Certain medications (e.g., heparin-induced thrombocytopenia (HIT), quinine, some antibiotics) can cause immune-mediated or direct platelet destruction.
- Disseminated Intravascular Coagulation (DIC): Widespread activation of coagulation leads to consumption of platelets (along with clotting factors).
- Sequestration: Enlarged spleen (splenomegaly) can sequester a significant portion of the body’s platelet pool, leading to a lower circulating count.
- Dilution: Massive transfusions of red blood cells or fluids that lack platelets can dilute the circulating platelet count.
- Detection of Thrombocytosis: Thrombocytosis (or thrombocythemia) refers to a platelet count above the normal reference range (typically >450×109/L). The primary concern of thrombocytosis is the increased risk of thrombosis. Thrombocytosis can be classified as:
- Reactive (Secondary) Thrombocytosis: The most common type, occurring in response to another underlying condition. It is typically benign and carries a lower thrombotic risk than essential thrombocythemia. Causes include acute and chronic infections, inflammation (e.g., inflammatory bowel disease, rheumatoid arthritis), splenectomy (removal of the spleen leads to a transient or persistent rise as platelets are no longer sequestered), trauma, and malignancy.
- Primary (Essential) Thrombocytosis: A myeloproliferative neoplasm characterized by autonomous overproduction of platelets by abnormal megakaryocytes in the bone marrow. This type carries a significantly higher risk of both thrombotic (arterial and venous) and hemorrhagic complications.
- Pre-operative Screening: A platelet count is routinely included in pre-operative assessments to ensure the patient has an adequate number of platelets to form a primary plug and minimize the risk of excessive bleeding during and after surgery.
Interpretation of Results (Low/High Platelet Count)
Low Platelet Count (Thrombocytopenia)
- Counts between 100−150×109/L are generally mild and usually asymptomatic, though patients with other hemostatic defects might have increased bleeding risk.
- Counts between 50−100×109/L may cause mild bleeding (e.g., easy bruising, prolonged bleeding from minor cuts).
- Counts between 20−50×109/L are associated with a moderate risk of spontaneous bleeding, particularly mucocutaneous bleeding.
- Counts below 20×109/L are associated with a high risk of spontaneous, clinically significant bleeding, including potentially life-threatening intracranial hemorrhage.
High Platelet Count (Thrombocytosis)
Clinical management depends on the cause (reactive vs. primary) and the associated thrombotic/hemorrhagic risk.
- Mild to moderate increases (450−800×109/L) are often reactive and may not require specific treatment, though the underlying cause should be addressed.
- Marked increases (e.g., >1000×109/L) are more concerning, especially in primary thrombocytosis, due to the increased risk of both thrombosis and, paradoxically, bleeding (due to acquired von Willebrand disease, where the very high number of platelets consume vWF).
In all cases, abnormal platelet counts require careful clinical correlation, assessment of symptoms, and often further investigation to determine the underlying etiology and guide appropriate management.
Summary of Coagulation Screening Panel Interpretation (Coagulation Panel)
The interpretation of a coagulation screening panel involves a systematic approach, moving from individual test results to their combined patterns, and finally, integrating them with the patient’s clinical context.
General Principles of Interpretation
Always Consider the Clinical Context
- Patient Symptoms: Is the patient presenting with bleeding (e.g., easy bruising, prolonged bleeding after injury/surgery, petechiae, hemarthroses, menorrhagia) or thrombotic events (e.g., DVT, PE, stroke)?.
- Medical History: Does the patient have a history of liver disease, kidney disease, malignancy, autoimmune conditions, or a family history of bleeding or clotting disorders?
- Medications: Is the patient on any anticoagulant drugs (warfarin, heparin, DOACs) or other medications that could affect coagulation (e.g., certain antibiotics, antiplatelet agents)? This is often the most straightforward explanation for abnormal results.
- Recent Events: Has the patient recently had trauma, surgery, sepsis, or massive transfusions? These can all significantly impact coagulation.
Understand the Pathways Assessed by Each Test
- PT/INR: Primarily evaluates the extrinsic pathway (Factor VII) and the common pathway (Factors X, V, II, I). Think of it as assessing how quickly coagulation is initiated from outside the bloodstream.
- aPTT: Primarily evaluates the intrinsic pathway (Factors XII, XI, IX, VIII) and the common pathway (Factors X, V, II, I). Think of it as assessing the amplification phase of coagulation.
- Platelet Count: Assesses the quantity of platelets, which are critical for primary hemostasis (forming the initial plug).
