TL;DR
Neutropenia is a quantitative deficiency of neutrophils, crucial for the innate immune system. Diagnosis relies on the absolute neutrophil count (ANC), classified by severity (mild, moderate, severe) and duration (acute, chronic).
- Causes ▾: Decreased bone marrow production (e.g., drugs, hematologic disorders), increased destruction/sequestration (e.g., autoimmune, splenomegaly), and increased utilization (overwhelming infection).
- Symptoms ▾: General signs of infection including fever, chills and fatigue.
- Laboratory investigations ▾: CBC with differential (ANC), peripheral blood smear, bone marrow biopsy/aspirate, microbiological cultures, and serological tests to identify the cause.
- Treatment ▾: Managing infections (antibiotics, antifungals, antivirals), using growth factors (G-CSF/GM-CSF), and addressing the underlying cause.
- Complications ▾: Mainly in severe and life-threatening infections, including sepsis and invasive fungal infections.
*Click ▾ for more information
Introduction
Neutropenia refers specifically to a condition characterized by a quantitative deficiency of neutrophils in the circulating blood.
Neutrophils are a critical component of the innate immune system, acting as the first line of defense against invading pathogens like bacteria and fungi. These highly mobile phagocytic cells are produced in the bone marrow and circulate in the bloodstream, ready to migrate to sites of infection to engulf and destroy foreign invaders.
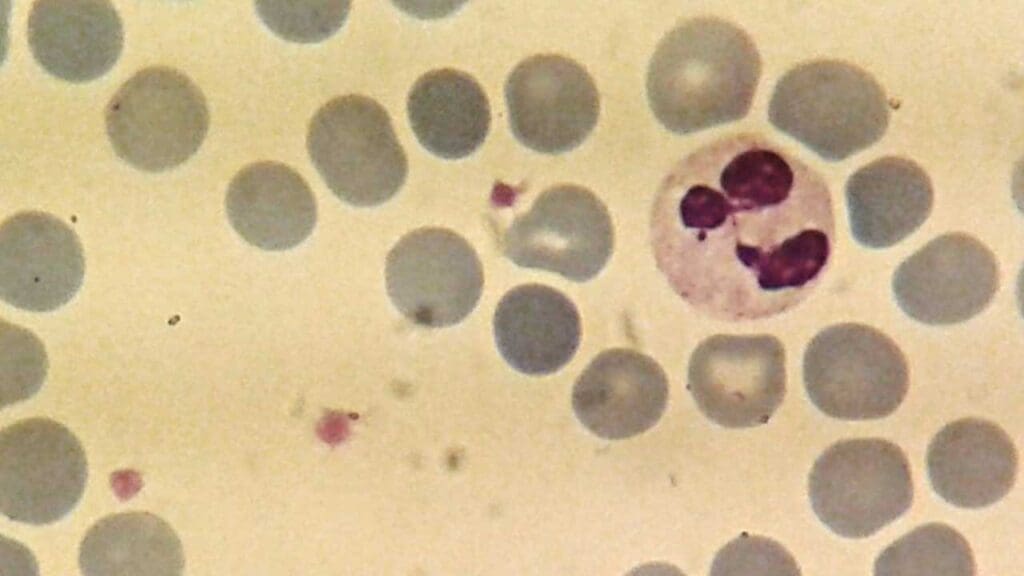
This reduction in the number of circulating neutrophils compromises the body’s ability to effectively combat infections, making individuals with neutropenia significantly more susceptible to a wide range of infectious agents. The severity of this increased risk directly correlates with the degree to which the neutrophil count is reduced. Clinical risks include:
- Impaired First Line of Defense: Neutropenia weakens the body’s initial response to infection. Pathogens can proliferate more easily and spread more rapidly without sufficient numbers of neutrophils to contain them at the site of entry.
- Increased Susceptibility to Opportunistic Infections: Individuals with neutropenia become vulnerable not only to common infections but also to opportunistic infections caused by organisms that typically do not cause disease in individuals with a healthy immune system (e.g., certain fungi, bacteria, and viruses).
- Severity of Infections: Infections in neutropenic patients can be more severe, progress rapidly, and be more difficult to treat. What might be a localized and easily managed infection in an immunocompetent individual can quickly become systemic and life-threatening in someone with neutropenia.
- Atypical Presentations: The typical signs and symptoms of infection (like pus formation, localized redness, and swelling, which are often neutrophil-mediated) may be blunted or absent in neutropenic patients, making early diagnosis challenging. Fever may be the only initial sign of a serious infection.
- Morbidity and Mortality: Neutropenic fever, defined as a fever (usually ≥ 38.3°C or ≥ 101°F) in a patient with neutropenia (absolute neutrophil count < 500 cells/µL or expected to fall below that level), is a medical emergency. It carries a significant risk of morbidity and mortality if not promptly and appropriately managed.
