TL;DR
Tachycardia is defined as when the heart rate > 100 bpm in adults. It is often a crucial sign of serious underlying issues in hematology patients.
- Anemia: Most frequent, heart compensates for low oxygen.
- Infections/Sepsis: Common in immunocompromised patients.
- Hypovolemia/Dehydration: Due to fluid loss or poor intake.
- Electrolyte Imbalances: e.g., low potassium or magnesium.
- Medication Side Effects: From chemo, growth factors, steroids.
- Thromboembolism: Especially pulmonary embolism (PE).
- Anxiety, fever, pain.
Symptoms ▾: Palpitations, shortness of breath, chest pain, dizziness, syncope, fatigue.
Key Investigations ▾: CBC (for anemia/infection), electrolytes, CRP/Procalcitonin (for infection), ECG (to differentiate types).
- Primary Goal: Treat the underlying cause (e.g., blood transfusion for anemia, antibiotics for infection, fluids for dehydration).
- Monitoring: Continuous cardiac monitoring, vital signs.
*Click ▾ for more information
Introduction
Tachycardia is defined as a heart rate exceeding 100 beats per minute (bpm) in adults.
In hematology, recognizing and addressing tachycardia is important because it often serves as a crucial clinical indicator of underlying conditions frequently encountered in hematological patients.
These conditions can range from anemia (where the heart compensates for reduced oxygen-carrying capacity by beating faster) and infection/sepsis (common in immunocompromised patients) to hypovolemia, electrolyte imbalances, or even serious thromboembolic events like pulmonary embolism.
Early detection and investigation of tachycardia can therefore lead to prompt diagnosis and intervention for these potentially life-threatening complications, significantly impacting patient outcomes.
Definition of Tachycardia
For most healthy adults, a normal resting heart rate (HR) falls within the range of 60 to 100 beats per minute (bpm). It’s important to note that this is a resting heart rate. During physical activity, stress, or other physiological demands, the heart rate will naturally increase. Factors like age, fitness level (athletes often have lower resting heart rates), and certain medications can influence an individual’s normal resting heart rate.
Different Grades of Tachycardia
While a universal, strictly defined “grade” system for tachycardia (mild, moderate, severe) based solely on heart rate isn’t as widely standardized as blood pressure classification, clinicians often interpret the severity based on the degree of elevation and the patient’s symptoms and hemodynamic stability.
However, for educational purposes, one can consider a pragmatic classification:
- Mild Tachycardia: Heart rates generally between 100-120 bpm. Often well-tolerated, especially if it’s a physiological response (e.g., due to mild anxiety, fever, or dehydration).
- Moderate Tachycardia: Heart rates typically between 121-150 bpm. At these rates, symptoms may become more noticeable (palpitations, some shortness of breath), and the underlying cause is more likely to require medical attention.
- Severe Tachycardia: Heart rates consistently above 150 bpm. These rates are more concerning and frequently lead to significant symptoms (e.g., chest pain, syncope, significant dyspnea) and/or hemodynamic instability, requiring urgent investigation and intervention.
However, clinical context is paramount. A heart rate of 120 bpm in a healthy, exercising individual is normal, whereas 120 bpm at rest in a patient with a hematological malignancy could be a sign of severe sepsis.
Distinction Between Sinus Tachycardia and Other Arrhythmias
This is a critical distinction in clinical practice, as the management differs significantly.
