TL;DR
Leukocytosis is an increase in white blood cell (WBC) count above the reference range.
Significance ▾
- Not a disease itself, but a marker of underlying infection, inflammation, or blood cancers.
Causes ▾
- Infections: Bacteria, viruses, fungi, or parasites battling your immune system.
- Inflammation: Chronic conditions like arthritis or injuries triggering immune response.
- Stress: Physical or emotional strain can temporarily elevate white blood cell count.
- Certain medications: Some drugs can stimulate white blood cell production.
- Spleen removal: This organ stores white blood cells, so its absence leads to a higher circulating count.
Symptoms ▾
- Fever
- Fatigue
- Malaise
Diagnosis ▾
- Requires a thorough history, physical exam, and complete blood count (CBC) with differential to identify the specific type of WBC elevated and potential causes.
Treatment & Management ▾
- Focuses on treating the underlying cause:
- Infections: Antibiotics, antifungals, or antivirals.
- Inflammation: Corticosteroids or immunosuppressants.
- Blood Cancers: Chemotherapy, radiation, or bone marrow transplant.
- Hyperleukocytosis Syndrome (HLS): Rare complication in some leukemias with extremely high WBC counts (>100,000/μL).
- Management involves:
- Hydration and urine alkalinization.
- Allopurinol or Rasburicase for uric acid control.
- Leukapheresis to remove excess WBCs (cautiously to avoid TLS).
- Hemodialysis in severe cases.
- Management involves:
*Click ▾ for more information
Introduction
Leukocytosis can be defined as a condition where you have an increased white blood cell (WBC) count in the blood. White blood cells, also known as leukocytes, are a critical part of the body’s immune system and help fight infection and inflammation.
Normally, the white blood cell count falls within a specific range. Leukocytosis is diagnosed when the WBC count goes above the normal range.
Normal White Blood Cell (WBC) Count Ranges
The white blood cell (WBC) count is part of a complete blood count (CBC) test, which measures various components of your blood. A CBC is frequently included in routine checkups to establish a baseline for your blood cell counts and potentially identify any underlying issues early on. Normal white blood cell (WBC) count and differential counts can vary depending on age.
| Age Group | Total WBC Count (x 109/L) | Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) | Basophils (%) |
| Newborn (0-1 day) | 9 – 34 | 30-60 | 20-60 | 3-10 | 1-6 | 0-2 |
| Infant (1 day – 1 month) | 5 – 19 | 40-70 | 20-50 | 3-10 | 1-6 | 0-2 |
| Child (1 month – 18 years) | 5 – 15 | 30-55 | 30-70 | 2-10 | 1-6 | 0-2 |
| Adult (>18 years) | 4.5 – 11 | 40-70 | 20-40 | 2-10 | 1-4 | 0-2 |
Significance of Leukocytosis
Leukocytosis, although not a specific diagnosis itself, holds significant importance as a diagnostic tool for several reasons:
Alerts to Potential Problems: An elevated white blood cell count is a red flag that something is going on in the body. It indicates the immune system is activated, potentially fighting off an infection, inflammation, or other underlying issue.
Guides Further Investigation: The presence of leukocytosis prompts further investigation to determine the cause. Doctors can consider the patient’s symptoms, medical history, and the specific type of leukocytosis (e.g., neutrophilia, lymphocytosis) to narrow down the possibilities. This can lead to a more efficient and targeted diagnostic approach.
Helps Differentiate Conditions: Leukocytosis patterns can be helpful in differentiating between different conditions.
* For example, a high neutrophil count (neutrophilia) is more suggestive of a bacterial infection, while a high lymphocyte count (lymphocytosis) might point towards a viral infection or an autoimmune disorder.
Monitors Treatment Response: In some cases, leukocytosis can be used to monitor how well a patient is responding to treatment. For instance, a decrease in white blood cell count after starting antibiotics for an infection might indicate the treatment is working.
Limitations to Consider
- Non-Specific: Leukocytosis can occur in various situations besides infection. Inflammation, tissue injury, and even some medications can cause a high WBC count.
- Variations: The normal white blood cell count can vary slightly depending on age, ethnicity, and even diurnal – it is lower in the morning and increases through the day.
