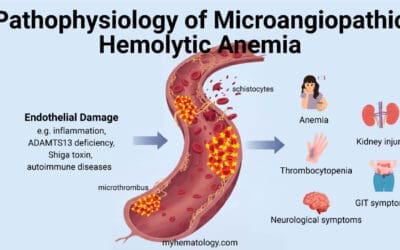
TL;DR
Hypochromic Microcytic Anemia is a condition where red blood cells are smaller than normal (microcytosis) and contain less hemoglobin, making them paler (hypochromia).
- Primary Causes:
- Iron Deficiency Anemia (IDA): The most common cause, due to insufficient iron.
- Anemia of Chronic Disease (ACD): Linked to chronic inflammatory conditions.
- Thalassemia: Genetic disorders affecting hemoglobin production.
- Sideroblastic Anemia: Characterized by impaired iron utilization in red blood cell precursors.
- Symptoms: Common symptoms of hypochromic microcytic anemia include fatigue, weakness, and pale skin, resulting from reduced oxygen delivery.
- Diagnostic Process: Diagnosis involves a thorough clinical history, physical examination, and key laboratory investigations (Complete Blood Count and Iron Studies).
*Click ▾ for more information
Introduction
Hypochromic microcytic anemia is a type of anemia characterized by red blood cells that have less hemoglobin than normal. Hemoglobin is the protein in red blood cells that carries oxygen throughout the body.
When red blood cells lack sufficient hemoglobin, they appear paler than usual (hypochromia) under a microscope, hence the prefix “hypo” (less) and “chromic” (color) in the name.
As the red blood cell is essentially a bag full of hemoglobins, the lack of hemoglobin also causes microcytosis – small sized cells. This deficiency in hemoglobin leads to reduced oxygen delivery to tissues, causing symptoms like fatigue, weakness, and shortness of breath.
Causes of Hypochromic Microcytic Anemia
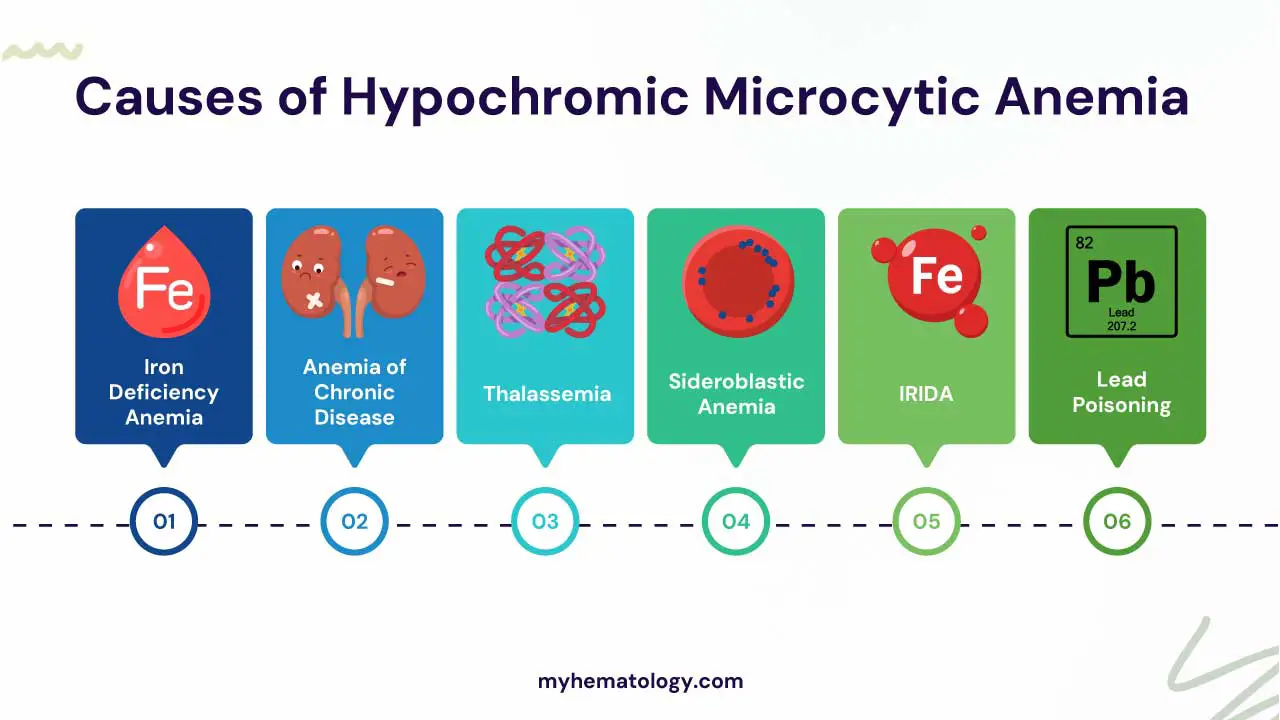
Iron Deficiency Anemia (IDA)
Iron deficiency anemia (IDA) is the most common type of anemia in the world and occurs when the body lacks sufficient iron to produce healthy red blood cells.