Sinus Tachycardia (ST)
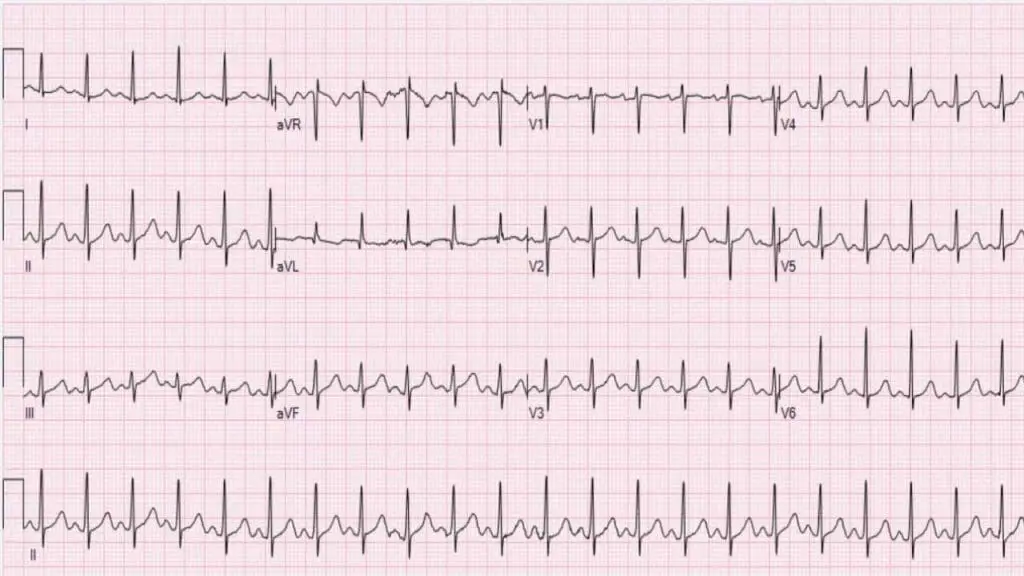
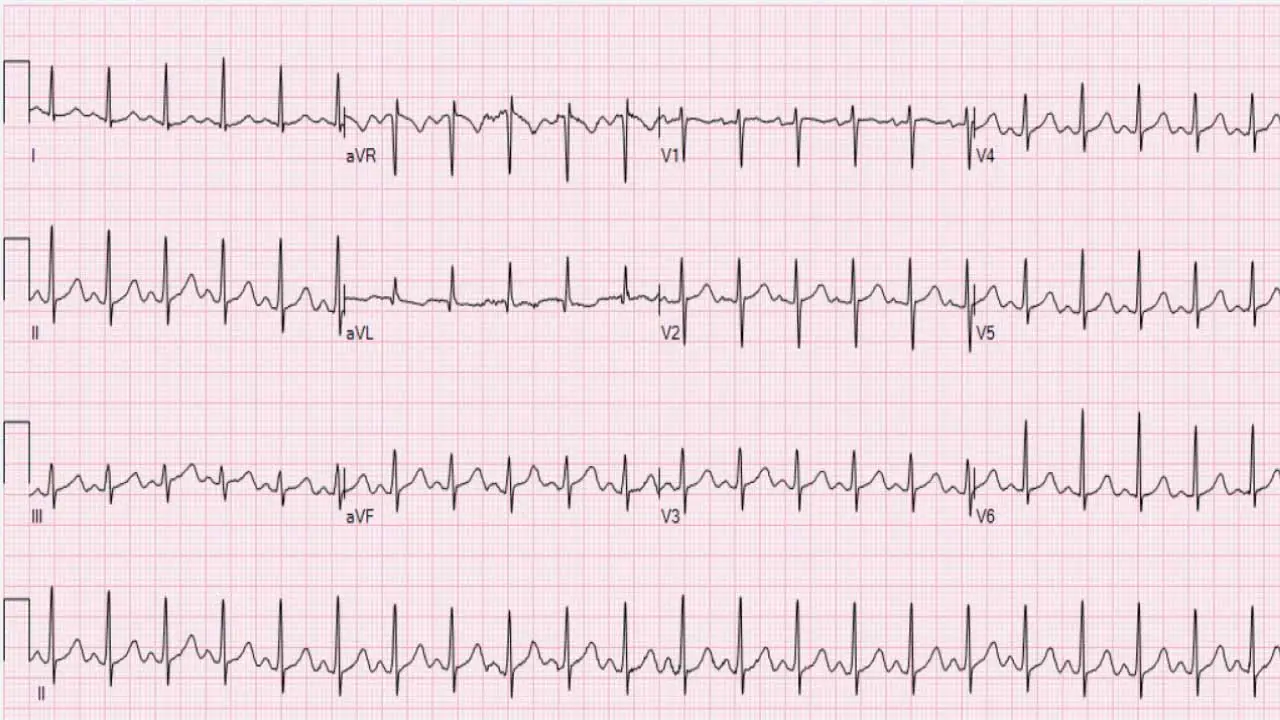
- Origin: Electrical impulses originate from the sinoatrial (SA) node, the heart’s natural pacemaker, but at an accelerated rate.