- Impact on Treatment Strategies: The presence of neutropenia can significantly influence treatment decisions for other underlying conditions, such as cancer. Chemotherapy regimens may need to be adjusted or delayed due to the risk of exacerbating or prolonging neutropenia and its associated infectious complications.
Definition and Classification
Absolute Neutrophil Count (ANC)
The absolute neutrophil count (ANC) is a crucial laboratory value that represents the actual number of neutrophils (both mature segmented neutrophils and immature band neutrophils) present in a microliter of blood. It provides a more accurate assessment of the body’s ability to fight infection compared to the total white blood cell (WBC) count alone, as it specifically quantifies the neutrophils, which are the primary effector cells in the early response to bacterial and fungal infections.
Calculation of Absolute Neutrophil Count (ANC)
The absolute neutrophil count (ANC) is calculated using the following formula:
ANC (cells/μL) = WBC count (cells/μL) × (% Neutrophils + % Bands)/100
Alternatively, if the WBC count is given in x109/L and percentages are used:
ANC (x109/L) = WBC count (x109/L) × (% Neutrophils + % Bands)/100
Or, if absolute numbers of neutrophils and bands are available (often given in x109/L):
ANC (cells/μL) = (Absolute Neutrophils + Absolute Bands) × 1000
Where:
- WBC count: The total number of white blood cells in a microliter of blood.
- % Neutrophils (also called polys or segmented neutrophils): The percentage of mature neutrophils reported in the differential count.
- % Bands (also called stabs): The percentage of immature neutrophils reported in the differential count.
- Absolute Neutrophils: The actual number of mature neutrophils per liter of blood (usually in x109/L).
- Absolute Bands: The actual number of immature neutrophils per liter of blood (usually in x109/L).
Understanding and calculating the absolute neutrophil count (ANC) is essential for assessing a patient’s risk of infection, especially in individuals undergoing chemotherapy, with hematologic disorders, or those with other conditions affecting bone marrow function. The absolute neutrophil count (ANC) helps in classifying the severity of neutropenia and guiding clinical management decisions.
Classification based on severity
The classification of neutropenia based on severity is a critical aspect of understanding and managing this condition. It directly correlates with the risk of infection and helps guide clinical decision-making. Neutropenia is typically categorized into three levels of severity based on the absolute neutrophil count (ANC).
The normal reference range for the absolute neutrophil count (ANC) in adults can vary slightly between laboratories, but generally falls within the range of 1,500 to 7,700 neutrophils/μL or 1.5 to 7.7 x 109/L.
Mild Neutropenia
- Absolute neutrophil count (ANC) range: 1000 to 1500 cells/μL or 1.0 to 1.5 × 109 cells/L.
- Clinical Significance: Individuals with mild neutropenia generally have a minimal increase in the risk of serious infections. They may be more susceptible to minor localized infections, but severe systemic infections are less common. Often, mild neutropenia may be asymptomatic and discovered incidentally during routine bloodwork. In some cases, it might not require specific interventions beyond monitoring and addressing the underlying cause if identified.
Moderate Neutropenia
- Absolute neutrophil count (ANC) range: 500 to 1000 cells/μL or 0.5 to 1.0 × 109 cells/L.
- Clinical Significance: Patients with moderate neutropenia have a noticeable increase in the risk of infection compared to those with mild or no neutropenia. They are more likely to develop bacterial and fungal infections, and these infections can be more severe and prolonged. Febrile neutropenia (fever with an absolute neutrophil count (ANC) below 1000 cells/μL) is a greater concern in this group and often warrants prompt medical attention, including antibiotic treatment.
Severe Neutropenia
- Absolute neutrophil count (ANC) range: Less than 500 cells/μL or < 0.5 × 109 cells/L.
- Clinical Significance: Severe neutropenia represents a significantly elevated risk of serious and life-threatening infections. In this range, the body’s ability to fight off even common environmental organisms is severely compromised. Patients are highly susceptible to bacterial, fungal, and even viral infections, which can rapidly progress to sepsis and other severe complications. Febrile neutropenia in this category is a medical emergency requiring immediate evaluation, blood cultures, and broad-spectrum antibiotic therapy. Prophylactic antimicrobial medications (antibacterial, antifungal, or antiviral) may be considered in certain situations of prolonged severe neutropenia.
| Severity | ANC (cells/μL) | Infection Risk | Clinical Implications |
| Mild | 1000 – 1500 | Minimal increase | May be asymptomatic, often requires monitoring and addressing underlying cause. |
| Moderate | 500 – 1000 | Noticeable increase | Increased risk of bacterial and fungal infections, febrile neutropenia is a concern, often requires prompt antibiotic treatment for fever. |
| Severe | < 500 | Significantly elevated | High risk of serious and life-threatening infections, febrile neutropenia is a medical emergency, prophylactic antimicrobials may be considered in prolonged cases. |
The duration of neutropenia also plays a significant role in the risk of infection. Prolonged neutropenia, even if not severely low at all times, carries a higher cumulative risk of infectious complications. Additionally, the underlying cause of neutropenia can influence the specific types of infections a patient might be susceptible to.
