TL;DR
Hemostasis, the intricate process of stopping and restarting blood flow after an injury, has different stages, each playing a crucial role and relying on the previous one for successful completion.
Vascular Spasm ▾
- Importance: The immediate constriction of injured blood vessels by the surrounding smooth muscle helps reduce blood flow to the wound, minimizing initial blood loss.
- Interdependence: This buys precious time for the next stages to kick in, and its effectiveness influences the amount of blood platelets needed to adhere to.
Platelet Adhesion and Activation ▾
- Importance: Platelets, sticky blood cells, adhere to exposed collagen in the vessel wall, forming a plug to slow down the bleeding. Activated platelets release signaling molecules that attract more platelets and initiate the next stage.
- Interdependence: Efficient adhesion depends on a healthy number of platelets and proper functioning of their surface receptors. It sets the foundation for building a robust clot.
Platelet Aggregation ▾
- Importance: Attracted by signaling molecules, activated platelets bind to each other, forming a dense, multi-layered plug that effectively seals the leak.
- Interdependence: This stage relies on the initial adhesion and efficient communication between platelets to amplify the clotting response.
Coagulation Cascade ▾
- Importance: A complex chain reaction of protein activations leads to the formation of fibrin, a fibrous protein that reinforces the platelet plug and solidifies the clot.
- Interdependence: This stage requires both internal and external triggers, and its efficiency impacts the strength and stability of the final clot.
Fibrinolysis ▾
- Importance: Once the wound starts to heal, plasmin, an enzyme, breaks down fibrin, dissolving the clot and allowing blood flow to resume.
- Interdependence: Proper timing and regulation of fibrinolysis are crucial to prevent excessive bleeding and ensure timely tissue repair.
Potential Problems ▾
- Excessive bleeding: Can occur if hemostasis is impaired due to deficiencies in platelets, clotting factors, or vascular problems.
- Abnormal clotting: Can lead to thrombosis (blood clots in healthy vessels), potentially causing serious complications like stroke or heart attack.
*Click ▾ for more information
Introduction
Hemostasis, a term derived from the Greek words for “blood” and “to stand,” is the remarkable biological process that keeps our blood flowing within the confines of our vascular network. It ensures that precious blood stays where it belongs, circulating and nourishing our organs, while swiftly plugging any leaks that might arise.
The mechanism of hemostasis acts as the rapid response team, ready to patch any punctures or tears that could disrupt the smooth flow. But its role goes beyond mere damage control. It also plays a crucial part in maintaining vascular integrity, preventing spontaneous bleeding and ensuring efficient distribution of oxygen and nutrients.
Here’s why hemostasis is so vital:
- Prevents excessive blood loss: Even a small cut can lead to significant blood loss if left unchecked. Hemostasis acts like a quick clot-forming system, minimizing blood escape and preventing potentially life-threatening situations.
- Promotes healing: The clot formed by hemostasis not only stops the bleeding but also serves as a scaffold for tissue repair. It provides a platform for cell migration and growth, facilitating wound healing and tissue regeneration.
- Combats infection: By sealing off the wound, hemostasis creates a physical barrier against invading pathogens. This reduces the risk of infections entering the bloodstream and causing further complications.
- Maintains vascular pressure: Hemostasis helps maintain optimal blood pressure within the vessels. Excessive bleeding can lead to a drop in pressure, compromising organ function and even causing circulatory shock.
In essence, hemostasis is a delicate balance between clotting and unclogging. It ensures that blood flows freely while keeping itself ready to form a barrier whenever needed. This dynamic process relies on the intricate interplay of platelets, clotting factors, and regulatory proteins, all working in perfect harmony to maintain our internal equilibrium.
History of Hemostasis
Our understanding of hemostasis has gone through fascinating transformations over centuries.
Ancient Times
- Egyptians treated wounds with honey and linen, likely relying on natural clotting mechanisms.
- Greeks and Romans attributed hemostasis to the balance of four humors (blood, phlegm, yellow bile, and black bile).
- Early surgeons used cauterization and compression to control bleeding, highlighting the practical approach.
Renaissance and Enlightenment
- Andreas Vesalius (16th century) revolutionized anatomy, paving the way for understanding the vascular system.
- William Harvey (17th century) discovered the circulation of blood, highlighting its dynamic nature.
- Surgeons experimented with ligatures and styptics, demonstrating the role of mechanical and chemical intervention.
19th and 20th Centuries
- Virchow proposed the “triad of Virchow” for thrombosis, including vessel wall injury, altered blood flow, and changes in blood composition.
- Morawitz distinguished between intrinsic and extrinsic coagulation pathways, laying the groundwork for modern understanding.
- Platelets were identified as key players in hemostasis, leading to research on their functions and interactions.
Present and Future
- Molecular biology unravels the intricate signaling pathways and protein interactions in hemostasis.
- Development of anticoagulant and antiplatelet drugs revolutionizes treatment of thrombosis and bleeding disorders.
- Personalized medicine seeks to tailor therapies based on individual variations in hemostasis.
Looking back, we see a remarkable evolution in our understanding of hemostasis. From ancient observations to advanced molecular insights, the journey continues, promising exciting discoveries and novel therapies for maintaining our precious blood flow.
