TL;DR
Hemolytic anemia is a blood disorder where red blood cells (RBCs) are destroyed faster than they can be replaced.
Types ▾
- Hereditary: Caused by genetic mutations affecting RBC structure, function, or enzyme production (e.g., sickle cell disease, thalassemia).
- Acquired: Develops later in life due to various factors (e.g., autoimmune hemolytic anemia, drug-induced immune hemolytic anemia, infections).
Symptoms ▾
- Fatigue and weakness
- Pallor (pale skin)
- Jaundice (yellowing of skin and eyes)
- Dark urine (in some cases)
- Shortness of breath
- Rapid heart rate
- Enlarged spleen (in some cases)
Complications ▾
- Gallstones
- Increased risk of infections
- Leg ulcers (sickle cell disease)
- Heart problems
- Delayed growth and development (in children)
Diagnosis ▾
- Medical history and physical examination
- Blood tests (CBC, peripheral blood smear, DAT, haptoglobin, bilirubin)
- Additional tests specific to suspected cause (e.g., hemoglobin electrophoresis for sickle cell disease)
Treatment ▾
- Address the underlying cause (if possible)
- Supportive care (folic acid)
- Blood transfusions (if needed)
- Medications (e.g., immunosuppressants for autoimmune hemolysis)
- Procedures (e.g., splenectomy in some cases)
- Lifestyle modifications (depending on cause)
*Click ▾ for more information
Introduction
Hemolytic anemia is a blood disorder where red blood cells (RBCs) are destroyed faster than the body can replace them. This rapid or premature destruction reduces the availability of enough red blood cells for the normal physiological function of the body, leading to anemia with various symptoms and complications.
Normal RBC function and lifespan
Red blood cells, also known as erythrocytes, play a critical role in the body’s oxygen transport system.
Function
- Oxygen Delivery: Red blood cells contain a protein called hemoglobin, which acts as a carrier for oxygen. Hemoglobin binds to oxygen in the lungs and transports it throughout the body.
- Carbon Dioxide Removal: They also play a role in removing carbon dioxide, a waste product of cellular respiration. Hemoglobin picks up carbon dioxide from tissues and carries it back to the lungs for exhalation.
Lifespan
- Production: Red blood cells are constantly produced in the bone marrow. This process, called erythropoiesis, takes about 7 days.
- Circulation: Mature red blood cells are released into the bloodstream and circulate for an average lifespan of 100-120 days. They lack a nucleus, allowing them to be flexible and squeeze through tiny blood vessels to deliver oxygen effectively.
- Destruction: As red blood cells age and become less efficient, they are removed from circulation by specialized cells called macrophages in the spleen and liver. The components of the destroyed red blood cells are then recycled by the body to create new ones.
What is extravascular hemolysis?
Extravascular hemolysis refers to the destruction of red blood cells (RBCs) outside the bloodstream, primarily occurring in the spleen and liver.

Location
- Spleen: This is the main site for extravascular hemolysis. The spleen has macrophages (white blood cells) that identify and remove old, damaged, or abnormal red blood cells from circulation.
- Liver: To a lesser extent, the liver can also remove damaged RBCs.
Process
- Targeting: Macrophages in the spleen and liver have receptors that recognize specific markers on the surface of red blood cells. These markers can be:
- Age-related changes: As RBCs age, their membranes become slightly altered, and macrophages recognize these changes.
- Abnormal shape or structure: Inherited disorders like sickle cell disease can cause RBCs to be abnormally shaped, making them more susceptible to destruction by macrophages.
- Antibody coating: In some cases, red blood cells may be coated with antibodies (proteins from the immune system). Macrophages have receptors that recognize these antibodies and target the coated RBCs for destruction.
- Phagocytosis: Once a macrophage identifies a target RBC, it engulfs and digests the cell, breaking down its components.