Causes of Neutropenia
Neutropenia can arise from a variety of underlying mechanisms. These causes are broadly categorized into three main groups: decreased production of neutrophils in the bone marrow, increased destruction or sequestration of neutrophils, and increased utilization of neutrophils.
Decreased Production of Neutrophils in the Bone Marrow
This is the most common cause of neutropenia and indicates a problem with the bone marrow’s ability to produce a sufficient number of healthy neutrophils.
Hematopoietic Stem Cell Disorders
- Aplastic Anemia: A severe condition where the bone marrow fails to produce all types of blood cells, including neutrophils. This can be idiopathic, autoimmune, drug-induced, or associated with viral infections.
- Myelodysplastic Syndromes (MDS): A group of disorders characterized by ineffective hematopoiesis, leading to cytopenias including neutropenia. There is an increased risk of progression to acute myeloid leukemia (AML).
- Congenital Bone Marrow Failure Syndromes: Inherited disorders like Fanconi anemia, Shwachman-Diamond syndrome, and Diamond-Blackfan anemia can affect neutrophil production.
Bone Marrow Infiltration
- Leukemia: Malignant proliferation of immature white blood cells can crowd out the normal hematopoietic cells, reducing neutrophil production.
- Lymphoma: Infiltration of the bone marrow by lymphoma cells can also impair normal blood cell production.
- Metastatic Cancer: Cancer cells from other parts of the body can metastasize to the bone marrow and disrupt neutrophil production.
- Myelofibrosis: Replacement of the bone marrow with fibrous tissue can hinder normal hematopoiesis.
Drug-Induced Neutropenia
Many medications can suppress bone marrow function and lead to neutropenia. This is a common cause and can be dose-dependent or idiosyncratic (unpredictable).
- Chemotherapy: Cytotoxic drugs target rapidly dividing cells, including hematopoietic precursors in the bone marrow.
- Certain Antibiotics: Examples include some penicillins, cephalosporins, sulfonamides, and trimethoprim-sulfamethoxazole.
- Psychotropic Medications: Some antipsychotics (e.g., clozapine) and antidepressants can cause neutropenia.
- Anticonvulsants: Certain anticonvulsant drugs like carbamazepine and phenytoin have been linked to neutropenia.
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): Rarely, NSAIDs can cause neutropenia.
- Other Medications: Various other drugs, including some cardiovascular medications and immunosuppressants, can also be implicated.
Nutritional Deficiencies
Certain nutrient deficiencies are essential for proper hematopoiesis.
- Vitamin B12 and Folate Deficiency: These are crucial for DNA synthesis in rapidly dividing cells in the bone marrow.
- Copper Deficiency: Copper is a cofactor for enzymes involved in hematopoiesis.
Congenital Neutropenia Syndromes
A group of rare inherited disorders characterized by chronic neutropenia from birth.
- Severe Congenital Neutropenia (Kostmann Syndrome): Characterized by very low absolute neutrophil count (ANC) and a high risk of severe infections and progression to myelodysplasia or leukemia.
- Cyclic Neutropenia: Characterized by regular, predictable fluctuations in neutrophil counts, typically with a 21-day cycle.
- Other Genetic Defects: Various other genetic mutations can affect neutrophil development and production.
Infections
Certain infections can directly suppress bone marrow function.
- Viral Infections: Parvovirus B19 (causes fifth disease), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human immunodeficiency virus (HIV) can suppress hematopoiesis.
- Severe Bacterial Infections: In some cases, overwhelming bacterial infections can temporarily suppress bone marrow output.
Radiation Therapy
Radiation to the bone marrow can damage hematopoietic stem cells and reduce neutrophil production.
Increased Destruction or Sequestration of Neutrophils
In these cases, the bone marrow is producing neutrophils adequately, but they are either being destroyed prematurely or are being trapped in other organs.
- Autoimmune Neutropenia: The body produces autoantibodies that target and destroy neutrophils in the peripheral blood. This can be primary (idiopathic) or secondary to other autoimmune diseases like systemic lupus erythematosus (SLE) or rheumatoid arthritis.