Identify if the Abnormality is Isolated or Combined
- An abnormality in only one test (e.g., prolonged aPTT, normal PT/INR) suggests a defect specific to that pathway.
- Abnormalities in multiple tests (e.g., prolonged PT/INR and aPTT) point towards a defect in the common pathway or a systemic consumption of factors (like DIC).
- An abnormal platelet count in isolation suggests a primary platelet disorder.
Reference Ranges
Always refer to the specific laboratory’s normal reference ranges, as these can vary slightly based on reagents, instrumentation, and local population.
Typical approximate normal ranges are:
- PT: 10-14 seconds
- INR: 0.8-1.2 (for non-anticoagulated patients)
- aPTT: 25-40 seconds
- Platelet Count: 150 – 450×109/L (or 150,000 – 450,000 /uL)
Common Patterns of Interpretation
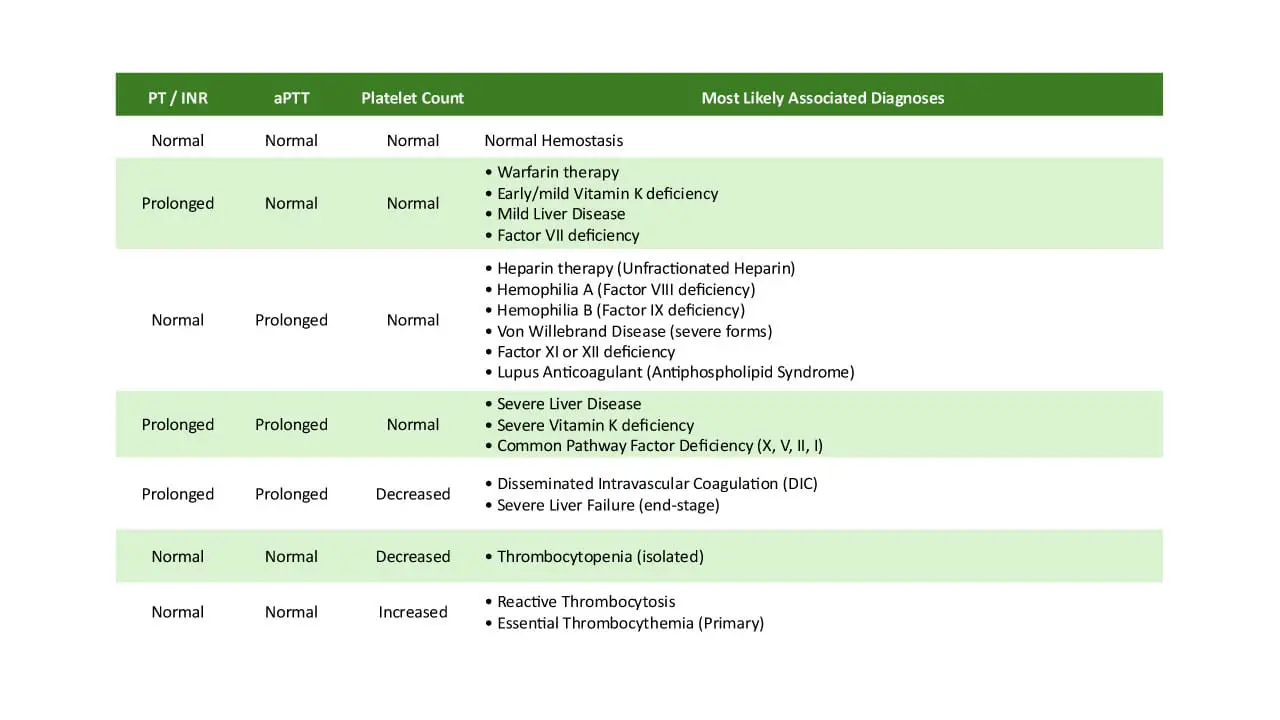
Normal PT/INR, Normal aPTT, Normal Platelet Count
- Interpretation: Indicates an intact coagulation system, as far as these screening tests can tell.
- Caveat: Does not rule out all bleeding disorders! Mild factor deficiencies, specific platelet function disorders (e.g., Glanzmann thrombasthenia, Bernard-Soulier syndrome), mild von Willebrand disease, or vessel wall abnormalities can cause bleeding even with normal screening tests. Further investigation might be needed if clinical suspicion remains high. It also doesn’t rule out hypercoagulable states.