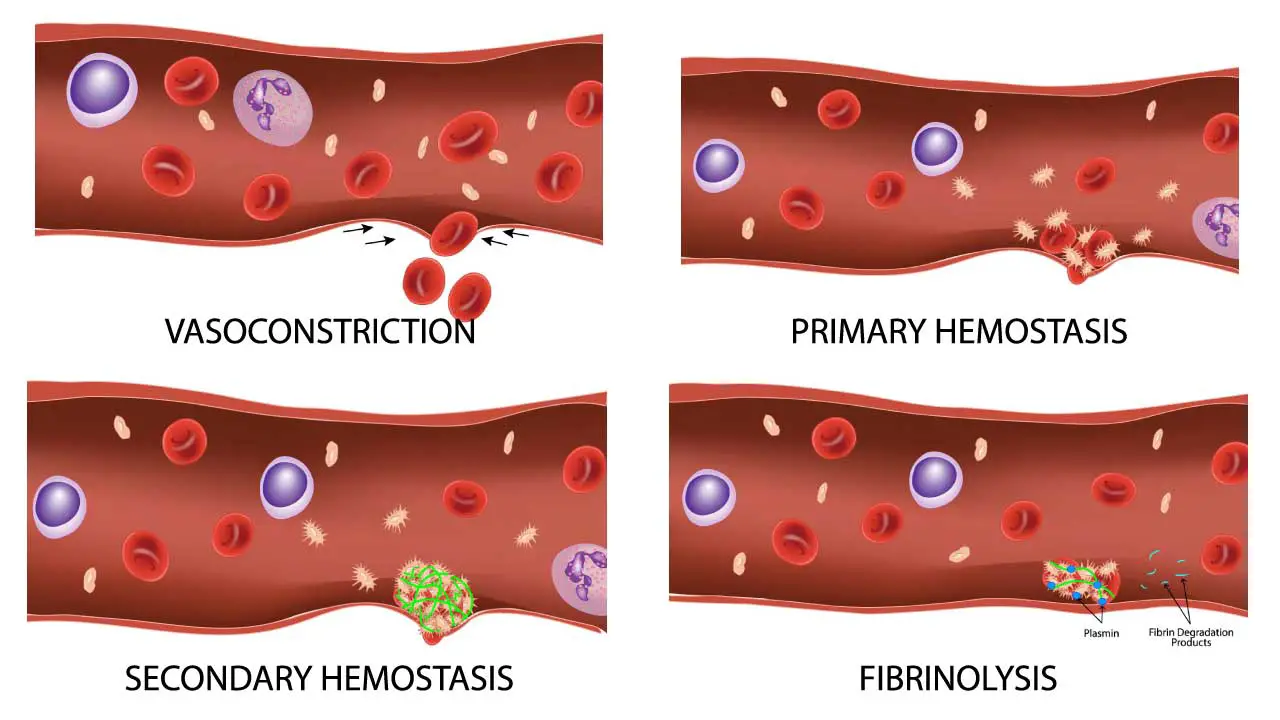
The mechanism of hemostasis involves 3 main processes – primary hemostasis, secondary hemostasis and fibrinolysis. Let’s take a closer look at each of these processes.
Primary Hemostasis
Primary hemostasis, our blood’s initial line of defense against leaks, acts like a lightning-fast patch-up crew.
- Vasoconstriction: Vasoconstriction plays a crucial role alongside platelet adhesion in primary hemostasis, acting like a temporary dam upstream to reduce blood flow to the injury site.
- Platelet adhesion: Platelets, drawn by distress signals from damaged vessels, arrive first. They adhere to collagen fibers exposed at the wound site.
- Platelet aggregation: Once anchored, platelets activate and attract more platelets, forming a dense plug to block the immediate flow of blood. Think of it as a microscopic sandbag wall holding back the tide by building a barricade.
- Reinforcing the barrier: Platelets release special messengers, calling for support from the next act in the hemostasis play – secondary hemostasis.
This rapid, platelet-driven response buys precious time for the more complex clotting cascade to kick in, ensuring a smooth transition from initial plugging to permanent sealing in hemostasis. It’s a remarkable display of teamwork, highlighting the body’s incredible ability to keep our blood flowing seamlessly, even in the face of minor injuries.