- Splenomegaly (Hypersplenism): An enlarged spleen can trap and destroy blood cells, including neutrophils, leading to neutropenia. This can be caused by various conditions like liver disease, hematologic malignancies, and infections.
- Drug-Induced Immune Hemolytic Anemia: Certain drugs can act as haptens, binding to neutrophils and triggering an antibody-mediated destruction.
- Neonatal Alloimmune Neutropenia: Maternal antibodies against fetal neutrophil antigens cross the placenta and destroy the baby’s neutrophils. This is usually transient.
Increased Utilization of Neutrophils
In some situations, neutrophils are consumed at a faster rate than they can be produced, leading to a transient neutropenia.
- Overwhelming Infections: During severe bacterial or fungal infections, a large number of neutrophils migrate to the site of infection and are consumed in the process of fighting the pathogen. If the bone marrow cannot keep up with this increased demand, neutropenia can develop. This is often a transient phenomenon that resolves as the infection is controlled.
Pseudoneutropenia (Redistribution)
This is not a true deficiency in the total number of neutrophils but rather a temporary shift of neutrophils from the circulating pool to the marginated pool (neutrophils that adhere to blood vessel walls).
- Early Stages of Sepsis: Cytokines released during sepsis can cause neutrophils to marginate.
- Hemodialysis: Interaction of neutrophils with the dialysis membrane can lead to transient margination.
- Anaphylaxis: Release of histamine can cause neutrophil margination.
Signs and Symptoms of Neutropenia
The signs and symptoms of neutropenia are primarily related to the increased susceptibility to infections. The severity and frequency of these manifestations often correlate with the degree and duration of the neutropenia. However, in severe neutropenia, the typical signs of inflammation (redness, swelling, pus) may be blunted or absent because these processes are often neutrophil-mediated.
General Signs of Infection
These are common to many types of infections and may be the initial indicators of a problem in a neutropenic patient.
- Fever: This is often the earliest and sometimes the only sign of infection in a neutropenic patient. Even a low-grade fever should be taken seriously. Febrile neutropenia (fever ≥ 38.3°C or ≥ 101°F with an absolute neutrophil count (ANC) < 1000 cells/µL, or expected to fall below that level) is a medical emergency.
- Chills and Rigors: Shaking chills can accompany fever and suggest a more significant infection, particularly a bloodstream infection.
- Malaise and Fatigue: A general feeling of being unwell, tired, and lacking energy.
Increased Susceptibility to Infections at Specific Sites
Due to the reduced number of neutrophils, infections can occur in various parts of the body.
Oral Cavity
- Mucositis: Painful inflammation and ulceration of the mucous membranes lining the mouth and throat. This can make eating and swallowing difficult.
- Gingivitis: Inflammation of the gums, which may be red, swollen, and bleed easily.
- Oral Ulcers (Stomatitis): Painful sores in the mouth.
- Fungal Infections (Thrush): White patches on the tongue or inner cheeks, often caused by Candida species.
Skin
- Cellulitis: A bacterial infection of the skin and underlying tissues, characterized by redness, warmth, swelling, and pain. In neutropenic patients, this can progress rapidly.
- Abscesses: Localized collections of pus. However, in severe neutropenia, pus formation may be minimal or absent.
- Skin Rashes: Can be a sign of infection or a drug reaction causing the neutropenia.
- Infections around indwelling catheters or other devices.
Respiratory Tract
- Pneumonia: Infection of the lungs, which can present with cough (may or may not be productive), shortness of breath, chest pain, and fever. In neutropenic patients, the typical signs of pneumonia might be subtle.
Gastrointestinal Tract
- Typhlitis (Neutropenic Enterocolitis): A serious and potentially life-threatening inflammation of the cecum (the beginning of the large intestine), characterized by abdominal pain (often in the right lower quadrant), fever, diarrhea, and sometimes nausea and vomiting.
- Perianal Infections: Pain, redness, and swelling around the anus, which can progress to abscess formation.
Bloodstream Infections (Sepsis)
A severe systemic response to infection, which can lead to organ dysfunction and is life-threatening. Symptoms can include fever, rapid heart rate, rapid breathing, altered mental status, and low blood pressure.
Other Less Common Sites
Infections can occur in virtually any part of the body, including the urinary tract, sinuses, and central nervous system.
Lack of Typical Inflammatory Signs
As mentioned earlier, the cardinal signs of inflammation (redness, heat, swelling, pain, and loss of function) may be less pronounced or even absent in individuals with severe neutropenia because these responses rely on the presence and function of neutrophils. Therefore, subtle signs like fever or a change in general well-being should be carefully evaluated.
Symptoms Related to the Underlying Cause of Neutropenia
In some cases, patients may also exhibit symptoms related to the underlying condition causing the neutropenia.