- Components: The breakdown products of the destroyed RBC, including hemoglobin, are released by the macrophages and processed further by the body. Hemoglobin, for example, is broken down into bilirubin, a yellow pigment that contributes to jaundice (yellowing of the skin) when present in high levels.
Examples of conditions that can cause extravascular hemolysis
- Hereditary spherocytosis
- Enzyme deficiencies
- Autoimmune hemolytic anemia (warm type)
What is intravascular hemolysis?
Intravascular hemolysis describes the destruction of red blood cells (RBCs) within the bloodstream itself. This rupturing of RBCs releases their contents, including hemoglobin, directly into the plasma, leading to different consequences compared to extravascular hemolysis.

Location
- Intravascular hemolysis happens directly within blood vessels. This can occur throughout the circulatory system, but some areas are more prone to it due to mechanical stress on the RBCs, such as:
- Heart valves (especially damaged or prosthetic valves)
- Blood vessels with narrowed passages due to diseases like atherosclerosis
Process
- Direct Damage: RBCs are susceptible to damage from various factors within the bloodstream.
- Mechanical Stress: Shearing forces in turbulent blood flow can tear fragile RBCs, especially in conditions like prosthetic heart valves or narrowed blood vessels.
- Chemical or Toxin Exposure: Certain chemicals, toxins, or medications can directly damage the RBC membrane, leading to rupture. Examples include snake venom, certain antibiotics, and severe infections.
- Immune Attack: In some autoimmune hemolytic anemia cases (particularly cold autoimmune hemolytic anemia), antibodies target RBCs in the bloodstream, marking them for destruction by the immune system.
- Parasites: Certain parasites, like Babesia, can invade and lyse (rupture) RBCs from within.
- Hemolysis: When damage occurs, the RBC membrane ruptures, releasing its contents, including hemoglobin, into the bloodstream. Free-floating hemoglobin can cause further complications.
Consequences
- Hemoglobinemia: The presence of free hemoglobin in the plasma is called hemoglobinemia. This can overwhelm the body’s natural processes for handling hemoglobin breakdown products.
- Hemosiderinuria: Free hemoglobin can be filtered by the kidneys, but it can damage them in the process. When excessive amounts are filtered, it can lead to hemosiderinuria, the presence of hemosiderin (iron from broken down hemoglobin) in the urine.
- Increased Bilirubin: Hemoglobin breakdown leads to the formation of bilirubin. Normally, the liver processes bilirubin, but in severe intravascular hemolysis, the liver may become overwhelmed, leading to high bilirubin levels and jaundice (yellowing of the skin and eyes).
Examples of conditions that can cause intravascular hemolysis
- Severe infections (sepsis)
- Autoimmune hemolytic anemia (cold type)
- Transfusion reaction (incompatibility between donor and recipient blood)
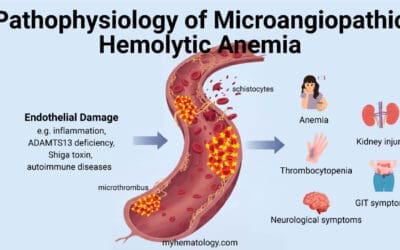
- Hemolytic uremic syndrome (HUS)
- Certain medications
- Prosthetic heart valves
Classification of Hemolytic Anemia Based on Cause
Hereditary hemolytic anemia
Hereditary hemolytic anemia is a group of blood disorders passed down through families (inherited) where there’s a defect in the red blood cells (RBCs) themselves. This defect makes them more fragile and prone to premature destruction compared to healthy RBCs.
Pathophysiology
- Genetic Basis: Mutations in genes responsible for red blood cell structure, function, or enzyme activity are inherited from one or both parents.
- Defect in RBCs: These mutations lead to abnormalities in the RBC membrane, enzymes involved in red blood cell function, or the structure of hemoglobin (the oxygen-carrying protein within RBCs).
- Increased RBC Destruction: Due to the defect, RBCs become more susceptible to premature destruction by the spleen (extravascular hemolysis) or within the bloodstream (intravascular hemolysis).