Vasoconstriction
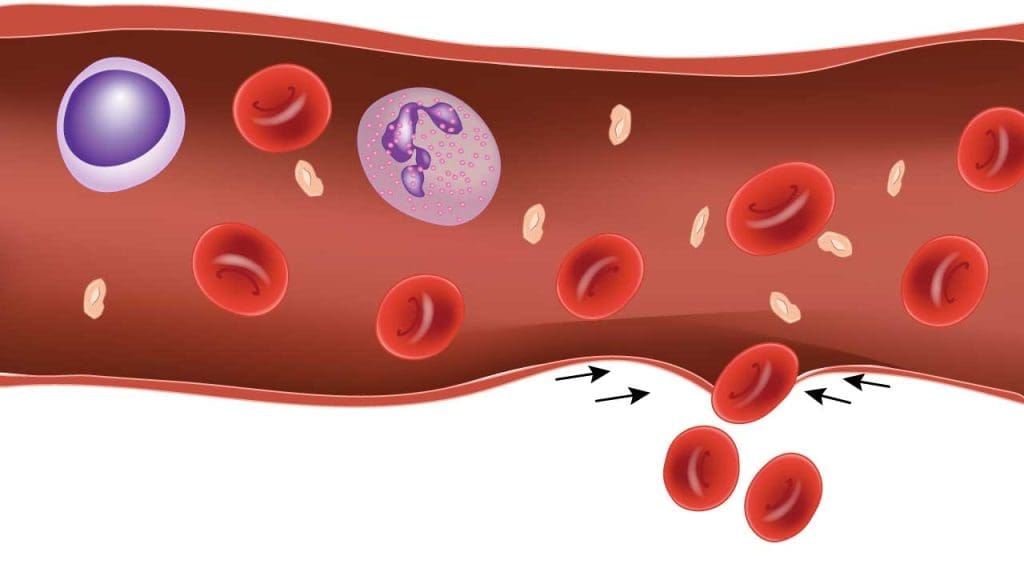
Vasoconstriction plays a crucial role in the initial stages of hemostasis.
Mechanism of action
- When a blood vessel wall is damaged, causing bleeding, the exposed subendothelial matrix (ECM) triggers a reflexive contraction of the surrounding smooth muscle cells in the vessel wall.
- This contraction narrows the blood vessel diameter, a process called vasoconstriction.
- Several factors contribute to this response:
- Direct mechanical effect: Damage to the vessel wall stretches the smooth muscle cells, triggering contraction.
- Chemical mediators: Platelets release substances like thromboxane A2, which acts on smooth muscle receptors, causing constriction.
- Endothelium-derived contracting factors: Damaged endothelial cells release substances like endothelin-1, further promoting vasoconstriction.
Key players
- Smooth muscle cells: Located in the blood vessel wall, they contract in response to various stimuli, including direct damage and chemical signals.
- Platelets: Release vasoconstrictors like thromboxane A2 upon activation.
- Endothelial cells: Respond to injury by releasing both vasoconstrictors (endothelin-1) and vasodilators (prostacyclin), balancing the local response.
- Subendothelial matrix (ECM): Exposed by injured endothelium, it triggers smooth muscle contraction directly through mechanical interaction.
Platelet Adhesion
Platelet adhesion is the crucial step in hemostasis, the process of stopping blood loss at the site of an injury.
Role of Platelets
Platelets are tiny, disc-shaped cells in the blood when resting. They lack nuclei and most organelles, allowing them to focus on their primary function: stopping bleeding. They circulate passively until an injury exposes collagen fibers in the vessel wall. These fibers act as a siren call, triggering platelet adhesion.
Structure of Platelets
Platelets have a unique structure perfectly suited for adhesion:
- Membrane: Covered with glycoproteins and adhesion receptors, which act like velcro, grabbing onto collagen and other platelets.
- Canaliculi: A complex network of internal channels allows for rapid release of signaling molecules and clotting factors once activated.
- Pseudopods: Flexible protrusions that extend and anchor the platelet to the collagen, like tiny grappling hooks.
Activation Triggers
Once platelets adhere to collagen, several triggers kickstart their activation:
- Surface contact: Collagen directly activates adhesion receptors on the platelet membrane.
- Adhesion receptors: Signalling molecules are released within the platelet, triggering internal changes.
- Vascular wall: Exposed endothelial cells at the wound site release additional chemicals that further activate platelets.
Consequences of Activation
Platelet activation leads to a cascade of events:
- Shape change: Platelets transform into spiky spheres, increasing their surface area for even stronger adhesion and interaction with other platelets.
- Granule release: They release stored molecules like thrombin and ADP, attracting and activating more platelets, building the initial plug.
- Signaling cascade: Further signals are sent to initiate secondary hemostasis, the formation of a stronger fibrin clot.