Iron is a critical component of hemoglobin, and without enough iron, red blood cells become smaller (microcytosis) and contain less hemoglobin, leading to hypochromia thus presenting as hypochromic microcytic anemia. This reduces the amount of oxygen delivered to tissues causing various symptoms.
Causes
- Insufficient iron intake: This can be due to a diet lacking iron-rich foods (e.g., red meat, poultry, fish), relying mainly on plant-based sources with lower iron bioavailability, or increased iron needs during pregnancy or menstruation leading to hypochromic microcytic anemia.
- Impaired iron absorption: Certain medical conditions like celiac disease, inflammatory bowel disease, or chronic diarrhea can hinder iron absorption from the intestines causing hypochromic microcytic anemia.
- Chronic blood loss: Blood loss, whether slow and ongoing (e.g., peptic ulcers, menorrhagia) or acute and severe, can significantly deplete iron stores.
Signs and Symptoms
The signs and symptoms of IDA can develop gradually and worsen over time. They are primarily caused by reduced oxygen delivery to tissues throughout the body due to hypochromic microcytic anemia. Common symptoms include:
- Fatigue and weakness
- Shortness of breath, especially with exertion
- Pale skin
- Dizziness or lightheadedness
- Cold hands and feet
- Headache
- Brittle nails or hair loss
- Restless legs syndrome (RLS)
- Pica (craving for non-food items like ice or dirt)
Complications
Untreated iron deficiency anemia causing hypochromic microcytic anemia can lead to various complications, including:
- Heart problems: An enlarged heart (cardiomegaly) may develop due to the heart working harder to pump oxygen-depleted blood.
- Increased risk of infection: Iron deficiency can impair immune function, making individuals more susceptible to infections.
- Pregnancy complications: IDA can increase the risk of premature birth and low birth weight.
- Developmental delays in children: Severe iron deficiency can affect cognitive development in children.
Laboratory Investigations
Diagnosis of IDA involves a combination of tests to assess iron stores and red blood cell health.
- Complete Blood Count (CBC): This test measures red blood cell count, size (mean corpuscular volume, MCV), and hemoglobin content (mean corpuscular hemoglobin, MCH). In IDA, MCV and MCH are typically low, indicating smaller red blood cells (microcytosis) with less hemoglobin (hypochromia).
- Iron Studies: These tests measure serum iron, ferritin (iron stores), transferrin (iron transport protein), and transferrin saturation (percentage of transferrin bound to iron). In IDA, serum iron and ferritin levels are usually low, while transferrin levels are high.
Treatment and Management
The primary treatment for IDA involves iron supplementation to alleviate hypochromic microcytic anemia. The type, dosage, and duration of iron supplementation depend on the severity of iron deficiency and the underlying cause.
- Iron supplements: Oral iron supplements are the most common treatment. Options include ferrous sulfate, ferrous fumarate, or ferrous gluconate.
- Dietary modifications: Increasing intake of iron-rich foods (red meat, poultry, fish, beans, lentils) and consuming vitamin C-rich foods (citrus fruits, tomatoes) to enhance iron absorption can be helpful.
- Addressing underlying causes: If chronic blood loss is identified, treating the source of bleeding is crucial. In cases of impaired iron absorption, managing the underlying condition may be necessary.
Anemia of Chronic Disease (ACD)
Anemia of chronic disease (ACD), also known as anemia of inflammation, is a type of anemia that develops due to chronic inflammatory conditions in the body. These conditions trigger various physiological changes that disrupt normal red blood cell production, leading to anemia.
Anemia of chronic disease (ACD) can sometimes present as hypochromic microcytic anemia. While typically normocytic and normochromic, prolonged inflammation associated with ACD can disrupt iron metabolism and erythropoiesis, leading to decreased hemoglobin production (hypochromia) and smaller red blood cells (microcytosis). This is less common than the normocytic form but should be considered in the differential diagnosis of hypochromic microcytic anemia.
Causes
ACD arises from various chronic inflammatory processes. Common causes include:
- Autoimmune diseases: Rheumatoid arthritis, lupus, inflammatory bowel disease (IBD)
- Infections: Bacterial, viral, or parasitic infections that persist for a long time
- Cancer: Different types of cancers can cause ACD
- Chronic kidney disease (CKD)
Signs and Symptoms
The signs and symptoms of ACD are often nonspecific and can be masked by the underlying chronic illness. They can include:
- Fatigue and weakness, a common symptom in many chronic conditions
- Pale skin
- Shortness of breath, especially with exertion
- Decreased appetite and weight loss
- Difficulty concentrating
- These symptoms may be similar to those of IDA (iron deficiency anemia), but they tend to be milder in ACD.
Complications
While ACD itself is rarely life-threatening, the underlying chronic condition can lead to serious complications. Additionally, ACD can worsen the symptoms and overall health of individuals with the underlying disease.
Laboratory Investigations
Diagnosis of ACD involves a combination of tests to assess red blood cell health and inflammatory markers.
- Complete Blood Count (CBC): Similar to IDA, CBC may show low red blood cell count, MCV, and MCH, indicating smaller red blood cells (microcytosis) with less hemoglobin (hypochromia) causing hypochromic microcytic anemia. However, unlike IDA, iron stores might be normal or even elevated in ACD.