- Severity Doesn’t Correlate: The degree of leukocytosis doesn’t necessarily reflect the severity of the underlying condition. Sometimes, severe infections might not cause a significant increase in WBCs.
- Can be Transient: Leukocytosis can sometimes be transient (short-lived) due to factors like stress, exercise, or certain medications.
- Can be Absent: Not all infections or inflammatory conditions lead to leukocytosis. Some individuals, especially those with compromised immune systems, might not experience a rise in WBC count despite an infection.
Physiological Leukocytosis
Leukocytosis can occur in various patient populations, but reference ranges for what constitutes “elevated” white blood cell (WBC) counts can differ depending on age and specific circumstances.
Newborns
- Higher Baseline WBC: Newborns naturally have higher baseline WBC counts compared to adults. This is due to their immature immune system and the stress of birth.
- Physiological Leukocytosis: In the first few days of life, newborns often experience a transient “physiological leukocytosis” with WBC counts reaching up to 30 x 109/L. This is normal and doesn’t necessarily indicate an infection.
- Leukocytosis in Newborns: However, a persistently high WBC count (above 30 x 109/L) or a rapid rise in WBC count can be a sign of infection, which needs prompt evaluation by a healthcare professional. The specific type of leukocytosis (neutrophilia, lymphocytosis, etc.) can offer clues about the potential type of infection.
Pregnant Women
- Physiological Leukocytosis: During pregnancy, women experience a physiological leukocytosis with WBC counts reaching up to 18 x 109/L. This is a normal adaptation to support the developing fetus and prepare for childbirth.
- Leukocytosis During Pregnancy: While a mild elevation in WBC count is expected, a significant or persistent increase can indicate an underlying infection, such as a urinary tract infection (UTI) or a vaginal infection. As with newborns, the specific type of leukocytosis can provide additional information.
- Considerations in Interpretation: When interpreting WBC counts in pregnant women, healthcare professionals consider factors like gestational age and potential sources of inflammation, such as labor or delivery.
Types of Leukocytosis
Predominant Cell Type
This classification focuses on the specific type of white blood cell that is predominantly elevated in the blood count.
- Neutrophilia: An increased number of neutrophils, the most common type of WBC. This often suggests a bacterial infection.
- Lymphocytosis: An elevated level of lymphocytes, which are involved in the immune response against viruses and other pathogens. This can be seen in viral infections, autoimmune diseases, or certain cancers.
- Monocytosis: An increase in monocytes, which are phagocytic WBCs and can indicate chronic infections, inflammatory conditions, or some cancers.
- Eosinophilia: An elevated number of eosinophils, which are involved in allergic reactions and parasite infections.
- Basophilia: The least common type of leukocytosis, characterized by an increase in basophils. This can be associated with allergic reactions, certain infections, or some bone marrow disorders.
Cell Maturity
This classification looks at the maturity of the white blood cells present in the blood.
- Shift to the Left (Leukocyte Left Shift): This indicates the presence of immature white blood cells (blasts and precursors) in the bloodstream. This can occur when the bone marrow is under stress and needs to rapidly increase WBC production, often seen in severe infections or chronic leukemia.
- Shift to the Right: A less common finding, a shift to the right indicates an increased number of mature white blood cells compared to immature ones. This is not typically associated with leukocytosis and might be seen in some vitamin B12 deficiencies.