- Fatigue, pallor, and shortness of breath may suggest anemia, which can occur with bone marrow disorders.
- Easy bruising or bleeding may suggest thrombocytopenia, another potential consequence of bone marrow dysfunction.
- Bone pain or lymph node enlargement might be present in hematologic malignancies.
- Symptoms of autoimmune diseases (e.g., joint pain, skin rashes) may be present in cases of autoimmune neutropenia.
Laboratory Investigations
Laboratory investigations are crucial for diagnosing neutropenia, determining its severity, and identifying the underlying cause. A systematic approach is necessary, often starting with basic hematological tests and potentially progressing to more specialized investigations.
Complete Blood Count (CBC) with Differential
This is the cornerstone of the initial evaluation for neutropenia.
- Absolute Neutrophil Count (ANC): As discussed earlier, this is the primary diagnostic marker for neutropenia. The CBC provides the total white blood cell (WBC) count and the differential count (percentage of different types of white blood cells). The absolute neutrophil count (ANC) is then calculated. Serial absolute neutrophil count (ANC) measurements can help determine if the neutropenia is transient, persistent, or cyclical.
- Total White Blood Cell (WBC) Count: A low WBC count (leukopenia) often accompanies neutropenia, but not always (e.g., in some cases of isolated neutropenia).
- Hemoglobin and Hematocrit: These assess for anemia, which can occur concurrently with neutropenia in bone marrow disorders.
- Platelet Count: Thrombocytopenia (low platelet count) can also be present in bone marrow failure or infiltrative processes. Evaluating all three cell lines (neutrophils, red blood cells, and platelets) is essential.
- Red Blood Cell Indices (MCV, MCH, MCHC): These can provide clues to underlying conditions like nutritional deficiencies (e.g., macrocytic anemia in B12 or folate deficiency).
Peripheral Blood Smear
- Neutrophil Morphology: Look for abnormal white blood cell morphology like toxic granulation, Döhle bodies (infections or inflammation), hypersegmentation (vitamin B12 or folate deficiency), or hypogranulation (myelodysplasia).
- Presence of Immature Cells: Blast cells suggest a hematologic malignancy like leukemia.
- Atypical Lymphocytes: May be seen in viral infections.
- Other Cell Line Abnormalities: Abnormal red blood cell or platelet morphology can also provide diagnostic clues.
Bone Marrow Biopsy and Aspirate
This is often necessary to evaluate the production of neutrophils and other blood cells in the bone marrow and to identify the underlying cause of neutropenia, especially if the cause is not readily apparent from the peripheral blood.
- Bone Marrow Cellularity: Assessment of the proportion of hematopoietic cells to fat cells. Hypocellularity suggests bone marrow failure (e.g., aplastic anemia), while hypercellularity with abnormal maturation may indicate leukemia or myelodysplasia.
- Myeloid Maturation: Evaluation of the different stages of neutrophil development. Arrest in maturation or dysplastic features can be seen in various disorders.
- Presence of Infiltrative Processes: Detection of abnormal cells like leukemia blasts, lymphoma cells, or metastatic cancer cells.
- Cytogenetic Analysis: Chromosomal abnormalities are common in myelodysplastic syndromes and leukemias.
- Flow Cytometry: Helps identify specific cell populations and can be crucial in diagnosing hematologic malignancies and paroxysmal nocturnal hemoglobinuria (PNH).
- Molecular Studies: Can detect specific gene mutations associated with congenital neutropenia syndromes, myelodysplasia, and leukemia.
Microbiological Cultures
In neutropenic patients, especially those with fever, identifying the source of infection is critical.
- Blood Cultures: To detect bacteremia or fungemia (bacteria or fungi in the bloodstream). Multiple sets of cultures (aerobic and anaerobic) should be drawn from different sites.
- Cultures from Other Potential Sites of Infection: Urine, sputum, wound drainage, stool (if diarrhea is present), and samples from indwelling catheters should be cultured as clinically indicated.
Serological Tests
These may be helpful in specific situations to identify the underlying cause of neutropenia.
- Autoimmune Markers: Antineutrophil antibodies (ANA) may be tested if autoimmune neutropenia is suspected. Other autoimmune markers (e.g., anti-dsDNA, rheumatoid factor) may be relevant if neutropenia is associated with an underlying autoimmune disease.
- Viral Serology: Tests for viruses like parvovirus B19, EBV, CMV, and HIV may be indicated if a viral infection is suspected as the cause of neutropenia.
Other Investigations Based on Suspected Underlying Cause
Depending on the clinical presentation and initial laboratory findings, further investigations may be necessary.