- Iron Studies: Serum iron and ferritin levels may be normal or elevated, reflecting trapped iron due to inflammation. Transferrin levels can be low or normal, and transferrin saturation is typically low.
- Inflammatory Markers: C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) are often elevated in ACD, indicating ongoing inflammation.
Treatment and Management
The primary focus of ACD treatment is managing the underlying chronic inflammatory condition. Different approaches are used depending on the specific cause.
- Treating the underlying disease: Controlling the chronic inflammatory condition through medications, surgery, or other interventions is crucial to improve red blood cell production.
- Erythropoietin-Stimulating Agents (ESAs): In severe cases of ACD, ESAs can stimulate the bone marrow to produce more red blood cells. These medications are typically used when other treatment options are not effective or if symptoms are debilitating.
- Blood transfusions: In rare instances, blood transfusions might be necessary if ACD is severe and causing life-threatening complications.
Thalassemia
Thalassemia is a group of inherited blood disorders affecting the hemoglobin causing hypochromic microcytic anemia. People with thalassemia trait inherit a single mutated gene for alpha or beta globin, the protein chains that make up hemoglobin in red blood cells. While they don’t have full-blown thalassemia disease, they carry the potential to pass the mutated gene on to their offspring. The severity of thalassemia can range from asymptomatic to transfusion dependent.
Causes
Thalassemia is caused by mutations in genes responsible for alpha or beta globin production. These mutations can be inherited from one parent (carrier) or both parents (each with a different type of thalassemia mutation).
- Alpha thalassemia trait: Caused by mutations in genes for alpha globin chains. There are four alpha globin genes, and the severity of the trait depends on the number of mutated genes inherited (one or two).
- Beta thalassemia trait: Caused by mutations in genes for beta globin chains. The severity can vary depending on the specific mutation inherited.
Signs and Symptoms
People with thalassemia trait are typically asymptomatic. In some cases, mild microcytosis and hypochromia might be detected on a routine blood test.
The general signs and symptoms of thalassemia primarily stem from hypochromic microcytic anemia, as the body struggles to produce sufficient healthy hemoglobin and red blood cells to deliver oxygen efficiently. This often leads to common symptoms like fatigue, weakness, shortness of breath, and pale or yellowish skin (jaundice).
More severe forms, often diagnosed in early childhood, can cause additional issues such as slow growth and delayed puberty, an enlarged spleen and liver, abnormal bone development (especially in the face), and dark urine, all resulting from the body’s attempt to compensate for chronic anemia and potential iron overload.
The specific symptoms and their severity vary greatly depending on the type and genetic severity of thalassemia.
Complications
Thalassemia trait itself rarely causes any complications. However, individuals with the trait have an increased risk of having children with thalassemia major or intermedia if their partner also carries a thalassemia trait.
In more severe forms, thalassemia can lead to a range of serious complications due to chronic hypochromic microcytic anemia and the body’s compensatory responses.
These include iron overload, primarily from frequent blood transfusions (hemosiderosis) and increased iron absorption, which can damage organs like the heart (leading to heart failure and arrhythmias), liver (cirrhosis), and endocrine glands (causing diabetes, thyroid issues, and delayed puberty).
The constant demand for red blood cell production can result in bone deformities (particularly facial and skull bones) and osteoporosis, making bones brittle. An enlarged spleen (splenomegaly) is common as it works harder to remove damaged red blood cells, potentially necessitating splenectomy.
Furthermore, individuals may experience an increased risk of infections, gallstones, and in severe cases, the formation of extramedullary hematopoietic masses outside the bone marrow.
Laboratory Investigations
Diagnosis of thalassemia includes:.
- Complete Blood Count (CBC): Low MCV, indicating microcytosis and low MCH, indicating hypochromia. Other than very mild cases, thalassemia usually presents with hypochromic microcytic anemia.
- Hemoglobin Electrophoresis or HPLC: This test analyzes the different types of hemoglobin present in red blood cells. In thalassemia, characteristic patterns are observed depending on the type of trait (alpha or beta).
- Genetic Testing: While not routinely performed, genetic testing can definitively identify the specific mutations responsible for the trait.
Treatment and Management
Thalassemia trait itself doesn’t require any specific treatment. However, here are some important considerations:
- Genetic counseling: If diagnosed with thalassemia trait, genetic counseling is highly recommended, especially if planning a family. This helps couples understand the risk of passing the trait to their children and make informed decisions about family planning.
- Prenatal screening: If both partners carry a thalassemia trait, prenatal testing can be performed during pregnancy to determine if the fetus has inherited thalassemia major or intermedia.
More severe forms, however, typically require regular blood transfusions to provide healthy red blood cells and alleviate hypochromic microcytic anemia symptoms. A critical aspect of transfusion therapy is iron chelation therapy, which is essential to remove excess iron that accumulates from repeated transfusions and prevent organ damage.
In some cases, a splenectomy (surgical removal of the spleen) may be performed if an enlarged spleen is contributing significantly to anemia.
For a potential cure, especially in severe cases like beta-thalassemia major, hematopoietic stem cell transplantation (bone marrow transplant) from a compatible donor offers the best long-term outcome. Emerging therapies like gene therapy also hold promise for the future.