Leukocytosis Causes
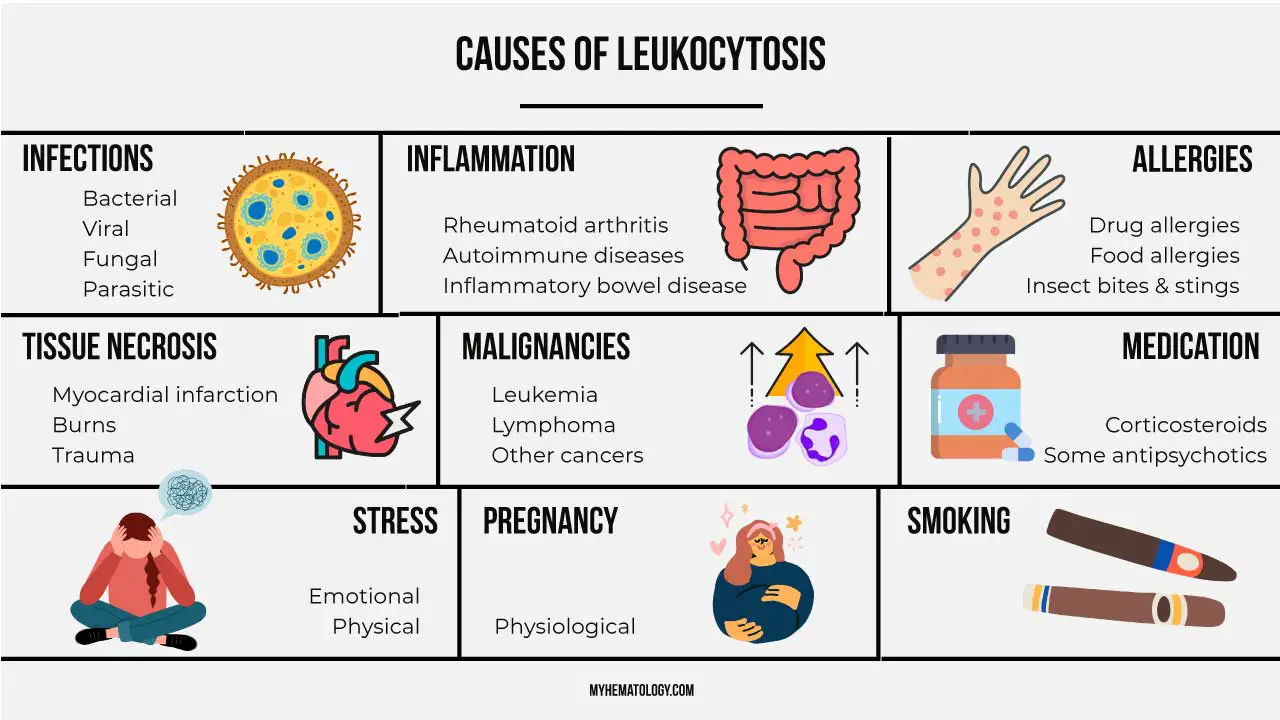
| Category | Examples |
| Infection | Bacterial infections (pneumonia, urinary tract infection) Viral infections (mononucleosis, cytomegalovirus) Parasitic infections (malaria) F ungal infections (aspergillosis) |
| Inflammation | Inflammatory bowel disease (IBD) Rheumatoid arthritis Systemic lupus erythematosus (SLE) Vasculitis |
| Autoimmune Diseases | Rheumatoid arthritis Systemic lupus erythematosus (SLE) Inflammatory bowel disease (IBD) Psoriasis |
| Tissue Injury | Burns Trauma Surgery |
| Neoplasms (Cancers) | Leukemias (acute and chronic) Lymphomas Multiple myeloma Solid tumors (in some cases) |
| Allergic Reactions | Drug allergies Food allergies Insect bites and stings Anaphylaxis |
| Stress Response | Major surgery Burns Severe emotional stress |
| Other Causes | Splenomegaly (enlarged spleen) Dehydration Smoking |
Leukocytosis Symptoms & Signs
Leukocytosis itself is often asymptomatic. However, the underlying cause of leukocytosis can manifest through various signs and symptoms.
General Symptoms
- Fever: An elevated body temperature is a common indicator of the body’s inflammatory response, potentially associated with infection.
- Fatigue: Increased metabolic demands during leukocytosis and the underlying condition can lead to tiredness and weakness.
- Malaise: A general feeling of discomfort, illness, or uneasiness can accompany leukocytosis.
Leukocytosis Symptoms Depending on the Cause
- Infection
- Localized pain, swelling, or redness at the infection site (e.g., sore throat with tonsillitis, urinary urgency with UTI).
- Cough, shortness of breath (respiratory infection).
- Diarrhea, nausea, vomiting (gastrointestinal infection).
- Inflammation
- Joint pain, swelling, and stiffness (rheumatoid arthritis).
- Skin rash (inflammatory skin conditions).
- Autoimmune Diseases
- Fatigue, weight loss, widespread pain (lupus).
- Cancer
- Unexplained weight loss, night sweats, easy bleeding or bruising (depending on the specific cancer).