- Symptoms: This increased destruction can lead to symptoms like fatigue, weakness, pallor (pale skin), jaundice (yellowing of the skin and eyes), and sometimes dark urine.
Types of Hereditary Hemolytic Anemia
Hereditary hemolytic anemias are classified based on the specific defect in the RBCs.
- Membrane Defects
- Hereditary spherocytosis: RBCs become sphere-shaped (spherocytes) and are more susceptible to destruction.
- Elliptocytosis: RBCs have an oval shape (elliptical) and may be less severe than spherocytosis.
- Enzyme Deficiencies:
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency: This enzyme helps protect RBCs from oxidative damage. Deficiency makes them vulnerable to destruction, especially with certain triggers like medications or infections.
- Pyruvate kinase (PK) deficiency: This enzyme plays a role in RBC energy production. Deficiency can lead to decreased RBC lifespan.
- Hemoglobin Abnormalities:
- Sickle cell disease: A mutation in the hemoglobin gene causes RBCs to sickle (become crescent-shaped) and block blood vessels.
- Thalassemia: Mutations reduce or eliminate the production of globin chains, protein components of hemoglobin. This leads to abnormal hemoglobin and decreased RBC production.
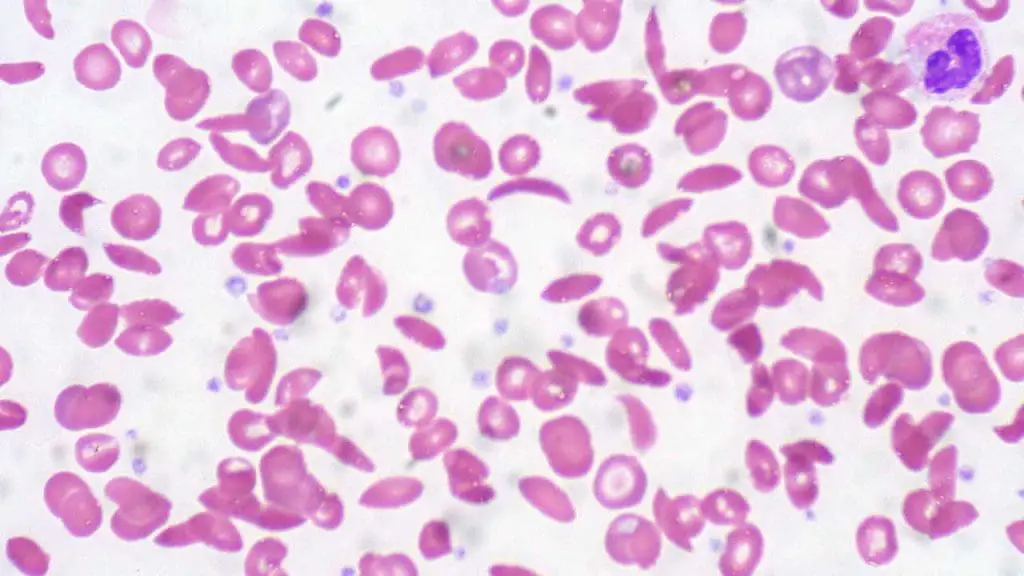
Acquired hemolytic anemia
Acquired hemolytic anemia, unlike hereditary hemolytic anemia, is not something you’re born with. It develops later in life due to various factors that damage or destroy healthy red blood cells (RBCs). This damage occurs outside of the inherent properties of the RBCs themselves.
Pathophysiology
The specific mechanism of RBC destruction depends on the cause. Here are some common scenarios:
- Antibody-mediated: In immune-mediated hemolytic anemia, antibodies attach to RBCs, marking them for destruction by macrophages in the spleen or liver.
- Complement activation: In some cases, the immune system may activate a protein cascade called the complement system, which directly damages the RBC membrane.