- Vitamin B12 and Folate Levels: To rule out nutritional deficiencies. Methylmalonic acid and homocysteine levels can further clarify B12 deficiency.
- Copper Levels: In cases of suspected copper deficiency, especially in patients with neurological symptoms or anemia.
- Liver and Kidney Function Tests: To assess for organ dysfunction that might be contributing to neutropenia or affecting treatment options.
- Splenic Size Assessment (e.g., Ultrasound, CT Scan): If hypersplenism is suspected as a cause of increased neutrophil destruction or sequestration.
- Genetic Testing: For suspected congenital neutropenia syndromes or other inherited bone marrow failure disorders. Specific gene mutations (e.g., ELANE in cyclic and severe congenital neutropenia) can be identified.
- Paroxysmal Nocturnal Hemoglobinuria (PNH) Testing: Flow cytometry can detect the absence of GPI-anchored proteins on blood cells, characteristic of PNH, which can be associated with bone marrow failure.
Treatment and Management
The treatment and management of neutropenia focus on two primary goals: managing and preventing infections and addressing the underlying cause of the neutropenia. The specific approach depends on the severity, duration, and etiology of the neutropenia, as well as the patient’s overall clinical status.
Management of Infections
Prompt recognition and aggressive treatment of infections are paramount in neutropenic patients due to their compromised immune system.
Febrile Neutropenia is a Medical Emergency
Any neutropenic patient with a fever (usually defined as ≥ 38.3°C or ≥ 101°F and absolute neutrophil count (ANC) < 1000 cells/µL, or expected to fall below that level) requires immediate evaluation and broad-spectrum antibiotic therapy.
- Blood Cultures: Should be drawn promptly before starting antibiotics to identify the causative organism.
- Empirical Broad-Spectrum Antibiotics: Therapy is initiated immediately, often targeting both Gram-positive and Gram-negative bacteria. Common regimens include combinations of beta-lactam antibiotics (e.g., cefepime, ceftazidime, piperacillin-tazobactam) with or without an aminoglycoside or fluoroquinolone.
- Antifungal Therapy: Should be considered if fever persists despite broad-spectrum antibiotics for several days, or if there is clinical suspicion of a fungal infection.
- Antiviral Therapy: Indicated if a specific viral infection is suspected or confirmed.
- Modification of Antibiotics: Once the causative organism is identified and sensitivities are known, the antibiotic regimen may be narrowed to target the specific pathogen.
- Duration of Therapy: Antibiotics are typically continued until the absolute neutrophil count (ANC) recovers to a safe level (usually > 500 cells/µL and trending upwards) and the patient is afebrile and clinically stable.
- Management of Localized Infections: Even localized infections in neutropenic patients can rapidly become systemic and require aggressive treatment with antibiotics or antifungals. Drainage of abscesses may be necessary, although pus formation might be minimal.
Granulocyte Colony-Stimulating Factor (G-CSF) and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
These are hematopoietic growth factors that stimulate the proliferation, differentiation, and activation of neutrophil precursors in the bone marrow, leading to an increase in the absolute neutrophil count (ANC).
G-CSF primarily stimulates the production of neutrophils, while GM-CSF has a broader effect on myeloid lineage cells, including neutrophils and monocytes. It is typically administered as a subcutaneous injection.
- Indications:
- Prevention of Febrile Neutropenia: Prophylactic use in patients receiving chemotherapy regimens with a high risk of causing significant and prolonged neutropenia.
- Treatment of Febrile Neutropenia: Can be used as adjunctive therapy in some cases of established febrile neutropenia, particularly in patients with prolonged severe neutropenia or those at high risk of complications. However, the routine use of G-CSF in all cases of febrile neutropenia is not universally recommended.
- Severe Chronic Neutropenia: Long-term G-CSF therapy is often used to increase the absolute neutrophil count and reduce the frequency and severity of infections in patients with severe congenital neutropenia, cyclic neutropenia, and some cases of acquired chronic neutropenia.
- Post-Hematopoietic Stem Cell Transplantation: To accelerate neutrophil recovery.
- Side Effects: Bone pain is the most common side effect. Other potential side effects include fever, myalgias, and rarely, splenic rupture.
Treatment of the Underlying Cause
Addressing the underlying condition responsible for the neutropenia is crucial for long-term management.
- Drug-Induced Neutropenia: Discontinuation of the offending medication is usually the first step. The absolute neutrophil count (ANC) typically recovers within days to weeks after stopping the drug, depending on the specific agent and the duration of exposure.
- Hematologic Malignancies (Leukemia, Lymphoma, Myelodysplasia): Treatment involves chemotherapy, radiation therapy, targeted therapies, or hematopoietic stem cell transplantation, depending on the specific malignancy and its stage. Management of neutropenia is an integral part of the overall treatment strategy.