Prolonged PT/INR, Normal aPTT, Normal Platelet Count
- Interpretation: Suggests a problem primarily in the extrinsic pathway, affecting Factor VII, or a mild defect in the common pathway that is more sensitively picked up by PT.
- Thought Process:
- Medication? Always first consider warfarin therapy. If the patient is on warfarin, is the INR within the therapeutic range for their indication?
- Vitamin K? Is there evidence of vitamin K deficiency (malnutrition, malabsorption, obstructive jaundice)? Vitamin K is needed for Factor VII synthesis.
- Liver? Is there liver dysfunction (acute or chronic)? The liver produces Factor VII, which has the shortest half-life, making PT/INR sensitive to early liver impairment.
- Isolated Factor VII Deficiency? This is rare congenitally, but consider it if other causes are ruled out.
Normal PT/INR, Prolonged aPTT, Normal Platelet Count
- Interpretation: Points to a problem primarily in the intrinsic pathway (Factors XII, XI, IX, VIII).
- Thought Process:
- Medication? Heparin therapy (unfractionated) is the most common cause.
- Factor Deficiencies? Is there a history of bleeding consistent with hemophilia (A or B)? Factor VIII and IX deficiencies are classic causes. Factor XI deficiency can also cause bleeding. Factor XII deficiency is usually asymptomatic. Specific factor assays would confirm.
- Von Willebrand Disease? Severe forms of vWD can lead to secondary Factor VIII deficiency, thus prolonging aPTT.
- Inhibitor Present? This is critical.
- Lupus Anticoagulant (LA): An antibody that prolongs aPTT in vitro but is associated with thrombosis in vivo. If suspected, a mixing study and specific LA assays are needed.
- Specific Factor Inhibitor: An antibody against a specific clotting factor (e.g., acquired Factor VIII inhibitor). This is a serious condition causing bleeding. A mixing study is key here.
Prolonged PT/INR, Prolonged aPTT, Normal Platelet Count
- Interpretation: Indicates a problem in the common pathway, or severe deficiencies affecting both intrinsic and extrinsic pathways, without significant platelet consumption.
- Thought Process:
- Severe Liver Disease: Impaired synthesis of multiple factors (II, V, VII, X, I), affecting both pathways.
- Severe Vitamin K Deficiency: Affects Factors II, VII, IX, X, thus impacting both PT/INR and aPTT.
- Common Pathway Factor Deficiency: Rare, but deficiencies in Factor X, V, II (prothrombin), or severe fibrinogen deficiency can cause this.
Prolonged PT/INR, Prolonged aPTT, Decreased Platelet Count
- Interpretation: This pattern strongly suggests a widespread, consumptive coagulopathy.
- Thought Process:
- Disseminated Intravascular Coagulation (DIC): This is the hallmark pattern. Widespread activation of coagulation consumes factors and platelets. Often accompanied by elevated D-dimer and decreased fibrinogen. Requires urgent investigation for underlying cause (sepsis, severe trauma, malignancy, obstetric complications).
- Severe Liver Failure (End-Stage): The liver cannot produce enough factors, leading to prolonged times, and sometimes also develops secondary thrombocytopenia due to hypersplenism or impaired thrombopoietin production.
Normal PT/INR, Normal aPTT, Decreased Platelet Count
- Interpretation: Indicates a primary platelet disorder (thrombocytopenia).
- Thought Process:
- Production Problem? Bone marrow issues (e.g., aplastic anemia, leukemia, chemotherapy effect, viral infection).
- Destruction Problem? Immune Thrombocytopenic Purpura (ITP), drug-induced thrombocytopenia, TTP/HUS (often with microangiopathic hemolytic anemia).
- Sequestration? Splenomegaly.
- Dilution? Massive transfusions.
- Pseudothrombocytopenia? An in vitro artifact due to platelet clumping in the EDTA tube. Always confirm with a peripheral blood smear.
Normal PT/INR, Normal aPTT, Increased Platelet Count
- Interpretation: Thrombocytosis.
- Thought Process:
- Reactive (Secondary)? This is most common. Look for underlying inflammation, infection, iron deficiency, post-splenectomy state, or malignancy. The high platelet count is a response to another condition.
- Primary (Essential Thrombocythemia)? If reactive causes are ruled out, consider a myeloproliferative neoplasm. This requires further hematological workup (e.g., JAK2 mutation, bone marrow biopsy).
The Role of Mixing Studies (Correction Studies)
When a PT or aPTT is prolonged (especially an isolated aPTT), a mixing study is often performed.