Sideroblastic Anemia
Sideroblastic anemia is a group of uncommon blood disorders characterized by abnormal iron metabolism within red blood cell precursors in the bone marrow.
Sideroblastic anemia leads to hypochromic microcytic anemia due to ineffective iron utilization. Despite having adequate or even excess iron, these patients cannot efficiently incorporate iron into hemoglobin.
This impaired heme synthesis results in decreased hemoglobin production and smaller (microcytosis), paler red blood cells (hypochromia), characteristic of hypochromic microcytic anemia.
Causes
Sideroblastic anemia can be caused by various factors, categorized into two main types:
- Inherited (Congenital) Sideroblastic Anemia: This type arises due to genetic mutations affecting enzymes involved in heme (iron-containing component of hemoglobin) synthesis within red blood cells. There are different subtypes depending on the specific gene mutation.
- X-linked sideroblastic anemia: The most common inherited form, affecting males and passed down through the X chromosome. It can be further divided into pyridoxine-responsive (responds to vitamin B6 supplementation) and pyridoxine-resistant subtypes.
- Autosomal recessive sideroblastic anemia: Less common, caused by mutations in genes on non-sex chromosomes and inherited from both parents.
- Acquired Sideroblastic Anemia: This type develops due to various non-genetic factors that disrupt heme synthesis.
- Medications: Certain medications (e.g., isoniazid, chloramphenicol) can interfere with iron utilization in red blood cells.
- Alcoholism: Chronic alcohol consumption can impair heme synthesis and lead to acquired sideroblastic anemia.
- Lead poisoning: Lead can disrupt iron metabolism and contribute to sideroblastic anemia.
- Bone marrow diseases: Myelodysplastic syndrome (MDS) and other bone marrow disorders can affect red blood cell production and cause sideroblastic anemia.
Signs and Symptoms
The signs and symptoms of sideroblastic anemia are similar to those of other hypochromic microcytic anemia and can develop gradually, worsening over time. These include:
- Fatigue and weakness
- Shortness of breath, especially with exertion
- Pale skin
- Dizziness or lightheadedness
- Headache
- Cold hands and feet
- In some cases, skin rash might be present.
Complications
Untreated sideroblastic anemia can lead to various complications due to hypochromic microcytic anemia, including:
- Heart problems: An enlarged heart (cardiomegaly) may develop due to the heart working harder to pump oxygen-depleted blood.
- Increased risk of infection: Iron overload in tissues can impair immune function, making individuals more susceptible to infections.
- Spleen and liver enlargement: In severe cases, the spleen and liver may become enlarged due to iron overload.
Laboratory Investigations
Diagnosis of sideroblastic anemia involves a combination of tests to assess iron stores, red blood cell health, and identify potential underlying causes.
- Complete Blood Count (CBC): Similar to other anemias, CBC may show low red blood cell count, MCV, and MCH, indicating smaller red blood cells (microcytosis) with less hemoglobin (hypochromia) leading to hypochromic microcytic anemia.
- Iron Studies: Serum iron and ferritin levels are typically elevated, reflecting iron overload despite microcytic hypochromic anemia.
- Bone Marrow Examination: This test is crucial for diagnosis. Microscopic examination of bone marrow reveals the presence of ring sideroblasts, which are red blood cell precursors with perinuclear iron deposits.
- Genetic Testing: Inherited forms might require genetic testing to identify specific gene mutations.
- Other Tests: Depending on the suspected cause, additional tests like blood tests for lead levels or alcohol markers might be performed.
Treatment and Management
The treatment approach for sideroblastic anemia depends on the underlying cause and severity.
- Treating the underlying cause: If an acquired cause like medication or alcoholism is identified, addressing that factor is important.
- Blood transfusions: In severe cases with significant anemia and symptoms of hypochromic microcytic anemia, blood transfusions might be necessary to increase red blood cell count and improve oxygen delivery.
- Chelation therapy: This treatment uses medications (chelating agents) to bind excess iron and remove it from the body. It might be helpful in cases of severe iron overload.
- Vitamin B6 supplementation: For patients with pyridoxine-responsive X-linked sideroblastic anemia, vitamin B6 supplements can help improve red blood cell production.
- Stem cell transplantation: In rare cases, especially with severe inherited forms or those associated with MDS, stem cell transplantation might be considered.
IRIDA (Iron-Refractory IDA with Ring Sideroblasts)
IRIDA, which stands for Iron-Refractory Iron Deficiency Anemia with Ring Sideroblasts, is a rare type of anemia characterized by a combination of features typically seen in two separate conditions: iron deficiency anemia (IDA) and sideroblastic anemia.
Causes
The exact cause of IRIDA remains under investigation, but it’s believed to be a complex interplay of factors.
- Genetic Predisposition: Mutations in specific genes, particularly those involved in iron regulation or heme synthesis, might play a role.
- Iron Deficiency: Similar to IDA, insufficient iron stores contribute to the anemia. However, the reasons for iron deficiency in IRIDA can be diverse.
- Ineffective Iron Utilization: Despite iron deficiency, there’s an issue with incorporating iron into hemoglobin within red blood cell precursors. This can be due to the underlying genetic mutations or other unknown factors.