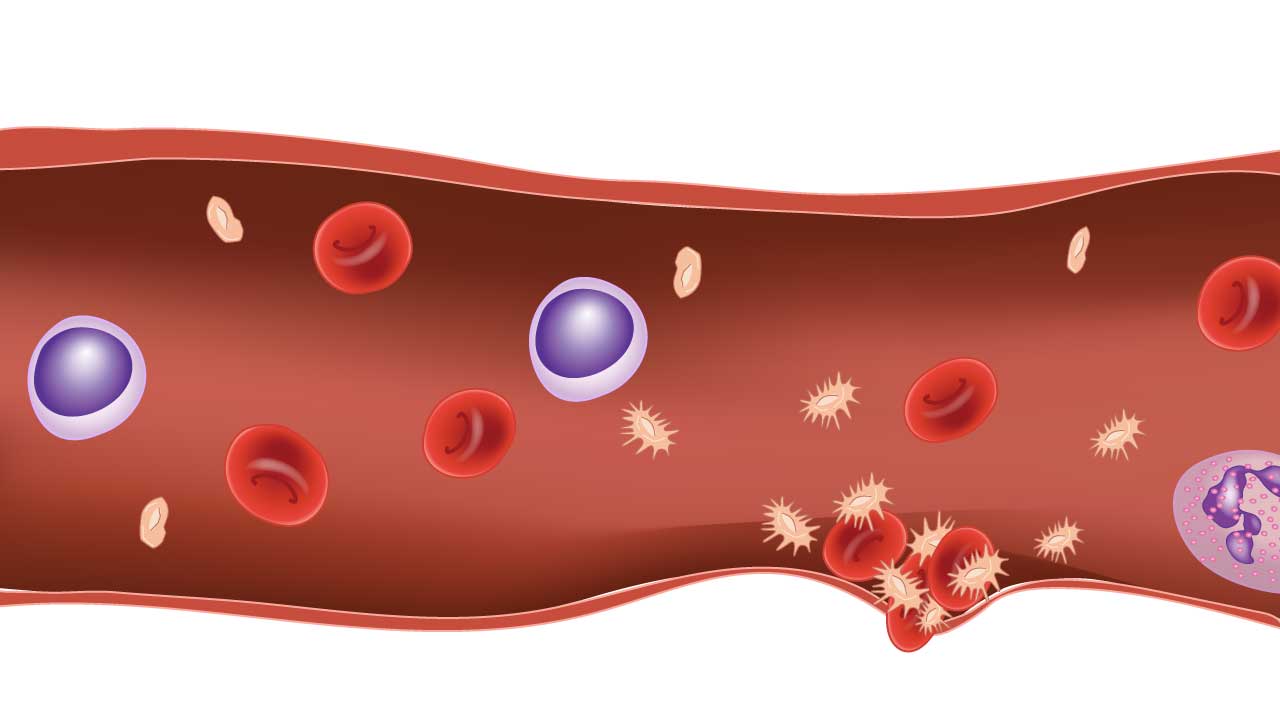
Platelet Aggregation
Activated platelets emit potent signals that attract and bind their fellow platelets, forming a dense, multi-layered plug over the injured area.
Signaling the Alarm
Once a platelet adheres to exposed collagen in the vessel wall, it undergoes activation. This triggers the release of several key signaling molecules:
- ADP: This acts like a chemical beacon, attracting nearby platelets to the site of injury.
- Thromboxane A2: This molecule further activates nearby platelets, amplifying the adhesion cascade.
Receptors on the Ready
Activated platelets express specific receptors on their surface, ready to capture the arriving platelets:
- GPIIb/IIIa: These receptors act like docking stations, grabbing onto the ADP and thromboxane released by their activated kin.
The Binding Chain Reaction
As arriving platelets bind to the ADP and thromboxane on the activated platelet surface, they too get activated, releasing more signaling molecules, attracting even more platelets. This creates a snowball effect, rapidly expanding the platelet plug as layer upon layer of platelets bind to each other, creating a growing cascade.
Strength in Numbers
Activated platelets also undergo a shape change, extending pseudopods like microscopic fingers. These protrusions not only help anchor the plug to the collagen but also intertwine with other platelets, further strengthening the bond.
The Fibrin Boost
As the platelet plug forms, it initiates the next stage of hemostasis, secondary hemostasis. Activated platelets release clotting factors that trigger the formation of a fibrin mesh, further solidifying and reinforcing the clot. Think of the platelet plug as a foundation and the fibrin mesh as a layer of cement, creating a robust barrier against bleeding.
Secondary Hemostasis
While primary hemostasis builds the initial sandbag wall with platelets, secondary hemostasis steps in like a skilled construction crew, laying down a sturdy brick-and-mortar foundation to permanently seal the wound.
The Coagulation Cascade
Imagine a tiny dam being built within your blood vessels, sealing a leak and preventing a flood. That’s the essence of the coagulation cascade, a complex chain reaction of proteins in your blood that forms a fibrin clot over an injury to stop bleeding.
The Domino Effect
Each step in the cascade involves a specific protein being activated, which then activates another, and so on, like a row of dominoes toppling one after another. Think of each protein as a domino, with a specific surface that needs to be “knocked over” (activated) by another molecule. Once activated, the protein changes shape and releases specific signals that “knock over” the next domino in the chain.
Key Players
The cascade has several key players:
- Thrombin: The conductor, responsible for activating fibrinogen and other clotting factors.
- Fibrinogen: The building blocks, transformed by thrombin into sticky fibrin threads that weave the clot’s mesh.
- Clotting factors: Helper proteins like factors VIII, IX, and X, assisting in various steps of the cascade.
Amplification and Control
The cascade cleverly self-amplifies, with each activated protein triggering the activation of several others. This ensures a rapid and robust clot formation. But it’s not a runaway reaction! Built-in control mechanisms, like anticoagulant proteins, keep the cascade in check, preventing excessive clotting and ensuring its timely dismantling once the wound heals.
Pathways
The traditional model of the coagulation cascades is a culmination of 2 pathways; the intrinsic and extrinsic pathways.