- Autoimmune Neutropenia: Treatment may involve corticosteroids, intravenous immunoglobulin (IVIG), rituximab (a monoclonal antibody targeting B cells), or other immunosuppressants.
- Nutritional Deficiencies (Vitamin B12, Folate, Copper): Supplementation with the deficient nutrient is essential.
- Congenital Neutropenia Syndromes: Long-term G-CSF therapy is often the mainstay of treatment. Hematopoietic stem cell transplantation may be considered in severe cases, particularly those with an increased risk of leukemia.
- Splenomegaly (Hypersplenism): Treatment focuses on managing the underlying cause of splenomegaly. Splenectomy may be considered in selected cases of chronic severe neutropenia due to hypersplenism.
- Infections: Treating the underlying infection may lead to the resolution of transient neutropenia caused by increased neutrophil utilization or bone marrow suppression.
Supportive Care
General measures to prevent infection and support the patient are essential.
- Meticulous Hygiene: Frequent handwashing is crucial for patients and caregivers.
- Avoidance of Sick Contacts: Patients should avoid individuals with known infections.
- Safe Food Handling: Following guidelines for safe food preparation and consumption to minimize the risk of foodborne infections.
- Avoidance of Invasive Procedures: Procedures that can introduce infection should be minimized when the acute neutrophil count (ANC) is low.
- Dental Care: Maintaining good oral hygiene is important to prevent oral infections.
- Vaccination: In general, live vaccines should be avoided in severely immunocompromised patients. Inactivated vaccines may be given when the acute neutrophil count (ANC) is adequate or improving, but the immune response may be suboptimal.
- Patient Education: Educating patients and their families about the signs and symptoms of infection and the importance of seeking prompt medical attention.
Prophylactic Measures
In certain high-risk patients with prolonged or severe neutropenia, prophylactic antimicrobial medications may be considered.
- Antibacterial Prophylaxis: May be used in patients with prolonged severe neutropenia, especially those undergoing hematopoietic stem cell transplantation or with certain hematologic malignancies. Fluoroquinolones or trimethoprim-sulfamethoxazole are sometimes used.
- Antifungal Prophylaxis: Often indicated in patients with prolonged severe neutropenia, particularly after hematopoietic stem cell transplantation or during intensive chemotherapy for hematologic malignancies. Azole antifungals (e.g., fluconazole, voriconazole, posaconazole) or echinocandins may be used.
- Antiviral Prophylaxis: May be considered in patients at risk for specific viral infections, such as herpes simplex virus reactivation in hematopoietic stem cell transplant recipients.
Prognosis and Potential Complications
The prognosis of neutropenia is not a single entity but rather a spectrum influenced by a complex interplay of factors. While mild and transient forms often resolve without significant long-term consequences, severe and chronic neutropenia, especially when associated with serious underlying conditions or recurrent severe infections, can have a more guarded prognosis. Careful monitoring, prompt management of infections, and effective treatment of the underlying cause are crucial for optimizing outcomes in patients with neutropenia.
The major complications of neutropenia revolve around the increased risk and severity of infections. These infections can be bacterial, fungal, or viral, and can lead to life-threatening conditions like sepsis. Additionally, the treatments used to manage neutropenia and prevent infections can have their own set of complications. Vigilant monitoring, prompt diagnosis and treatment of infections, and careful management of the underlying cause are crucial to minimize these risks and improve outcomes for patients with neutropenia.
Severe and Life-Threatening Infections
This is the most immediate and dangerous complication of neutropenia. Without adequate neutrophils, the body cannot effectively contain and eliminate invading pathogens.
- Bacterial Infections: Neutropenic patients are highly susceptible to a wide range of bacterial infections, which can rapidly progress. Common sites include the bloodstream (bacteremia, sepsis), lungs (pneumonia), skin and soft tissues (cellulitis, abscesses), and gastrointestinal tract (typhlitis). These infections can quickly become systemic and life-threatening.
- Fungal Infections: Patients with prolonged and severe neutropenia are at increased risk of invasive fungal infections, such as aspergillosis, candidiasis, and mucormycosis. These infections can be difficult to diagnose and treat and often have high mortality rates.
- Viral Infections: While neutrophils are primarily involved in bacterial and fungal defense, severe neutropenia can also increase susceptibility to certain viral infections or reactivation of latent viruses.
- Opportunistic Infections: As the absolute neutrophil count (ANC) drops, individuals become vulnerable to infections caused by organisms that typically do not cause disease in immunocompetent individuals (e.g., Pneumocystis jirovecii pneumonia, certain molds).