- Mechanical damage: In non-immune causes like red cell fragmentation syndromes, RBCs are physically damaged by shearing forces in the circulation.
- Chemical damage: Toxins or infections can directly damage the RBC membrane or alter their internal environment, leading to destruction.
Types of Acquired Hemolytic Anemia
A wide range of factors can trigger acquired hemolytic anemia. These can be broadly categorized as:
- Immune-mediated: The immune system mistakenly attacks healthy RBCs. This can be further classified as:
- Warm autoimmune hemolytic anemia (WAIHA): Antibodies target RBCs at body temperature.
- Cold agglutinin disease: Antibodies target RBCs at cooler temperatures.
- Alloimmune-related: Antibodies from a different blood type cause hemolysis, such as in a hemolytic transfusion reaction.
- Drug-induced: Certain medications can damage RBCs or trigger an immune reaction against them.
- Red cell fragmentation syndromes: Mechanical shearing forces fragment RBCs in the bloodstream (e.g., microangiopathic hemolytic anemia).
- Paroxysmal nocturnal hemoglobinuria (PNH): A rare blood disorder where RBCs are more susceptible to complement-mediated destruction.
- Infections: Some infections can damage RBCs or trigger an immune response leading to hemolysis.
- Chemical and physical agents: Severe burns, exposure to certain toxins, or snakebites can damage RBCs.
- Secondary causes: Underlying conditions like liver disease or severe kidney disease can contribute to hemolysis.
- March hemoglobinuria: A rare condition triggered by strenuous exercise in cold weather, causing RBC breakdown.
Signs and Symptoms
Hemolytic anemia, whether inherited or acquired, disrupts the normal function of red blood cells (RBCs) by causing their premature destruction. This disruption leads to a range of signs and symptoms that can vary depending on the severity of the anemia.
Common Symptoms
- Fatigue and weakness: This is a hallmark symptom due to reduced oxygen delivery to tissues throughout the body. People with hemolytic anemia may experience tiredness, difficulty concentrating, and a decreased ability to perform physical activities.
- Pallor (pale skin): A lack of healthy RBCs circulating in the blood can cause the skin to appear pale or whitish.
- Jaundice (yellowing of the skin and eyes): When red blood cells break down, they release a yellow pigment called bilirubin. If the body can’t eliminate bilirubin fast enough, it builds up in the bloodstream, causing the skin and whites of the eyes to turn yellow.
Other Potential Symptoms
- Dark urine (hemoglobinuria): In some cases, free hemoglobin released from destroyed RBCs can be filtered by the kidneys and appear in the urine, causing a reddish or dark brown discoloration.
- Shortness of breath: This can occur as the body struggles to get enough oxygen to tissues, especially during exertion.
- Rapid heart rate (tachycardia): The heart may beat faster to try and compensate for the reduced oxygen-carrying capacity of the blood.
- Enlarged spleen (splenomegaly): The spleen is an organ that filters blood and removes damaged RBCs. In hemolytic anemia, the spleen may become enlarged due to increased workload from excessive RBC destruction.
Complications
Hemolytic anemia, if left untreated or if severe, can lead to various complications. These complications arise from the ongoing destruction of red blood cells (RBCs) and the body’s struggle to compensate.
- Gallstones: Increased breakdown of RBCs leads to the production of excess bilirubin. When bilirubin levels are high, it can solidify and form gallstones in the gallbladder.
- Increased risk of infections: A shortage of healthy RBCs can impair the immune system’s ability to fight infections effectively.
- Heart problems: Chronic anemia forces the heart to work harder to pump oxygen-deficient blood throughout the body. This can lead to heart enlargement, arrhythmias (irregular heartbeat), and eventually heart failure.
- Delayed growth and development (in children): In children with chronic hemolytic anemia, oxygen deficiency can impede growth and development.
- Splenic complications: An enlarged spleen due to excessive RBC destruction can rupture, although rare.