Leukocytosis Physical Examination Findings
- Lymphadenopathy: Enlarged lymph nodes, which are part of the immune system and can become palpable during leukocytosis, especially when infection is the cause.
- Splenomegaly: An enlarged spleen, another immune organ, can sometimes be felt on physical examination in some cases of leukocytosis.
Approach to Leukocytosis
Points to consider when evaluating a patient with leukocytosis.
- Detailed History: A thorough history, including current symptoms, past medical history, medications, recent exposures (infectious agents, allergens), and social habits, is crucial.
- Physical Examination: A comprehensive physical exam can reveal signs suggestive of the underlying cause, such as fever, lymphadenopathy (swollen lymph nodes), rash, or organ-specific findings.
- Correlation with WBC Count and Type: The specific type of leukocytosis (neutrophilia, lymphocytosis, etc.) can offer clues about the potential cause. For example, neutrophilia is more suggestive of a bacterial infection, while lymphocytosis might point towards a viral infection.
- Other Laboratory Tests: Additional tests like blood cultures, urinalysis, chest X-ray, or other investigations might be necessary depending on the suspected cause.
Additional Considerations
- Shift to the Left: The presence of immature WBCs in the peripheral blood smear (shift to the left) can indicate a more severe infection and the body’s rapid attempt to produce more WBCs.
- Specific Leukocytosis Types: The type of leukocytosis (neutrophilia, lymphocytosis, etc.) can offer clues about the underlying cause.
Complications of Leukocytosis
Leukocytosis itself isn’t necessarily a disease, but rather an indicator of an underlying condition. However, depending on the severity and type of leukocytosis, there can be potential complications.
Vascular Occlusion
- Hyperleukocytosis: Extremely high WBC counts (>100 x 109/L) can lead to a condition called hyperleukocytosis.
- Increased Blood Viscosity: In hyperleukocytosis, the massive number of WBCs can thicken the blood, increasing its viscosity. This can impair blood flow and potentially lead to:
- Ischemic Organ Damage: Reduced blood flow to organs like the brain, lungs, kidneys, or heart can cause tissue damage and organ dysfunction.
- Stroke: If blood flow to the brain is compromised, a stroke can occur.
- Pulmonary Edema (Fluid in the Lungs): In severe cases, impaired blood flow to the lungs can lead to fluid buildup.
Disseminated Intravascular Coagulopathy (DIC)
- This is a serious condition where abnormal blood clotting occurs throughout the bloodstream.
- Certain types of leukocytosis, particularly those associated with some cancers, can increase the risk of DIC.
- DIC can lead to:
- Bleeding complications
- Blockage of blood vessels by clots
Tumor Lysis Syndrome (TLS)
- In certain cancers, particularly leukemias, aggressive treatment can lead to the rapid death of a large number of cancer cells.
- The breakdown products can overwhelm the body’s ability to handle them, leading to:
- Electrolyte imbalances (e.g., high potassium, low calcium)
- Kidney failure
- Heart rhythm disturbances
Increased Risk of Infection (Paradoxical)
- While leukocytosis indicates the body’s attempt to fight infection, very high WBC counts can sometimes impair the function of certain white blood cells, making the body paradoxically more susceptible to opportunistic infections.
Organ Infiltration
- In some cases of leukemias, the abnormal white blood cells can infiltrate organs like the liver, spleen, and lymph nodes, causing:
- Enlargement of these organs
- Disruption of their normal function
Laboratory Investigations for Leukocytosis
Leukocytosis is often identified through a complete blood count (CBC). However, a thorough evaluation often requires additional laboratory investigations to pinpoint the underlying cause.