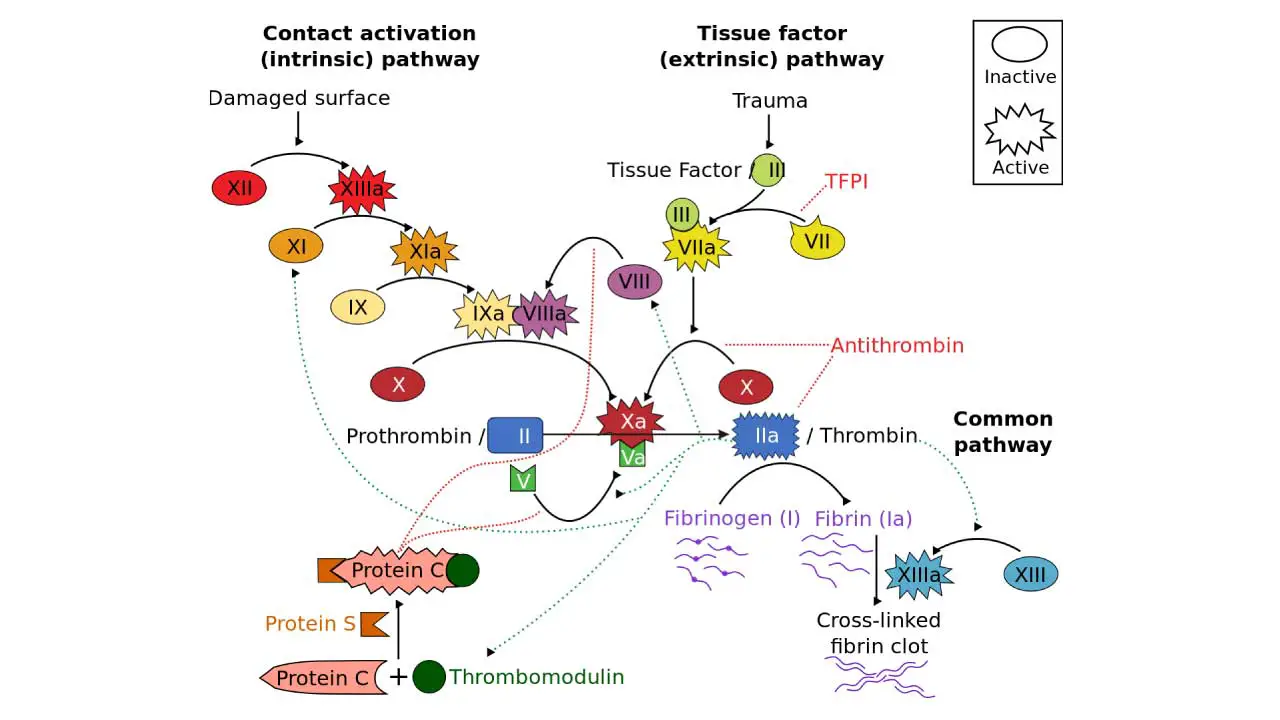
Intrinsic Pathway
Think of it as the “internal alarm system.” It gets activated within the bloodstream itself, when activated platelets release clotting factors like factor XII. This sets off a domino effect of protein activations, eventually leading to thrombin production and fibrin clot formation. It’s slower than the extrinsic pathway but is constantly “on patrol” within the blood, ready to respond to internal injuries.
Extrinsic Pathway
Imagine it as the “external alarm system.” It kicks in when tissue outside the blood vessels is injured, exposing a protein called tissue factor (TF). TF interacts with factor VII, initiating a rapid cascade of protein activations, leading to thrombin and fibrin clot formation much faster than the intrinsic pathway. This pathway is crucial for immediate response to external injuries, where rapid clotting is essential.
Common Pathway
Both pathways can converge and amplify each other, ensuring a robust and efficient clot formation in hemostasis regardless of the initial trigger. The intrinsic pathway provides a sustained response, while the extrinsic pathway delivers a quick burst of clotting activity.
Unified Cascade
While the traditional model of the coagulation cascade separates the intrinsic and extrinsic pathways, there’s growing evidence for a more nuanced understanding. This newer concept emphasizes a single, unified cascade triggered by both internal and external tissue factor (TF), offering a more dynamic and integrated picture of hemostasis.
Key Points of the Unified Cascade
- Shared Pathway: Both internal and external TF initiate the same basic cascade of protein activations, ultimately leading to thrombin generation and fibrin clot formation. Think of it as a single pathway with two doors – internal and external TF – leading to the same room (clot formation).
- Internal TF: Previously thought to be absent in healthy blood, internal TF has been found in low levels on circulating monocytes and activated endothelial cells. This “hidden” TF can trigger the cascade in response to internal injuries or inflammatory processes.
- Amplification Loop: TF, regardless of its origin, activates factor VIIa, which then directly activates factor X. This bypassing of some initial steps in the traditional model creates a faster and more robust clotting response.
- Cross-talk between Pathways: The intrinsic and extrinsic pathways can still exert distinct influences on the cascade. Factors from the intrinsic pathway can amplify the signal from external TF, and vice versa, ensuring a fine-tuned response to different types of injuries.