- Iron overload (hemochromatosis): The body normally recycles iron from broken-down RBCs. However, in chronic hemolytic anemia, excessive iron absorption can occur, leading to a condition called hemochromatosis, which can damage organs like the liver, heart, and pancreas.
Additional complications specific to certain causes of hemolytic anemia
- March hemoglobinuria: This rare condition can lead to kidney damage if severe and prolonged.
- Paroxysmal nocturnal hemoglobinuria (PNH): This rare blood disorder can also cause symptoms like abdominal pain, blood clots, and bone marrow problems.
- Sickle cell disease: In sickle cell disease, a specific type of hemolytic anemia, abnormally shaped RBCs can block blood flow, leading to painful leg ulcers.
Laboratory Investigations
Diagnosing hemolytic anemia involves a multi-step approach, combining information from a patient’s medical history and physical examination with various laboratory tests.
Medical History and Physical Examination
- Enquire for symptoms like fatigue, weakness, shortness of breath, and any history of jaundice or dark urine.
- Family history of blood disorders is also important, especially for hereditary hemolytic anemia.
- Physical examination will assess for signs like pale skin, jaundice, and an enlarged spleen (splenomegaly).
Common Laboratory Investigations
These tests provide initial clues about the presence and severity of hemolytic anemia.
- Complete Blood Count (CBC)
- Measures red blood cell count (RBC count), hemoglobin level, hematocrit (percentage of blood volume occupied by RBCs), and other cell types.
- A low RBC count, hemoglobin, and hematocrit are indicative of anemia.
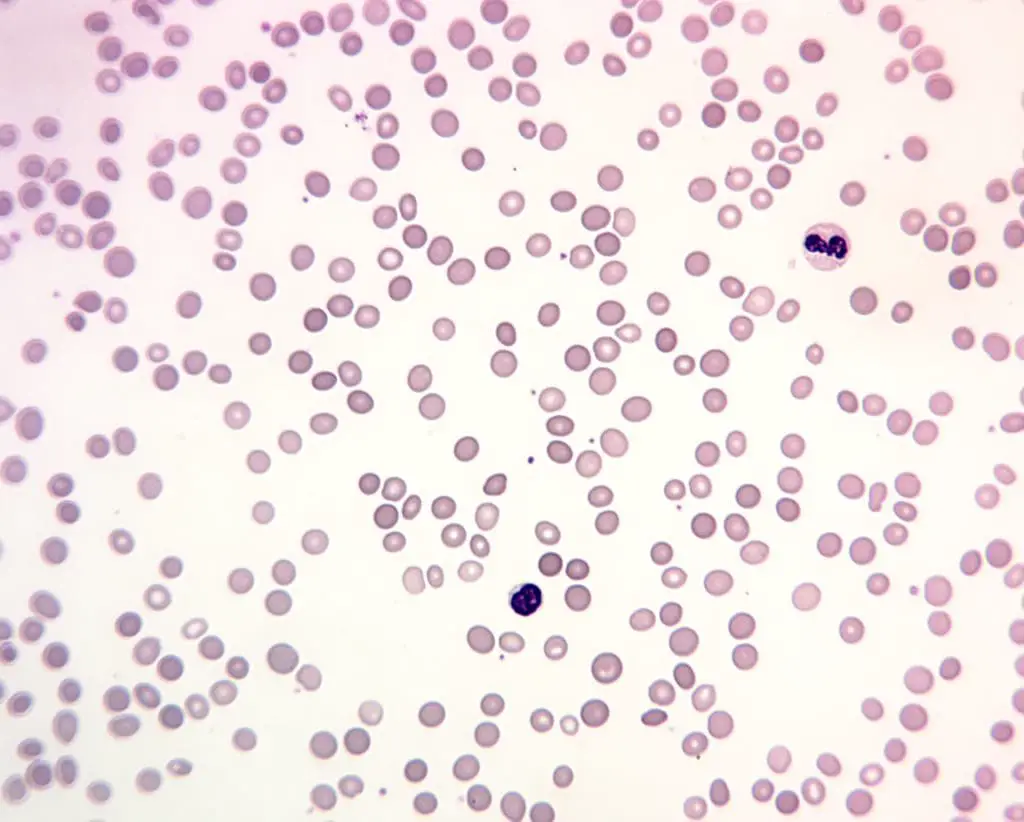
- Peripheral Blood Smear
- Examines the size, shape, and color of red blood cells, the RBC morphology under a microscope.