Signs and Symptoms
The signs and symptoms of IRIDA can be similar to those of IDA and sideroblastic anemia, and may include:
- Fatigue and weakness, often more severe than in typical IDA
- Shortness of breath, especially with exertion
- Pale skin
- Dizziness or lightheadedness
- Headache
- Cold hands and feet
- In some cases, skin rash might be present, similar to sideroblastic anemia.
Complications
Untreated IRIDA can lead to complications associated with both iron deficiency and impaired red blood cell production.
- Heart problems: An enlarged heart (cardiomegaly) due to the heart working harder to pump oxygen-depleted blood.
- Increased risk of infection: Iron deficiency can impair immune function.
- Spleen and liver enlargement: In severe cases, iron overload in tissues might occur.
Laboratory Investigations
Diagnosis of IRIDA involves a combination of tests to assess iron stores, red blood cell health, and identify the presence of ring sideroblasts.
- Complete Blood Count (CBC): Similar to other anemias, CBC shows low red blood cell count, MCV, and MCH, indicating smaller red blood cells (microcytosis) with less hemoglobin (hypochromia) leading to hypochromic microcytic anemia.
- Iron Studies: Serum iron and ferritin levels might be low or borderline, reflecting iron deficiency. Transferrin levels can be high, similar to IDA.
- Bone Marrow Examination: This is crucial for diagnosis. Microscopic examination reveals the presence of ring sideroblasts, characteristic of sideroblastic anemia.
- Genetic Testing: While not routinely performed, genetic testing can help identify mutations associated with IRIDA. This can be helpful for diagnosis and family planning.
Treatment and Management
Treatment for IRIDA can be challenging due to its complex nature.
- Addressing Iron Deficiency: Oral iron supplementation is typically the initial approach. However, individuals with IRIDA often have poor iron absorption or utilization, making oral iron less effective.
- Erythropoietin-Stimulating Agents (ESAs): These medications can stimulate the bone marrow to produce more red blood cells. They might be combined with iron therapy or used alone.
- Luspatercept: This newer medication helps regulate hepcidin, a protein that controls iron levels. It can improve iron utilization and red blood cell production in IRIDA patients.
- Blood Transfusions: In severe cases with significant anemia and symptoms, blood transfusions might be necessary.
- Chelation therapy: This treatment, using medications to remove excess iron, might be considered if iron overload develops despite iron deficiency.
Lead Poisoning
Lead poisoning is one of the less common causes of hypochromic microcytic anemia. It is a serious condition caused by elevated levels of lead in the body. Lead is a highly toxic metal that can cause a wide range of health problems, particularly in young children and developing fetuses.
Lead poisoning interferes with heme synthesis in the process of creating hemoglobin. This disruption leads to reduced hemoglobin production (hypochromia) and smaller red blood cells (microcytosis). Additionally, lead shortens the lifespan of red blood cells, further contributing to anemia. These combined effects result in hypochromic microcytic anemia in lead poisoning.
Causes
Lead exposure can occur through various routes:
- Lead paint: Ingesting lead paint chips or dust from deteriorating lead-based paint in older homes is a major risk factor for children.
- Contaminated soil: Lead can be present in soil, especially near industrial sites or areas with past use of leaded gasoline. Children playing in contaminated soil can ingest lead-laden dust.
- Contaminated water: Lead pipes or lead solder in plumbing fixtures can leach lead into drinking water.
- Other sources: Lead exposure can also occur through imported toys with lead paint, lead-glazed pottery, certain cosmetics, or traditional medicines.
Signs and Symptoms
The signs and symptoms of lead poisoning can vary depending on the level of exposure and the duration.
- In children
- Irritability, hyperactivity, or learning difficulties
- Abdominal pain, constipation, or vomiting
- Loss of appetite and weight loss
- Developmental delays
- Headaches
- Fatigue and weakness
- Behavioral problems
- Seizures or coma (in severe cases)
- In adults
- Headaches
- Muscle weakness or joint pain
- High blood pressure
- Memory problems
- Fertility problems
- Abdominal pain
- Mood swings or depression
Complications
Lead poisoning can lead to various complications, some of which can be permanent.
- Brain damage: Lead exposure can impair cognitive development, leading to learning disabilities, memory problems, and decreased IQ.
- Nervous system damage: Lead can damage nerves, causing peripheral neuropathy (weakness, numbness, or tingling in hands and feet).
- Kidney damage: Lead can damage the kidneys, leading to chronic kidney disease in severe cases.
- Stunted growth and development: Lead exposure can hinder growth and development in children.
- Hearing problems: Lead poisoning can cause hearing loss.
- Miscarriage and premature birth: Lead exposure in pregnant women can increase the risk of miscarriage, premature birth, and low birth weight babies.
Laboratory Investigation
Diagnosis of lead poisoning relies on measuring blood lead levels.
- Blood Lead Level Test: This blood test measures the amount of lead present in the bloodstream. Levels above a specific threshold indicate lead poisoning.
- Complete Blood Count (CBC): While not diagnostic for lead poisoning, a CBC might reveal hypochromic microcytic anemia due to reduced red blood cell size (microcytosis) and hemoglobin content (hypochromia) as a potential consequence of lead exposure.