- ECG Characteristics:
- Regular rhythm: The R-R intervals (time between consecutive QRS complexes) are typically consistent.
- Normal P wave morphology: Each QRS complex is preceded by a P wave, and the P waves generally have a normal shape and axis (positive in lead II).
- 1:1 P:QRS ratio: Every P wave is followed by a QRS complex.
- Gradual onset and offset: The heart rate typically speeds up and slows down gradually in response to the physiological stimulus (e.g., with exercise, the heart rate increases progressively; upon stopping, it decreases gradually).
- Nature: Sinus tachycardia is usually a physiological response to an underlying stressor or condition, rather than an arrhythmia in itself. It’s the heart’s way of compensating for an increased metabolic demand or decreased cardiac output.
- Common Causes: Fever, pain, anxiety, exercise, hypovolemia, anemia, hyperthyroidism, hypoxemia, sepsis, certain medications.
Other Arrhythmias (Pathological Tachycardias)
These are characterized by abnormal electrical activity not originating solely from a fast SA node firing, or by abnormal conduction pathways. They can be broadly categorized by their origin.
Supraventricular Tachycardias (SVTs)
Originating above the ventricles (in the atria or AV node). They generally result in a narrow QRS complex on an ECG (indicating normal ventricular conduction).
- Atrial Fibrillation (AFib)
- Irregularly irregular rhythm: The most distinctive feature.
- Absence of discernible P waves: Instead, there’s chaotic atrial activity (fibrillatory waves).
- Variable ventricular rate: Often rapid and unpredictable.
- Atrial Flutter
- “Sawtooth” P waves (flutter waves): Especially visible in inferior leads (II, III, aVF).
- Regular or irregular rhythm: Can have fixed (e.g., 2:1, 3:1) or variable AV block.
- AV Nodal Reentrant Tachycardia (AVNRT) & AV Reentrant Tachycardia (AVRT) (e.g., Wolff-Parkinson-White Syndrome)
- Abrupt onset and termination: Often described as “paroxysmal.”
- Very regular rhythm.
- P waves may be absent or hidden within the QRS complex (AVNRT) or appear retrograde (AVRT).
- Rates often higher than typical sinus tachycardia (e.g., 150-250 bpm).
Ventricular Tachycardia (VT)
Originating in the ventricles. These are generally wide QRS complex tachycardias (indicating abnormal, slower ventricular conduction).
- Characteristics: Rapid, regular or irregular wide QRS complexes.
- Severity: Can be life-threatening and often requires immediate intervention as it can degenerate into ventricular fibrillation (VFib).
- Causes: Often associated with structural heart disease, myocardial ischemia/infarction, electrolyte imbalances.
| Feature | Sinus Tachycardia | Other Arrhythmias (Examples) |
| Origin | SA node | Atria, AV node, or Ventricles (ectopic foci or reentrant circuits) |
| ECG Rhythm | Regular | Can be regular (e.g., SVT, VT) or irregularly irregular (e.g., AFib) |
| P Waves | Present, normal morphology, precede every QRS | Absent (AFib), “sawtooth” (Atrial Flutter), hidden/retrograde (SVTs), or dissociated from QRS (VT) |
| QRS Complex Width | Narrow (usually) | Narrow (SVTs) or Wide (VT, SVT with aberrancy) |
| Onset/Offset | Gradual (“warm up” and “cool down”) | Often abrupt/paroxysmal |
| Physiological Role | Compensatory response to an underlying stressor | Pathological electrical abnormality |
| Typical Rate | Rarely exceeds 150-160 bpm in adults at rest | Can be much higher (e.g., 150-250+ bpm for SVTs, VT) |
Causes of Tachycardia in Hematological Patients
Tachycardia in hematological patients can arise from a complex interplay of direct effects of their underlying disease, side effects of treatments, and common complications.
Direct Effects of Hematological Conditions and Their Complications
Anemia
This is perhaps the most common cause of tachycardia in hematology. Anemia, defined as a reduced red blood cell count or hemoglobin concentration, leads to a decrease in the oxygen-carrying capacity of the blood. To compensate for this reduced oxygen delivery to tissues, the heart works harder and faster, increasing cardiac output by elevating the heart rate.