Complete Blood Count (CBC) with Differential
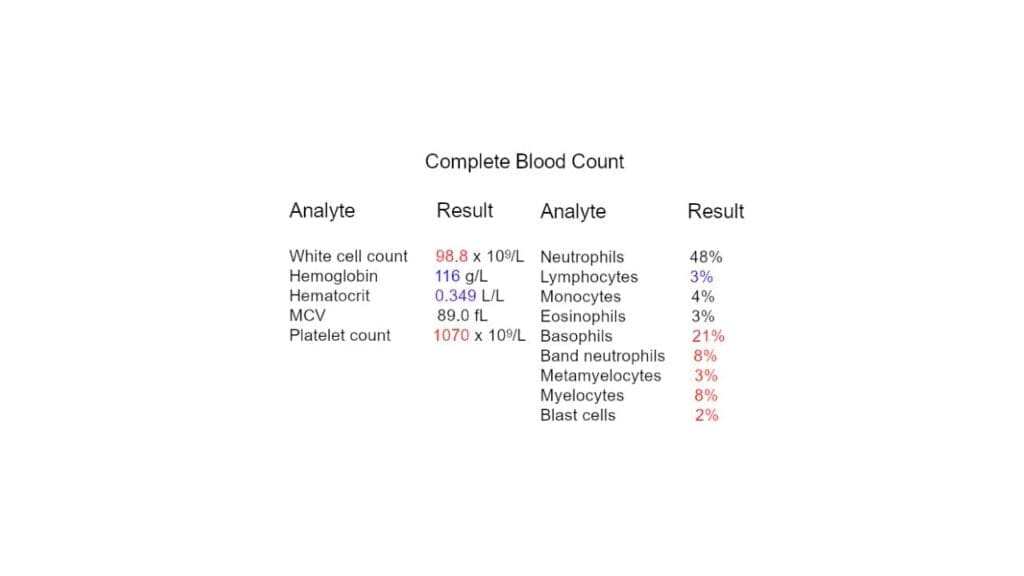
This is the cornerstone of diagnosing leukocytosis.
- WBC Count: It measures the total number of WBCs per liter (L) of blood.
- Differential: This provides a breakdown of the different types of WBCs (neutrophils, lymphocytes, monocytes, eosinophils, basophils) as percentages or absolute numbers.
- Interpretation: The specific type of leukocytosis (neutrophilia, lymphocytosis, etc.) offers valuable clues about the potential cause.
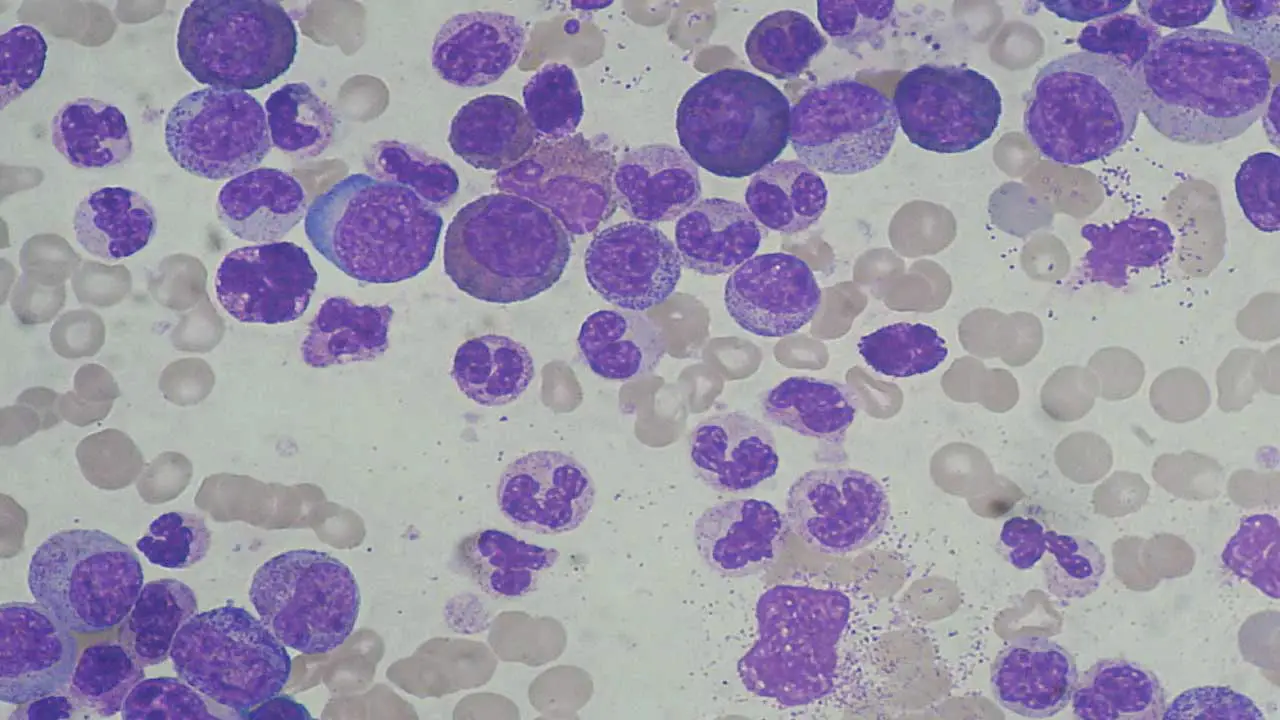
Peripheral Blood Smear
A blood smear allows for visualization of individual blood cells under a microscope.