Treatment and Management
Treatment for lead poisoning depends on the severity of exposure and the blood lead level.
- Chelation therapy: Medications (chelating agents) bind to lead in the bloodstream and promote its removal from the body through urine. This is typically used for moderate to severe lead poisoning.
- Supportive care: Treating symptoms of anemia, ensuring adequate nutrition, and managing any developmental delays are essential aspects of care.
- Environmental lead removal: Identifying and eliminating the source of lead exposure is crucial to prevent further poisoning. This might involve removing lead paint, replacing lead pipes, or remediating contaminated soil.
Prevention
The primary focus should be on preventing lead exposure, especially in children.
- Lead paint abatement: Older homes should be inspected for lead paint and have it safely removed if present.
- Safe drinking water: Ensure the drinking water supply is lead-free. Consider using a water filter if lead contamination is a concern.
- Dietary precautions: Avoid sources of lead exposure, such as lead-glazed pottery or imported toys with lead paint.
- Handwashing: Frequent handwashing, especially before eating, can help reduce lead ingestion from dust or contaminated surfaces.
- Healthy diet: A diet rich in calcium and iron can help reduce lead absorption in the body.
- Regular checkups: Children living in high-risk environments should have regular blood lead level testing.
Public health initiatives like lead screening programs, education campaigns, and regulations on lead paint use play a crucial role in reducing the overall burden of lead poisoning.
Diagnosing Hypochromic Microcytic Anemia
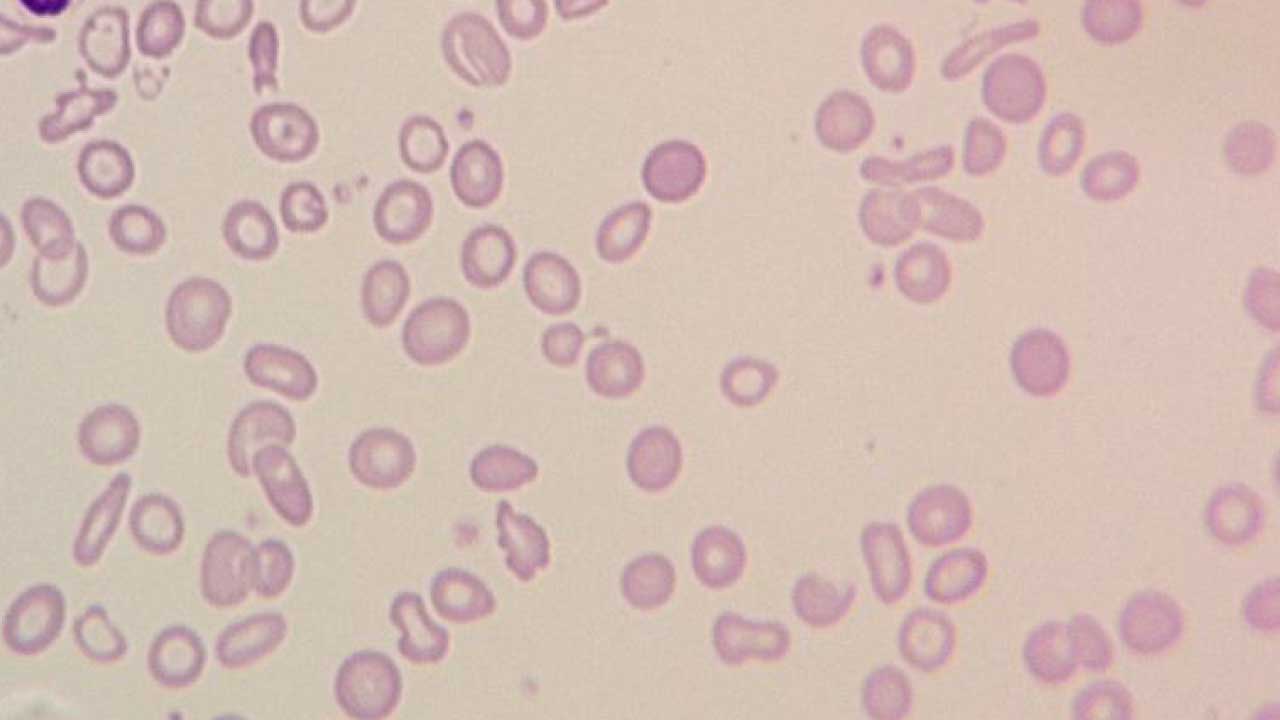
Hypochromic microcytic anemia is a type of anemia characterized by smaller red blood cells (microcytosis) with reduced hemoglobin content (hypochromia), causing a deficiency in oxygen-carrying capacity. Diagnosing it involves a combination of clinical evaluation, considering factors like ethnicity and family history, physical examination findings, and laboratory investigations.
Clinical History
- Symptoms: A detailed inquiry about symptoms like fatigue, weakness, shortness of breath, pale skin, dizziness, or pica (craving for non-food items) is crucial. The onset, severity, and progression of symptoms can provide clues.
- Dietary Habits: Understanding dietary patterns, including iron intake (red meat, poultry, fish, beans, lentils), vegetarian or vegan diet, and history of restrictive diets can be important.