Tachycardia can be seen in various types of anemia, including:
- Iron deficiency anemia: Chronic blood loss (e.g., gastrointestinal bleeding in a patient with a hematological malignancy), poor absorption.
- Aplastic anemia: Bone marrow failure leading to pancytopenia.
- Hemolytic anemia: Accelerated destruction of red blood cells (e.g., autoimmune hemolytic anemia, paroxysmal nocturnal hemoglobinuria, sickle cell disease). In sickle cell disease, chronic anemia combined with vaso-occlusive crises can frequently induce tachycardia.
The degree of tachycardia generally correlates with the severity of anemia; profound anemia will elicit a more significant compensatory heart rate increase.
Infections/Sepsis
- Immunocompromised State: Hematological patients are often severely immunocompromised due to their underlying disease (e.g., leukemia, lymphoma, multiple myeloma) or aggressive treatments like chemotherapy and stem cell transplantation.
- Systemic Inflammatory Response: Infections, especially systemic ones like sepsis, trigger a widespread inflammatory response. This leads to increased metabolic demand, vasodilation, and a compensatory increase in heart rate and cardiac output to maintain tissue perfusion. Tachycardia can be an early and critical sign of developing sepsis.
Hypovolemia/Dehydration
Patients may experience significant fluid loss due to:
- Gastrointestinal side effects of treatment: Nausea, vomiting, diarrhea from chemotherapy or mucositis.
- Poor oral intake: Due to illness, pain, or side effects.
- Hemorrhage: Patients with thrombocytopenia or coagulopathies (common in hematological malignancies or myelosuppressive treatments) are at high risk for bleeding (e.g., gastrointestinal hemorrhage, epistaxis, intracranial hemorrhage), leading to acute blood loss and hypovolemia.
Decreased intravascular volume reduces venous return to the heart, leading to a compensatory increase in heart rate to maintain blood pressure and cardiac output.
Electrolyte Imbalances
Several electrolyte disturbances can disrupt normal cardiac electrical activity and lead to tachycardia.
Common Imbalances:
- Hypokalemia (low potassium): Can prolong the QT interval and predispose to ventricular arrhythmias.
- Hypomagnesemia (low magnesium): Often accompanies hypokalemia and can exacerbate arrhythmogenicity.
- Hypercalcemia (high calcium): Can be a complication of certain hematological malignancies (e.g., multiple myeloma) due to bone destruction, leading to various arrhythmias, including tachycardia.
Thromboembolic Events
Cancer patients, including those with hematological malignancies, are at an increased risk of developing venous thromboembolism (VTE) due to disease-related factors (e.g., inflammation, tumor-induced procoagulant factors) and treatment-related factors (e.g., central venous catheters, certain chemotherapies, immobility).
In pulmonary embolism, where a blood clot lodges in the pulmonary arteries, it causes an acute increase in pulmonary vascular resistance. The right ventricle has to work harder, leading to reflex tachycardia. Tachycardia, often accompanied by dyspnea and pleuritic chest pain, is a hallmark symptom of pulmonary embolism.
Paraneoplastic Syndromes (Rare)
In rare instances, tumors can produce hormones or other substances that directly affect the heart or the autonomic nervous system, leading to various cardiac manifestations, including tachycardia.
Anxiety/Stress
Hematological diagnoses and treatments are inherently stressful, leading to significant anxiety and emotional distress.
Emotional stress activates the sympathetic nervous system, releasing catecholamines that increase heart rate. This can exacerbate existing tachycardia or be the sole cause of sinus tachycardia, especially in the absence of other physiological stressors.
Medication Side Effects
Many drugs used in hematology can directly or indirectly cause tachycardia.
Chemotherapeutic Agents
While many chemotherapies are associated with various forms of cardiotoxicity (e.g., cardiomyopathy), some can cause acute or chronic tachycardia or arrhythmias.
- Anthracyclines (e.g., Doxorubicin): While famous for their long-term cardiomyopathy risk, they can acutely cause arrhythmias, including tachycardia.