- Principle: Patient plasma is mixed 1:1 with normal pooled plasma (which contains 100% of all clotting factors). The PT/aPTT is re-measured.
- Interpretation:
- Correction: If the prolonged PT/aPTT corrects (i.e., falls back into or near the normal range), it suggests a factor deficiency. The normal plasma provided the missing factor(s).
No Correction (or Partial/Delayed Correction): If the prolonged PT/aPTT does not correct, or corrects only partially after incubation, it suggests the presence of an inhibitor. Something in the patient’s plasma is inactivating the factors in the normal plasma (e.g., lupus anticoagulant, specific factor inhibitors). This is a crucial distinction as management differs significantly.
Factors Affecting Coagulation Screening Panel Results
Accurate interpretation of coagulation screening panel (coagulation panel) results is important for patient care. These tests are highly sensitive to various factors that can lead to erroneous results. These factors can broadly be categorized into pre-analytical, analytical, and patient-related variables.
Pre-analytical Variables
These are factors that occur before the actual laboratory analysis, from the time the blood is drawn until it is prepared for testing. Errors in this phase are the most common cause of inaccurate coagulation screening panel (coagulation panel) results.
Sample Collection
- Venipuncture Technique (Traumatic Draw): A difficult or “traumatic” venipuncture can activate coagulation in vivo by releasing tissue factor from damaged tissue or causing hemolysis. This can falsely shorten PT/aPTT or consume factors, leading to falsely prolonged results, and can also activate platelets.
- Needle Gauge: Using too small a needle can cause hemolysis and platelet activation due to shear stress, affecting results.
- Order of Draw: Coagulation tubes (light blue top, citrate) should ideally be drawn before tubes containing other anticoagulants or additives (e.g., EDTA, heparin from flush lines) to avoid contamination. If a “discard tube” is not used for a difficult draw, the coagulation tube should be the second tube drawn after a non-additive tube to clear any tissue factor from the venipuncture site.
- Contamination with Heparin: If blood is drawn from an intravenous line that has been flushed with heparin, even small amounts of heparin can significantly prolong aPTT and, to a lesser extent, PT.
- Tourniquet Application: Prolonged tourniquet application (stasis) can lead to localized hemoconcentration and activation of coagulation, potentially shortening clotting times.
- Patient Identification and Labeling: Mislabeling of tubes is a critical error that can lead to severe patient harm due to misdiagnosis and inappropriate treatment.
Sample Volume and Anticoagulant Ratio
- Correct Blood-to-Anticoagulant Ratio (9:1): Coagulation tubes contain a specific amount of sodium citrate anticoagulant. It’s critical to fill the tube to the indicated fill line.
- Underfilled Tube (“Short Draw”): Too little blood means an excess of citrate, which binds more calcium than necessary. When calcium is added back in the lab, there’s insufficient free calcium to fully reactivate coagulation, leading to falsely prolonged PT and aPTT. This is a very common error.
- Overfilled Tube: Too much blood relative to the anticoagulant means insufficient citrate to prevent clotting in the tube, potentially leading to microclots within the sample that consume factors and platelets, falsely prolonging clotting times or causing falsely low platelet counts.
- High Hematocrit: In patients with very high hematocrit (e.g., >55−60%), the plasma volume is proportionally reduced. This means the standard amount of citrate is effectively in excess for the reduced plasma volume, leading to falsely prolonged results. Special tubes with adjusted citrate volumes are sometimes used for these patients.
Sample Handling and Processing
- Mixing: Tubes must be gently inverted 5-8 times immediately after collection to ensure adequate mixing of blood with the anticoagulant. Vigorous shaking can cause hemolysis and platelet activation.
- Transportation and Storage:
- Temperature: Samples should be transported and processed promptly, ideally at room temperature. Extreme temperatures can affect factor stability.
- Time to Processing: Coagulation factors, especially Factor V and Factor VIII, are labile and degrade over time. Delays in processing can lead to falsely prolonged PT and aPTT. Platelet count can also be affected by clumping if not processed promptly or properly.
- Centrifugation: Proper centrifugation (speed and duration) is essential to obtain platelet-poor plasma (PPP), which is critical for accurate PT and aPTT results. Residual platelets in the plasma can release phospholipids, neutralizing lupus anticoagulants and potentially shortening clotting times or affecting anti-Xa assays.
- Interfering Substances:
- Hemolysis: Hemolyzed samples (ruptured red blood cells) release intracellular components that can interfere with optical clot detection methods and activate coagulation, leading to inaccurate results.