- Menstrual History: In women, heavy menstrual bleeding can be a significant source of iron loss, contributing to hypochromic microcytic anemia.
- Medical History: Previous surgeries, chronic illnesses (inflammatory bowel disease, celiac disease), or blood loss due to peptic ulcers or gastrointestinal malignancies can be potential causes.
- Medication Use: Certain medications (aspirin, nonsteroidal anti-inflammatory drugs) can irritate the stomach lining and lead to chronic blood loss.
Ethnicity and Family History
- Ethnicity: Certain ethnicities, like people with Mediterranean or Southeast Asian ancestry, have a higher prevalence of genetic traits like beta thalassemia trait, which can cause mild microcytosis (smaller red blood cells) without significant anemia.
- Family History: A family history of anemia, especially iron deficiency anemia, can suggest a genetic predisposition or shared environmental factors.
Physical Examination
- General appearance: Pallor (pale skin, mucous membranes) is a common finding in hypochromic anemia.
- Cardiovascular: Tachycardia (fast heart rate) might be present due to the body’s attempt to compensate for reduced oxygen delivery.
- Spleen examination: An enlarged spleen (splenomegaly) might be present in some cases, particularly with chronic blood loss.
Laboratory Investigations
- Complete Blood Count (CBC): This is a key test. It reveals decreased red blood cell count, mean corpuscular volume (MCV, typically below 80 fL) (microcytosis), and mean corpuscular hemoglobin (MCH, typically below 27 pg) (hypochromia).
- Iron Studies: Serum iron and ferritin levels are typically low in iron deficiency anemia. Transferrin levels might be high, reflecting increased iron-binding capacity due to iron deficiency.
- Reticulocyte count: This measures the production of young red blood cells by the bone marrow. A low reticulocyte count suggests a problem with red blood cell production, while a high count might indicate the body’s attempt to compensate for anemia.
- Other Tests: Depending on the suspected cause, additional tests like stool tests for occult blood (hidden blood loss), upper endoscopy or colonoscopy to investigate the gastrointestinal tract, or celiac disease testing might be performed.
Putting It All Together
By combining the information gathered from clinical history, ethnicity, family history, physical examination findings, and laboratory tests, the specific type of hypochromic microcytic anemia can be ascertained.
- Iron deficiency anemia: This is the most common cause of hypochromic microcytic anemia. The clinical picture, dietary habits, and iron studies will point towards this diagnosis.
- Anemia of chronic disease: If chronic inflammatory conditions are present and iron studies show normal or elevated ferritin despite anemia, this possibility is considered.
- Other causes: Less common causes like lead poisoning, sideroblastic anemia, or thalassemia might be investigated based on specific findings and further tests.
| Parameter | Iron Deficiency Anemia (IDA) | Anemia of Chronic Disease (ACD) | Thalassemia | Sideroblastic Anemia | IRIDA (Iron-Refractory IDA with Ring Sideroblasts) | Lead Poisoning |
| MCV (< 80 fL) /MCH (< 27 pg) | Low | Low (may be normal) | Low | Low | Low | Low |
| Serum Iron (µg/dL) | Low | Variable (may be normal or elevated) | Normal | Raised | Low or Borderline | Variable |
| TIBC (µg/dL) | High | Low | Normal or High | Normal or High | Low | Normal or High |
| Serum Ferritin (ng/mL) | Low | Normal or High | Normal | Normal or High | Variable | Variable |
| Bone Marrow Iron Stores | Depleted | Normal or Increased | Normal | Increased | May be Depleted or Increased | May be Normal or Increased |
| Erythroblast Iron | Absent | Absent | Normal | Ring sideroblasts present | Ring sideroblasts present | Absent |
| Hemoglobin Electrophoresis / HPLC | Normal | Normal | Characteristic alpha or beta globin chain pattern | Normal | Normal | Normal |
| Other Relevant Parameters | May be low reticulocyte count | Elevated inflammatory markers (CRP, ESR) | – | – | May be low reticulocyte count | Elevated Blood Lead Level |
Importance of Accurate Diagnosis
Accurately diagnosing the underlying cause of hypochromic microcytic anemia is crucial for several reasons.
- Targeted Treatment: Different types of hypochromic microcytic anemia require distinct treatment approaches. For instance, iron deficiency anemia responds well to iron supplementation, whereas anemia of chronic disease requires addressing the underlying inflammatory condition. A misdiagnosis can lead to inappropriate treatment, potentially delaying recovery or even worsening symptoms.
- Optimizing Treatment Effectiveness: Knowing the specific cause allows for tailoring treatment strategies for optimal results. For example, the type and dosage of iron supplements in IDA may differ depending on the severity of iron deficiency and the presence of blood loss.
- Preventing Complications: Left untreated, some causes of hypochromic microcytic anemia can lead to serious complications. For instance, chronic iron deficiency can increase the risk of heart problems, while untreated thalassemia can cause bone deformities and growth delays. Early diagnosis allows for intervention to prevent such complications.
- Addressing Underlying Conditions: Hypochromic microcytic anemia can sometimes be a sign of an underlying health issue. Diagnosing the specific type of hypochromic microcytic anemia can help identify the root cause, allowing for its treatment and potentially improving overall health.