- Tyrosine Kinase Inhibitors (TKIs): Several TKIs, especially those used for chronic myeloid leukemia (CML) or other hematological malignancies (e.g., ibrutinib used in chronic lymphocytic leukemia), can be associated with arrhythmias, including atrial fibrillation and, less commonly, ventricular arrhythmias, which present as tachycardia. They can affect ion channels in the heart.
- Other agents: Some other chemotherapeutic agents may also contribute to tachycardia through various mechanisms, including systemic inflammatory responses or direct effects on cardiac electrical activity.
Growth Factors
- Granulocyte Colony-Stimulating Factor (G-CSF) (e.g., Filgrastim, Pegfilgrastim): Used to stimulate neutrophil production to prevent neutropenia, G-CSF can cause bone pain (which can lead to pain-mediated tachycardia), and sometimes more systemic inflammatory responses that might result in tachycardia. There are also reports of G-CSF inducing cardiac events in susceptible individuals.
- Erythropoietin-Stimulating Agents (ESAs) (e.g., Epoetin alfa, Darbepoetin alfa): Used to treat anemia, ESAs can increase blood viscosity and blood pressure, which may indirectly lead to a compensatory increase in heart rate. While not a direct tachycardic agent, associated adverse events like hypertension or thromboembolism could lead to it.
Corticosteroids (e.g., Dexamethasone, Prednisone)
Commonly used in many hematological conditions (e.g., multiple myeloma, lymphomas), corticosteroids can directly increase heart rate through sympathetic activation and can also cause electrolyte imbalances (e.g., hypokalemia), contributing to tachycardia.
Transfusion Reactions
Febrile non-hemolytic transfusion reactions, allergic reactions, or acute hemolytic transfusion reactions are immune-mediated responses to blood products that can cause fever, chills, and systemic inflammation, leading to compensatory tachycardia.
Other Contributing Factors
- Fever: Any source of fever, whether infectious or related to the underlying malignancy (e.g., tumor fever in lymphoma), will increase metabolic rate and cause compensatory tachycardia.
- Pain: Severe pain, common in patients with bone involvement from malignancy or post-procedural pain, activates the sympathetic nervous system and can significantly elevate heart rate.
- Hypoxemia: Respiratory compromise from pneumonia, acute respiratory distress syndrome (ARDS), or large pleural effusions can lead to hypoxemia, triggering compensatory tachycardia to try and deliver more oxygen.
- Cardiac Conditions: While not specific to hematology, patients may have pre-existing cardiac conditions (e.g., heart failure, coronary artery disease, arrhythmias) that can be exacerbated by the stress of their hematological disease or its treatment, leading to tachycardia.
Clinical Signs & Symptoms of Tachycardia
Some patients may be asymptomatic, particularly with mild or transient tachycardia, others can experience a wide range of manifestations depending on the underlying cause, the rate of the tachycardia, its duration, and the patient’s overall cardiac health.
Subjective Symptoms (What the patient feels)
- Palpitations: This is the most common complaint. Patients describe an unpleasant awareness of their own heartbeat. They might feel their heart “pounding,” “racing,” “skipping beats,” “fluttering,” or “thumping” in their chest, neck, or throat. While subjective, palpitations are a direct indicator of an altered heart rhythm or rate and warrant further investigation.
- Shortness of Breath (Dyspnea): Difficulty breathing or a feeling of not getting enough air. At very high heart rates, the heart has less time to fill with blood between beats, reducing cardiac output. This can lead to pulmonary congestion (fluid buildup in the lungs) and increased respiratory effort to compensate for reduced oxygen delivery to tissues. It can also be a symptom of the underlying cause (e.g., anemia, pulmonary embolism).
- Chest Pain or Discomfort (Angina): A pressure, tightness, squeezing, or aching sensation in the chest. A rapid heart rate increases myocardial oxygen demand. If the blood supply through the coronary arteries cannot meet this increased demand (e.g., in patients with underlying coronary artery disease or severe anemia), myocardial ischemia (lack of oxygen to the heart muscle) can occur, leading to angina.
- Dizziness/Lightheadedness (Presyncope): A feeling of being faint, unsteady, or about to lose consciousness. Reduced cardiac output due to very rapid heart rates can lead to decreased blood flow and oxygen delivery to the brain.