Fibrin Clot Formation
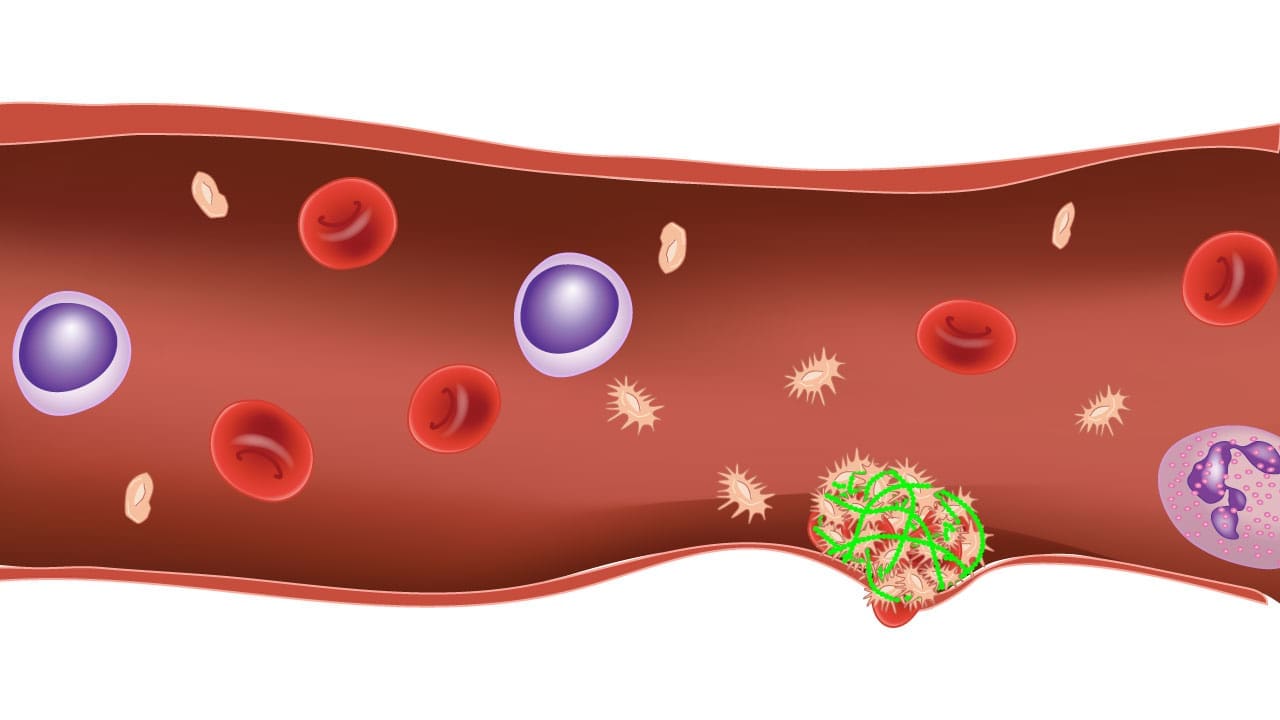
Fibrin clot formation, the grand finale of hemostasis, is where proteins and platelets culminate in a sticky mesh, plugging the leak in the blood vessel, sealing the wound and allowing healing to begin.
The Key Players
- Fibrinogen: This large, inactive protein acts as the building block of the clot.
- Thrombin: Thrombin is responsible for transforming fibrinogen into sticky fibrin threads.
- Clotting factors: They assist thrombin in the conversion process and provide additional stability to the clot.
The Transformation
- Activation: Thrombin, generated by the coagulation cascade, cleaves specific parts of fibrinogen molecules, causing them to change shape and stick together.
- Polymerization: These activated fibrin molecules, now called fibrin monomers, bind to each other in a branching pattern, creating a dense three-dimensional mesh.
- Strengthening: Clotting factors further stabilize the fibrin mesh by cross-linking the fibrin strands.
The Result
- A robust fibrin clot effectively plugs the damaged vessel, trapping blood cells and stopping further bleeding. This fibrin seal provides a temporary barrier for wound healing while the body slowly dismantles the clot once repairs are complete.