- Prognosis and Future Management: Understanding the cause of hypochromic microcytic anemia helps determine the long-term prognosis and guide future management strategies. For example, iron deficiency anemia caused by dietary factors might require ongoing monitoring and potential adjustments to diet, while a one-time blood loss event would have a different management approach.
- Genetic Counseling: In cases of genetic conditions like thalassemia trait, a proper diagnosis allows for genetic counseling. This empowers individuals and families to make informed decisions regarding future pregnancies and family planning.
Frequently Asked Questions (FAQs)
What is microcytosis?
Microcytosis is a medical term that describes the condition where a person’s red blood cells (RBCs) are abnormally smaller than their normal size.
It’s primarily identified by a blood test called a Complete Blood Count (CBC), specifically by the Mean Corpuscular Volume (MCV). An MCV value below the normal reference range (typically less than 80 femtoliters, or fL, for adults) indicates microcytosis.
Microcytosis itself is a descriptive finding, not a definitive diagnosis. It prompts further investigation to determine the underlying cause, as it can be a sign of various medical conditions, most commonly types of hypochromic microcytic anemia.
What is hypochromia?
Hypochromia is a medical term that describes the condition where a person’s red blood cells (RBCs), when viewed under a microscope, appear paler than normal.
A healthy red blood cell has a biconcave disc shape and a central area of pallor (paleness) that typically takes up about one-third of the cell’s diameter. In hypochromic cells, this central pale area is enlarged, and the red color is concentrated more on the outer rim of the cell.
Clinically, hypochromia is often assessed by the Mean Corpuscular Hemoglobin (MCH) and, more accurately, the Mean Corpuscular Hemoglobin Concentration (MCHC) in the Complete Blood Count (CBC). A low MCHC indicates that the red blood cells have a lower-than-normal concentration of hemoglobin, directly reflecting hypochromia.
Hypochromia often occurs alongside microcytosis, a combination known as hypochromic microcytic anemia. This is because problems with hemoglobin production often lead to both smaller (microcytosis) and paler red blood cells (hypochromia).
How to differentiate between microcytic and macrocytic anemia?
The primary way to differentiate between microcytic and macrocytic anemia is by looking at the Mean Corpuscular Volume (MCV), a key parameter measured in a standard Complete Blood Count (CBC) test.
- Microcytic Anemia: The MCV is lower than normal, typically less than 80 femtoliters (fL). This indicates that the red blood cells are smaller than their usual size (microcytosis). Common causes of microcytic anemia include iron deficiency anemia, thalassemia, and anemia of chronic disease.
- Macrocytic Anemia: The MCV is higher than normal, typically greater than 100 fL. This indicates that the red blood cells are larger than their usual size. Common causes of macrocytic anemia include Vitamin B12 deficiency and folate deficiency, as well as liver disease, alcoholism, and certain medications.
Is hypochromic microcytic anemia serious or not?
While some cases of hypochromic microcytic anemia (like mild iron deficiency) may not be immediately alarming and are easily treatable, it’s crucial to seek medical evaluation to determine the specific cause. Untreated hypochromic microcytic anemia, even seemingly “mild” cases can worsen over time, and severe cases or those caused by serious underlying conditions can lead to significant health problems and even be life-threatening.
Can microcytic anemia lead to leukemia?
Microcytic anemia is not a cause of leukemia, it can sometimes be a symptom or an indicator that a more serious underlying condition, such as leukemia or another cancer, needs to be ruled out. If microcytic anemia is diagnosed and the typical causes (like iron deficiency) are not readily apparent or responsive to treatment, further investigations for other underlying conditions, including malignancies, are often pursued.
Can microcytic anemia be cured?
Microcytic anemia is often curable, especially if it’s due to iron deficiency. However, if the microcytic anemia is caused by a chronic disease or a genetic condition, management focuses on treating the underlying issue or controlling symptoms, and a “cure” may only be possible with specific, advanced interventions like a stem cell transplant for certain cases.
What is the difference between anemia and microcytic anemia?
All microcytic anemia are a form of anemia.
However, not all anemias are microcytic anemia. Anemia can also be:
- Macrocytic anemia: Red blood cells are larger than normal (high MCV), often due to Vitamin B12 or folate deficiency.
- Normocytic anemia: Red blood cells are normal in size (normal MCV), but there aren’t enough of them, or they are not functioning correctly. This can be seen in hemolytic anemia, acute blood loss, kidney disease, or some chronic diseases.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Chaudhry HS, Kasarla MR. Microcytic Hypochromic Anemia. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470252/
- Massey A. C. (1992). Microcytic anemia. Differential diagnosis and management of iron deficiency anemia. The Medical clinics of North America, 76(3), 549–566. https://doi.org/10.1016/s0025-7125(16)30339-x
- Kharche, K. V., Bhake, A. and Vagha, S. (2021) “A Study Protocol for Evaluation of Microcytic Hypochromic Anemia by High-Performance Liquid Chromatography (HPLC)”, Journal of Pharmaceutical Research International, 33(60B), pp. 3399–3406. doi: 10.9734/jpri/2021/v33i60B35024.