- Syncope (Fainting/Loss of Consciousness): A transient loss of consciousness due to insufficient blood flow to the brain, followed by spontaneous recovery. This is a more severe manifestation of cerebral hypoperfusion than dizziness. It indicates a significant and sudden drop in cardiac output, which can occur with very fast or hemodynamically significant tachyarrhythmias (e.g., sustained ventricular tachycardia).
- Fatigue/Weakness: A generalized feeling of tiredness, lack of energy, or muscle weakness. Chronic or recurrent tachycardia, even if not critically high, can lead to reduced cardiac efficiency and overall decreased tissue perfusion, resulting in persistent fatigue. It can also be a prominent symptom of the underlying cause (e.g., anemia, chronic infection).
- Reduced Exercise Tolerance: Inability to perform physical activities that were previously manageable, or experiencing symptoms like dyspnea and palpitations with minimal exertion. The heart is already working fast at rest, leaving less reserve for increased demands during activity.
Objective Signs (What the clinician observes)
- Elevated Heart Rate: Directly measured via pulse palpation (radial, carotid), auscultation (stethoscope over the apex), or continuous cardiac monitoring. The primary diagnostic sign. A resting heart rate consistently above 100 bpm is the definition of tachycardia.
- Hypotension (Low Blood Pressure): If the heart rate is so fast that there isn’t enough time for the ventricles to fill adequately between beats, cardiac output drops significantly, leading to a fall in blood pressure. This indicates hemodynamic instability and is an ominous sign.
- Altered Mental Status: Confusion, disorientation, lethargy, or decreased level of consciousness. Similar to dizziness and syncope, this is due to decreased blood flow and oxygen delivery to the brain (cerebral hypoperfusion).
- Cool, Clammy Skin (Peripheral Hypoperfusion): Skin that feels cold and moist to the touch, often pale or mottled. The body shunts blood away from the periphery to prioritize vital organs (brain, heart) in response to decreased cardiac output, leading to reduced peripheral perfusion.
- Delayed Capillary Refill Time: When pressure is applied to a nail bed and released, the return of color takes longer than 2 seconds. This is another indicator of poor peripheral perfusion.
- Oliguria/Anuria (Significantly Reduced or Absent Urine Output): Reduced renal perfusion due to decreased cardiac output can impair kidney function.
- Diaphoresis (Excessive Sweating): A non-specific sign of sympathetic nervous system activation, often accompanying stress, pain, or significant hemodynamic compromise.
- Signs Related to the Underlying Cause:
- Pallor: (Pale skin/mucous membranes) – often indicating anemia.
- Fever/Chills: Suggestive of infection/sepsis.
- Signs of Dehydration: Dry mucous membranes, decreased skin turgor.
- Signs of Pulmonary Embolism: Tachypnea (rapid breathing), pleuritic chest pain, cough, decreased oxygen saturation.
- Signs of Heart Failure: Jugular venous distension (JVD), peripheral edema, crackles (rales) on lung auscultation.
General Laboratory Approach to Tachycardia in Hematological Patients
When approaching laboratory investigations for tachycardia in hematological patients, the goal is to identify the underlying cause, which is often multifactorial in this complex patient population. The investigations should be guided by the patient’s clinical presentation, vital signs, and recent medical history.
Initial & Essential Investigations (First-Line)
These tests are typically ordered immediately to rule out common and treatable causes.
- Complete Blood Count (CBC) with Differential and Peripheral Smear: These are crucial for assessing anemia, a very common cause of compensatory tachycardia in hematology. It also helps detect signs of infection (elevated white blood cell count, abnormal differential), myelosuppression, or active bleeding (low hemoglobin/hematocrit, platelet count). A peripheral smear can reveal abnormal cell red cell morphologies or white cell morphologies indicative of certain hematological disorders (e.g., schistocytes in hemolytic anemia, blasts in leukemia).
- Electrolytes (Sodium, Potassium, Magnesium, Calcium, Phosphate): Imbalances in these electrolytes can directly affect cardiac electrical activity and lead to arrhythmias, including various forms of tachycardia. Hypokalemia and hypomagnesemia are particularly arrhythmogenic. Hypercalcemia is common in certain hematological malignancies (e.g., multiple myeloma) and can also cause arrhythmias.