Invasive Fungal Infections (IFIs)
As mentioned above, IFIs are a particularly serious complication in patients with prolonged severe neutropenia, especially those undergoing chemotherapy or hematopoietic stem cell transplantation. These infections can be difficult to diagnose early and often require aggressive and prolonged antifungal therapy. Mortality rates associated with IFIs in neutropenic patients can be high.
Sepsis and Septic Shock
Uncontrolled infections in neutropenic patients can lead to sepsis, a life-threatening condition characterized by a dysregulated host response to infection. This can progress to septic shock, with organ dysfunction, dangerously low blood pressure, and a high risk of death. The lack of typical inflammatory signs in neutropenic patients can delay the recognition of sepsis.
Treatment-Related Complications
The treatments used to manage the underlying cause of neutropenia or to prevent infections can also have their own complications.
- Complications of G-CSF/GM-CSF Therapy: While generally well-tolerated, these growth factors can cause bone pain, fever, myalgias, and rarely, splenic rupture or allergic reactions.
- Complications of Antibiotic and Antifungal Prophylaxis: Long-term use of prophylactic antimicrobials can lead to the development of drug-resistant organisms, gastrointestinal side effects, and potential drug interactions.
- Complications of Immunosuppressive Therapy: Used in autoimmune neutropenia, these drugs can increase the risk of opportunistic infections and have other systemic side effects.
- Complications of Hematopoietic Stem Cell Transplantation: This treatment, used for some causes of severe neutropenia, carries significant risks, including graft-versus-host disease, infections, and transplant failure.
- Complications of Chemotherapy: Chemotherapy, a common cause of neutropenia, has numerous potential side effects, including nausea, vomiting, hair loss, mucositis, and other organ toxicities.
Delayed or Atypical Presentation of Infections
Due to the lack of sufficient neutrophils, the typical signs of inflammation (redness, swelling, pus formation) may be blunted or absent in neutropenic patients. This can make it challenging to diagnose infections early, potentially leading to delays in treatment and a poorer prognosis. Fever may be the only early sign of a serious infection.
Increased Morbidity and Mortality
Overall, neutropenia, particularly when severe and prolonged, is associated with increased morbidity (illness and complications) and mortality (risk of death) due to the heightened susceptibility to infections and their potentially rapid and severe progression.
Frequently Asked Questions (FAQs)
Can neutropenia turn into leukemia?
Neutropenia generally does not directly turn into leukemia, but certain types, particularly severe congenital neutropenia, carry a significantly increased risk of developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) over time, potentially influenced by genetic factors and long-term G-CSF treatment. Other forms of neutropenia, such as drug-induced or autoimmune, typically do not transform into leukemia, although underlying bone marrow disorders causing neutropenia may themselves have leukemic potential.
What is a Stage 4 neutropenia?
While there isn’t a universally recognized “Stage 4” for neutropenia in the same way that cancer is staged, the term is sometimes informally used to refer to the most severe form of neutropenia, which is more accurately classified as severe neutropenia or sometimes even agranulocytosis. This corresponds to an absolute neutrophil count (ANC) of less than 200 cells/µL (< 0.2 x 109/L), and in some classifications, less than 100 cells/µL (< 0.1 x 109/L). At this level, the risk of severe, life-threatening infections, including opportunistic infections, is extremely high, and the body’s inflammatory response may be significantly impaired.
Can neutropenia fix itself?
Yes, in many cases, neutropenia can fix itself, depending on the underlying cause.
For example:
- Neutropenia caused by a transient viral infection often resolves spontaneously as the infection clears.
- Drug-induced neutropenia typically improves and neutrophil counts return to normal after the offending medication is discontinued. The recovery time can vary from days to weeks depending on the drug and the duration of exposure.
- Neutropenia due to nutritional deficiencies (like vitamin B12 or folate deficiency) can improve with appropriate supplementation.
- Acute, transient neutropenia following certain acute illnesses might resolve on its own.
However, chronic neutropenia due to underlying conditions like severe congenital neutropenia, autoimmune disorders, or bone marrow failure syndromes often does not resolve spontaneously and may require ongoing management or treatment. The likelihood of neutropenia fixing itself is heavily dependent on identifying and addressing the root cause.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Justiz Vaillant AA, Rout P, Reynolds SB, et al. Neutropenia. [Updated 2024 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- Blumenreich MS. The White Blood Cell and Differential Count. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 153.
- Trevisani GFM, Bento VFV, Salles Rosa Neto N. Late-Onset Neutropenia Induced by Rituximab in Rheumatic Diseases: A Report of Two Cases of Severe Presentation and a Literature Review. Cureus. 2025 Mar 5;17(3):e80074. doi: 10.7759/cureus.80074. PMID: 40190867; PMCID: PMC11970875.