- Shift to the Left: The presence of immature WBCs (blasts and precursors) can indicate a more severe infection and the body’s rapid attempt to produce more WBCs.
- Abnormal Cell Morphology: Certain abnormalities in the appearance of WBCs can suggest specific causes, such as leukemia.
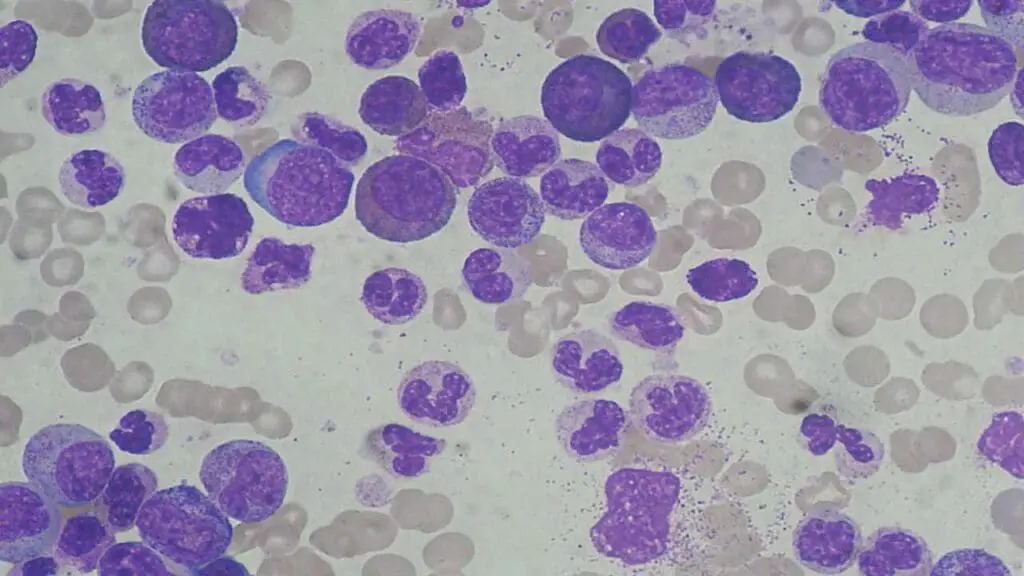
Additional Tests Based on Suspected Cause
- Blood Cultures: If infection is suspected, blood cultures are crucial to identify the specific causative organism.
- Urinalysis and Urine Culture: These tests can help diagnose urinary tract infections (UTIs), a common cause of leukocytosis.
- Chest X-Ray: Useful for evaluating potential lung infections like pneumonia, which can cause neutrophilia.
- Sputum Culture: If a respiratory infection is suspected, a sputum culture can help identify the causative organism.
- Viral Serology: Blood tests can be performed to detect specific viruses that can cause lymphocytosis.
- Bone Marrow Aspiration and Biopsy: In some cases, particularly when there’s suspicion of a bone marrow disorder or certain cancers, a bone marrow examination might be necessary.
- Autoimmune Disease Workup: If an autoimmune condition is suspected as the cause of leukocytosis, specific autoantibody tests might be ordered.
- Imaging Studies: Depending on the suspected cause, imaging studies like X-rays, CT scans, or MRIs might be used to visualize potential sources of infection or inflammation.
- Other Tests: Depending on the clinical scenario, additional tests like coagulation studies or electrolyte panels might be necessary.
Choosing the Right Tests
The specific laboratory investigations will be guided by the patient’s clinical presentation, medical history, and the type of leukocytosis identified on the CBC. A targeted approach is crucial to ensure a cost-effective and efficient evaluation.