- Renal Function Tests (Urea and Creatinine): Kidney dysfunction can lead to electrolyte imbalances.
- Inflammatory Markers (C-reactive protein (CRP) and Procalcitonin): To screen for systemic inflammation and infection, especially sepsis, which is a major cause of tachycardia in immunocompromised hematology patients. Procalcitonin is often more specific for bacterial infections compared to CRP.
- Blood Cultures: If infection/sepsis is suspected based on fever, elevated inflammatory markers, or clinical signs, blood cultures are essential to identify the causative organism and guide targeted antibiotic therapy.
- Blood Glucose: Both hypoglycemia and hyperglycemia can cause stress responses leading to tachycardia.
Secondary & Targeted Investigations (Depending on Clinical Suspicion)
Once initial results are available or if the cause remains unclear, further targeted tests may be needed.
- Thyroid Function Tests (TSH, free T3, free T4): These tests are to rule out hyperthyroidism, which is a well-known cause of sinus tachycardia and other arrhythmias.
- Cardiac Markers (Troponin, BNP/NT-proBNP): If myocardial ischemia (chest pain, ECG changes) or heart failure is suspected.
- Troponin: Indicates myocardial injury.
- BNP/NT-proBNP: Elevated in heart failure, which can be exacerbated or present with tachycardia.
- Arterial Blood Gas (ABG): Assesses oxygenation (hypoxemia can cause compensatory tachycardia) and acid-base status (acidosis or alkalosis can influence heart rate and rhythm).
- Coagulation Profile (PT, aPTT, D-dimer): Coagulation profile is important if a thromboembolic event, particularly pulmonary embolism (PE), is suspected. Elevated D-dimer is a sensitive, though not specific, marker for PE and deep vein thrombosis (DVT). Hemostasis abnormalities are common in hematological malignancies.
- Liver Function Tests (LFTs): Liver dysfunction can affect drug metabolism and contribute to electrolyte imbalances or coagulopathies.
- Urinalysis & Urine Culture: To screen for urinary tract infections, especially in febrile patients.
- Toxicology Screen: If drug overdose, illicit drug use, or specific medication toxicities (e.g., cardiotoxic drugs) are suspected.
- Specific Hematological Disease Markers: Depending on the known or suspected hematological diagnosis, further specialized tests might be indicated (e.g., serum protein electrophoresis/immunofixation for multiple myeloma, specific genetic tests for myeloproliferative neoplasms, direct antiglobulin test for hemolytic anemia).
Imaging and Other Diagnostic Modalities (Beyond Lab Tests, but often guided by them)
While not “laboratory investigations,” these are crucial diagnostic steps that often follow or are prompted by lab findings:
- Electrocardiogram (ECG): Essential for differentiating sinus tachycardia from other arrhythmias (e.g., atrial fibrillation, supraventricular tachycardia, ventricular tachycardia). It can also show signs of ischemia, electrolyte abnormalities, or drug effects.
- Chest X-ray (CXR): To assess for pulmonary infiltrates (pneumonia), pleural effusions, signs of heart failure, or mediastinal widening (suggesting large lymphadenopathy or tumor).
- Echocardiogram (Echo): To assess cardiac structure and function, including ejection fraction, valvular abnormalities, and pericardial effusions. This is particularly relevant if heart failure or structural heart disease is suspected.
- CT Pulmonary Angiogram (CTPA): If pulmonary embolism is strongly suspected (e.g., positive D-dimer with clinical probability).
Treatment and Management of Tachycardia in Hematological Patients
The treatment and management of tachycardia in hematological patients requires a comprehensive approach, as it fundamentally hinges on identifying and addressing the underlying cause. Simply slowing the heart rate without resolving the precipitating factor can be ineffective or even detrimental.
Addressing the Underlying Cause (Primary Management)
This is the cornerstone of managing tachycardia in hematological patients. Treatment should be tailored to the specific etiology.
Anemia
- Blood Transfusion: For symptomatic or severely anemic patients (e.g., hemoglobin < 7-8 g/dL or higher if symptomatic). This is the most rapid way to improve oxygen-carrying capacity.
- Iron Supplementation: For iron-deficiency anemia (oral or IV).