Fibrinolysis
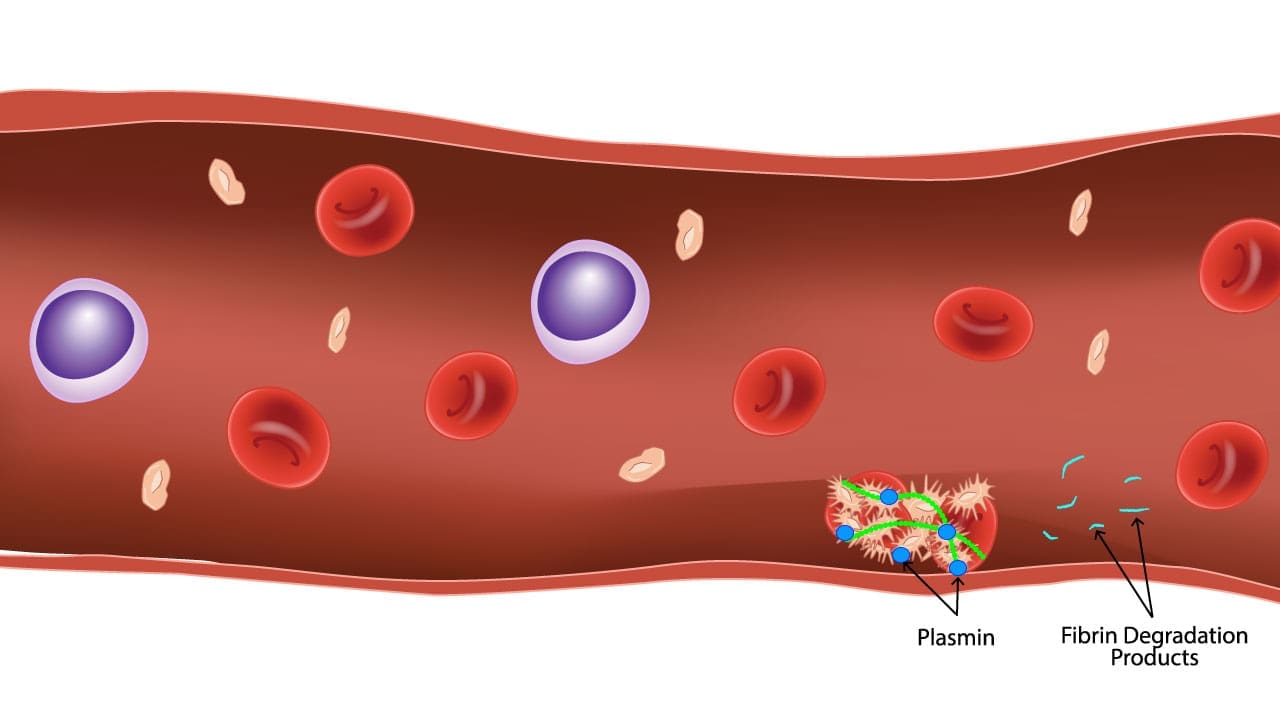
Fibrinolysis is the enzymatic breakdown of fibrin, a key protein component of blood clots. It’s the crucial counterpart to the clotting process, ensuring a delicate balance between hemostasis and maintaining healthy blood flow.
Dissolving the Clot
Once the initial injury has healed and the tissues are repairing themselves, removing the fibrin clot becomes equally important as forming it in the first place. This process, known as fibrinolysis, plays a crucial role in maintaining healthy blood flow and preventing unnecessary tissue damage. Here’s why clot removal is essential:
Maintaining Circulation
A lingering clot can obstruct blood flow to the surrounding tissues, depriving them of vital oxygen and nutrients. This can lead to delayed healing, inflammation, and even tissue death in severe cases. Think of it like a clogged drain preventing water from flowing – the tissue cells become analogous to thirsty plants deprived of water. Fibrinolysis ensures the timely dismantling of the clot, allowing blood to flow freely and nourish the healing tissues.
Preventing Tissue Damage
Prolonged presence of clotting factors and activated platelets can trigger inflammatory responses within the tissues. This can lead to scar tissue formation and impair the regeneration process. Removing the clot through fibrinolysis minimizes inflammation and promotes optimal tissue repair.
Balancing the System
Leaving clots intact creates an ongoing risk of them breaking off and traveling through the bloodstream. This can cause dangerous blockages in distant vessels, potentially leading to strokes or heart attacks. Fibrinolysis keeps the system in check, dissolving unnecessary clots and preventing these potentially life-threatening complications.
Promoting Tissue Remodeling
Fibrinolysis doesn’t just dissolve the clot; it also plays a role in tissue remodeling during the healing process. Plasmin, the enzyme responsible for clot breakdown, also activates other molecules that facilitate tissue repair and regeneration.
Plasminogen and Plasmin
Plasminogen is an inactive precursor molecule that is produced by the liver and circulates throughout the bloodstream. Under the influence of tissue plasminogen activators, plasminogen converts into plasmin. Plasmin cleaves fibrin molecules into smaller fragments which weakens the fibrin mesh. This will ultimately dissolve the clot and allow blood to flow freely again. Plasminogen activator inhibitors ensure plasmin doesn’t go on a rampage and dissolve unnecessary clots. Plasmin, besides targeting fibrin, also activates other molecules involved in tissue remodeling and the inflammatory response.
Related Disorders
The intricate process of hemostasis can be disrupted at various stages, leading to a spectrum of disorders:
Bleeding Disorders
- Platelet disorders: Deficiencies in platelet number or function can lead to excessive bleeding (eg., von Willebrand disease, hemophilia).
- Clotting factor deficiencies: Defects in specific clotting factors (eg., hemophilia A and B) result in impaired clot formation.
- Vascular disorders: Weak blood vessel walls can cause spontaneous bleeding (eg., telangiectasia).
Clot formation disorders
- Hypercoagulability: Increased risk of blood clots can lead to thrombosis (e.g., deep vein thrombosis).
- Disseminated intravascular coagulation (DIC): Uncontrolled clot formation and breakdown throughout the bloodstream.
Related Laboratory Tests
- Bleeding Time: Measures the time it takes for a small skin incision to stop bleeding, assessing platelet function.