Leukocytosis Treatment and Management
Leukocytosis generally is a sign of the body fighting an infection or inflammation and does not require specific treatment. Doctors typically focus on addressing the underlying cause of the high white blood cell count.
- Targeting the Root Cause: This is the main approach. If it’s an infection, antibiotics for bacteria, antifungals for fungus, or antivirals for viruses might be prescribed. For allergies, antihistamines could be helpful. In cases of inflammatory diseases, steroids or other anti-inflammatory drugs might be used.
- Management for Specific Conditions: Leukemia or other blood cancers would require specialized treatment plans like chemotherapy or radiation therapy.
- Addressing Hyperleukocytosis Syndrome: In rare instances of extremely high white blood cell counts, interventions like leukapheresis (filtering white blood cells from blood) along with hydration and medication to manage uric acid levels might be necessary.
- Symptom Relief: Medications to manage fever, pain, or inflammation caused by the underlying condition might be prescribed.
Frequently Asked Questions (FAQs)
How long does it take for WBC to return to normal?
The time it takes for your WBC to return to normal after leukocytosis depends on the underlying cause:
- Infections: For common bacterial infections, WBC count usually normalizes within 5 to 25 days after starting antibiotics and as the infection resolves.
- Viral infections: WBC can take longer to return to normal after viral illnesses, sometimes up to several weeks.
- Inflammation: If caused by an inflammatory condition, WBC will normalize as the inflammation is controlled with medication.
- Blood Cancers: In blood cancers, the timeframe for WBC normalization depends on the type and severity of the cancer and the treatment plan.
Why does leukocytosis occur after splenectomy?
Leukocytosis after a splenectomy is a well-documented phenomenon and is generally considered physiologic (normal response) rather than a sign of infection.
The Spleen’s Role
- The spleen is a vital organ in the lymphatic system that acts as a filter for blood.
- It removes old or damaged blood cells, including some white blood cells.
- It also serves as a reservoir for white blood cells, storing them until they’re needed to fight infection.
Splenectomy and White Blood Cells
- After a splenectomy, this storage pool for white blood cells is removed. The body compensates by increasing production of white blood cells in the bone marrow to maintain adequate levels in circulation.
- This leads to a temporary elevation in circulating white blood cells, a condition known as post-splenectomy leukocytosis.
Types of Leukocytosis after Splenectomy
- Early: Immediately after surgery, there’s a transient rise in neutrophils (a type of white blood cell). This usually peaks within a day or two and gradually returns to near normal within a week.
- Long-term: A mild to moderate elevation in white blood cells, including lymphocytes and monocytes (other types of white blood cells), can persist for months or even years after a splenectomy. This is considered a normal physiological response.
What is the main cause of leukocytosis?
Leukocytosis itself doesn’t have a single main cause because it’s a response, not a specific disease. The most common triggers are infection and inflammation. Your body ramps up white blood cell production to fight off invaders or deal with inflamed tissues. There are other causes as well, but these are the most frequent culprits.
Can leukocytosis be cured?
Leukocytosis isn’t actually cured because it’s not a disease itself. It’s a sign that your body’s immune system is activated. The focus is on treating the underlying cause that’s driving the high white blood cell count.
What level of WBC is alarming?
A white blood cell count is generally considered alarming when it falls outside the normal range, which can vary slightly between labs. Adults typically have a WBC between 4,500 and 11,000 per microliter. A high WBC (leukocytosis) above 11,000 might indicate infection or inflammation, and very high levels (hyperleukocytosis) over 50,000 require urgent medical attention. However, the specific level that’s concerning can depend on factors like age and recent surgery.
Is leukocytosis life threatening?
Leukocytosis itself isn’t usually life-threatening, but the underlying cause can be. The severity depends on the cause – a common infection likely isn’t a threat, but very high WBC or blood cancers require prompt attention. Early diagnosis and treatment are key.
Can leukocytosis turn into leukemia?
Leukocytosis itself doesn’t directly turn into leukemia. It’s a general rise in white blood cells, while leukemia is a specific cancer of the blood cells. However, leukocytosis can be a sign of leukemia, especially if the white blood cell count is very high or there are abnormal types of white blood cells present.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Mank V, Azhar W, Brown K. Leukocytosis. [Updated 2024 Feb 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program (2012) 2012 (1): 475–484.
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.