- Erythropoietin-Stimulating Agents (ESAs): In select cases of anemia of chronic disease or chemotherapy-induced anemia, though their use requires careful consideration of potential risks (e.g., thrombosis).
- Addressing underlying cause of anemia: e.g., treating bleeding source, managing chronic kidney disease.
Infections/Sepsis
- Prompt Broad-Spectrum Antibiotics: Initiate immediately in suspected sepsis, especially in febrile neutropenic patients.
- Targeted Antimicrobials: Once culture results are available, narrow the antibiotic spectrum.
- Source Control: Drain abscesses, remove infected lines/catheters.
- Supportive Care: IV fluids, vasopressors (if hypotensive), oxygen, fever control.
Hypovolemia/Dehydration
- Intravenous Fluid Resuscitation: Administer crystalloids (e.g., normal saline) rapidly to restore intravascular volume and improve perfusion.
- Address Fluid Losses: Antiemetics for nausea/vomiting, antidiarrheals if appropriate, management of bleeding.
- Monitor Fluid Balance: Strict input and output charting.
Electrolyte Imbalances
- Correction of Deficiencies: Replete potassium, magnesium, calcium, and phosphate intravenously or orally as needed.
- Monitor Electrolyte Levels: Frequent re-checks to ensure correction and prevent overcorrection.
- Address underlying causes: e.g., managing tumor lysis syndrome, treating renal insufficiency.
Medication Side Effects
- Dose Adjustment or Discontinuation: If the implicated medication is not critical or an alternative is available.
- Symptomatic Treatment: If the medication must be continued and the tachycardia is manageable (e.g., low-dose beta-blocker if appropriate and not contraindicated).
- Careful monitoring: Especially for drugs known for cardiotoxicity.
Thromboembolic Events (e.g., Pulmonary Embolism)
- Anticoagulation: Immediate initiation of therapeutic anticoagulation (e.g., low molecular weight heparin, unfractionated heparin, followed by oral anticoagulants).
- Thrombolysis/Embolectomy: In cases of massive PE with hemodynamic instability.
- Oxygen Support: To improve oxygenation.
Anxiety/Stress
- Reassurance and Education: Explain the causes of tachycardia and management plan.
- Anxiolytics: Short-term use of benzodiazepines (e.g., lorazepam) if severe anxiety is contributing significantly.
- Non-pharmacological approaches: Relaxation techniques, counseling.
General Supportive Care and Monitoring
- Continuous Cardiac Monitoring: To track heart rate and rhythm, detect arrhythmias, and monitor response to interventions. Essential in unstable patients or those with severe tachycardia.
- Vital Signs Monitoring: Frequent monitoring of blood pressure, respiratory rate, oxygen saturation, and temperature to assess hemodynamic stability and identify worsening conditions or new complications.
- Oxygen Therapy: Administer supplemental oxygen if the patient is hypoxic (SpO2 < 92-94%) to reduce myocardial workload and improve tissue oxygenation.
- Fluid Balance Monitoring: Strict input and output charting, daily weights, to guide fluid resuscitation or restriction.
- Pain Management: Adequate analgesia is crucial, as pain is a significant contributor to tachycardia.
- Nutritional Support: Ensure adequate nutrition to support recovery and overall patient well-being, especially in chronically ill hematological patients.
- Patient Education: Educate patients and their families about the symptoms of tachycardia, when to report them, and the importance of adherence to treatment plans.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Metivier, F., Marchais, S. J., Guerin, A. P., Pannier, B., & London, G. M. (2000). Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association, 15 Suppl 3, 14–18. https://doi.org/10.1093/oxfordjournals.ndt.a027970
- PORTER, W. B., & JAMES, G. W., 3rd (1953). The heart in anemia. Circulation, 8(1), 111–116. https://doi.org/10.1161/01.cir.8.1.111
- SANGHVI, L. M., SHARMA, R., & MISRA, S. N. (1957). Cardiovascular disturbances in chronic severe anemia. Circulation, 15(3), 373–378. https://doi.org/10.1161/01.cir.15.3.373
- Corwin, D. J., & Scarfone, R. J. (2018). Supraventricular Tachycardia Associated With Severe Anemia. Pediatric emergency care, 34(4), e75–e78. https://doi.org/10.1097/PEC.0000000000001134