- Platelet Count and Function: Quantifies platelet number and their ability to aggregate and adhere. (Platelet count, platelet aggregation testing)
- Coagulation Tests: Assesses various clotting factors and the overall function of the coagulation cascade. (Prothrombin time (PT), activated partial thromboplastin time (aPTT), specific factor assays)
- Genetic Testing: Identifies mutations in genes related to specific clotting factors or platelet function. (Hemophilia A and B genetic testing)
Related Therapeutic Treatments
Targeting Platelet Adhesion and Activation
- Desmopressin: Increases release of von Willebrand factor, enhancing platelet adhesion.
- Antiplatelet drugs: Aspirin, clopidogrel, reduce platelet activity to prevent excessive clotting.
Targeting Coagulation Cascade
- Heparin: Blocks thrombin activity, preventing fibrin clot formation.
- Warfarin: Inhibits vitamin K-dependent clotting factors, slowing clot formation.
- Direct oral anticoagulants (DOACs): Target specific steps in the cascade for precise antithrombotic action.
Targeting Fibrinolysis
- Tranexamic acid: Inhibits plasminogen activation, reducing fibrin breakdown and excessive bleeding.
Note: These are just a few examples, and the specific tests and medications will vary depending on the suspected disorder and individual patient needs.
Frequently Asked Questions (FAQs)
Is hemostasis the same as blood clotting?
Hemostasis and blood clotting are closely related, but they’re not exactly the same thing. Here’s the key difference:
Hemostasis
- Broader term: Refers to the entire process of stopping bleeding after an injury.
- Involves multiple steps: These include:
- Vasoconstriction (narrowing of blood vessels) to reduce blood flow to the injured area.
- Platelet plug formation: Platelets clump together to form a temporary seal at the injury site.
- Coagulation (blood clotting): A cascade of enzymatic reactions that converts fibrinogen (a soluble protein) into fibrin (an insoluble mesh) to form a stable clot.
- Clot retraction and fibrinolysis: The clot shrinks and eventually dissolves after the injury heals.
Blood clotting
- Specific step within hemostasis: Refers specifically to the process of fibrin formation, which creates the visible clot.
- Part of the coagulation cascade: Involves a series of protein activations that ultimately lead to the production of fibrin.
What happens if hemostasis fails?
If hemostasis fails, it can lead to a variety of serious consequences, depending on the severity and cause of the failure. Here are some potential outcomes:
Excessive bleeding: This is the most obvious consequence. Without proper hemostasis, even minor injuries can lead to significant blood loss, which can be life-threatening. This can happen due to:
- Defective platelet function: This could be due to low platelet count (thrombocytopenia) or dysfunctional platelets (e.g., in hemophilia).
- Impaired coagulation: This could be caused by deficiencies in clotting factors, like in hemophilia A or B, or by medications that interfere with clotting, like warfarin.
- Vascular problems: Damage to blood vessels, like in vasculitis, can make them more prone to bleeding.
Internal bleeding: Hemostasis failures can also lead to internal bleeding, which can be more dangerous than external bleeding because it’s often harder to diagnose and treat. This can occur in the brain, gastrointestinal tract, or other organs, causing symptoms like pain, swelling, and even shock.
Formation of abnormal blood clots: Sometimes, hemostasis failure can lead to the formation of abnormal blood clots in healthy blood vessels, called thrombosis or deep vein thrombosis (DVT). These clots can block blood flow and damage tissues, potentially leading to:
- Heart attack: If a clot blocks a coronary artery.
- Stroke: If a clot blocks blood flow to the brain.
- Pulmonary embolism: If a clot travels to the lungs.
Other complications: Depending on the cause and severity of hemostasis failure, there may be other complications, such as:
- Infection: If bleeding is severe, it can weaken the immune system and make you more susceptible to infections.
- Anemia: Chronic blood loss can lead to anemia, which can cause fatigue and other symptoms.
- Tissue damage: Internal bleeding can damage organs and tissues, leading to long-term health problems.
It’s important to note that this is not an exhaustive list, and the specific consequences of hemostasis failure can vary depending on the individual and the underlying cause.
What are the differences between hemostasis and thrombosis?
| Feature | Hemostasis | Thrombosis |
|---|---|---|
| Definition | Process of stopping blood loss after injury | Formation of blood clots within healthy blood vessels |
| Outcome | Controlled bleeding, clot formation, healing | Blockage of blood flow, potential tissue damage, serious complications |
| Mechanism | Platelet aggregation, vasoconstriction, coagulation cascade | Abnormal activation of coagulation, stasis of blood flow |
| Causes | Injuries, surgery, certain medications | Inherited disorders, acquired conditions, lifestyle factors |
| Symptoms | Excessive bleeding, easy bruising | Pain, swelling, redness, difficulty breathing (depending on clot location) |
| Treatment | Varies depending on cause, may include lifestyle changes, medications, transfusions, surgery | Varies depending on clot type and severity, may include anticoagulants, clot busters, surgery |
| Importance | Crucial for preventing blood loss and promoting healing | Early diagnosis and management are essential to prevent complications |
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Kaushansky K, Levi M. Williams Hematology Hemostasis and Thrombosis (McGraw-Hill). 2017.