- Icterus (High Bilirubin): High bilirubin levels can make the plasma turbid, interfering with optical clot detection.
- Lipemia (High Lipids): High lipid levels can also make the plasma turbid, interfering with optical clot detection.
Analytical Variables
These factors relate to the actual laboratory analysis process, including instrumentation, reagents, and quality control.
Reagent Quality and Lot Variation
- Reagent Sensitivity: Different manufacturers’ reagents (especially thromboplastin for PT) can have varying sensitivities to clotting factor deficiencies, requiring the use of INR for standardization.
- Reagent Storage and Handling: Improper storage (temperature, light exposure) or expired reagents can lead to degradation and inaccurate results.
- Calibration and Quality Control (QC): Laboratories must regularly calibrate instruments and run quality control samples to ensure the accuracy and precision of their tests. Failure to do so can lead to systemic errors.
Instrumentation
- Clot Detection Method: Different automated analyzers use various methods to detect clot formation (e.g., optical, mechanical). Each method has its own sensitivities and potential interferences (e.g., optical methods are affected by hemolysis, icterus, lipemia).
- Instrument Malfunction: Mechanical or electrical issues with the analyzer can lead to erroneous results.
Technical Expertise
- Operator Skill: While automated, manual steps like sample loading, reagent preparation, and result validation still require trained personnel to ensure accuracy and identify potential issues.
Patient-Related Factors
These are inherent patient characteristics or conditions that influence coagulation test results, often reflecting their actual hemostatic status.
Medications
- Anticoagulants: Warfarin (prolongs PT/INR), unfractionated heparin (prolongs aPTT, TT), low molecular weight heparin (LMWH – generally does not affect PT/aPTT, but affects anti-Xa), Direct Oral Anticoagulants (DOACs – variable effects, dabigatran prolongs TT and aPTT, rivaroxaban/apixaban prolong PT to varying degrees).
- Antiplatelet Agents: Aspirin, clopidogrel, etc., affect platelet function, but not typically the platelet count, PT, or aPTT.
- Antibiotics: Some broad-spectrum antibiotics can affect gut flora and reduce vitamin K production, leading to a prolonged PT/INR.
- Other Drugs: Certain drugs can induce thrombocytopenia or affect liver function, thereby impacting coagulation tests.
Underlying Medical Conditions
- Liver Disease: As discussed, impairs synthesis of clotting factors (prolongs PT/INR and aPTT).
- Vitamin K Deficiency: Impairs synthesis of functional vitamin K-dependent factors (prolongs PT/INR, and aPTT in severe cases).
- Disseminated Intravascular Coagulation (DIC): Consumes clotting factors and platelets (prolongs PT/INR, aPTT, decreases platelet count).
- Renal Failure/Uremia: Can cause platelet dysfunction, and sometimes affect clotting factor levels.
- Autoimmune Diseases: Presence of circulating inhibitors (e.g., lupus anticoagulant can prolong aPTT, specific factor inhibitors).
- Malignancies: Can cause DIC, hypercoagulability, or affect bone marrow function.
- Infections/Sepsis: Can trigger DIC or affect hemostasis.
- Thyroid Disorders: Can influence clotting factor synthesis.
- Pregnancy: Physiologically, pregnancy is a hypercoagulable state with increased levels of some clotting factors (e.g., FVIII, fibrinogen), which can shorten PT/aPTT slightly.
- Age: Neonates have physiologically lower levels of vitamin K-dependent factors, leading to prolonged baseline PT/aPTT. Factor levels generally reach adult levels by 6 months of age.
Physiological Variations
- Hemodilution/Hemoconcentration: Significant changes in fluid status (e.g., overhydration, severe dehydration) can dilute or concentrate clotting factors.
- Recent Transfusion: Massive transfusions of packed red blood cells or crystalloids can dilute clotting factors and platelets, leading to coagulopathy.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Raber MN. Coagulation Tests. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 157. Available from: https://www.ncbi.nlm.nih.gov/books/NBK265/
- Zaidi SRH, Rout P. Interpretation of Blood Clotting Studies and Values (PT, PTT, aPTT, INR, Anti-Factor Xa, D-Dimer) [Updated 2024 Jun 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK604215/
- J. J. van Veen, D. R. Spahn, M. Makris, Routine preoperative coagulation tests: an outdated practice?, BJA: British Journal of Anaesthesia, Volume 106, Issue 1, 1 January 2011, Pages 1–3, https://doi.org/10.1093/bja/aeq357
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.