- Can reveal abnormalities like spherocytosis (round cells), elliptocytosis (oval cells), sickle cells, or fragmented cells, which can point towards specific types of hemolytic anemia.
- Reticulocyte Count
- Measures the percentage of immature red blood cells (reticulocytes) in the bloodstream.
- Increased reticulocyte count indicates the bone marrow is attempting to compensate for increased red blood cell destruction.
- Direct Antiglobulin Test (DAT)
- Also known as the Coombs’ test, this test detects antibodies or complement proteins attached to red blood cells.
- A positive DAT suggests an immune-mediated cause of hemolytic anemia.
- Haptoglobin Levels
- Haptoglobin is a protein that binds to free hemoglobin released from destroyed red blood cells.
- Low haptoglobin levels indicate recent hemolysis.
- Serum Bilirubin Levels
- Measures the level of bilirubin, a yellow pigment produced from the breakdown of hemoglobin.
- Elevated bilirubin levels can indicate hemolysis and possible liver dysfunction.
Additional Tests Specific to Underlying Conditions
Depending on the suspected cause of hemolytic anemia, additional tests might be recommended.
- Hereditary Hemolytic Anemia
- Hemoglobin Electrophoresis: Identifies abnormal hemoglobin types like those seen in sickle cell disease or thalassemia.
- Genetic Testing: Can confirm specific gene mutations causing various hereditary hemolytic anemia types.
- Enzyme Deficiencies
- G6PD Deficiency Test: Measures the activity of the glucose-6-phosphate dehydrogenase (G6PD) enzyme in red blood cells.
- Immune-mediated Hemolytic Anemia
- Cold Agglutinin Test: Detects antibodies that react with red blood cells at colder temperatures (cold autoimmune hemolytic anemia).
- Warm Autoantibody Testing: Identifies antibodies active at body temperature (warm autoimmune hemolytic anemia).
- Alloimmune Hemolytic Anemia
- Antibody Identification Testing: Determines the type of antibody causing the hemolysis in a blood transfusion reaction.
- Infectious Hemolytic Anemia
- Blood Culture: Identifies bacteria causing infection that might be contributing to hemolysis.
- Paroxysmal Nocturnal Hemoglobinuria (PNH):
- Flow Cytometry: A specialized test that detects specific markers on red blood cells to diagnose PNH.
Treatment and Management
The general principle for treatment and management of hemolytic anemias depends on the underlying cause. However, some broad approaches aim to address the consequences of red blood cell (RBC) destruction and improve the overall well-being of the patient.
Supportive Care
- Folic Acid Supplementation: Folic acid is essential for healthy red blood cell production. Supplementation can be beneficial in most types of hemolytic anemia to ensure adequate RBC production by the bone marrow.
Addressing the Underlying Cause
- Hereditary Hemolytic Anemia: Treatment often focuses on reducing RBC destruction, for example, by:
- Splenectomy: Removing the spleen, a major site of RBC destruction, can be helpful in severe cases.
- Medications: Hydroxyurea, for example, may be used in sickle cell disease to increase the production of normal red blood cells.
- Acquired Hemolytic Anemia: The goal is to address the specific trigger or cause, such as:
- Stopping a problematic medication.
- Treating an underlying infection.
- Immunosuppressive therapy: Medications to suppress the immune system might be used in autoimmune hemolytic anemia.
Blood Transfusions
- In cases of severe anemia causing significant symptoms or complications, blood transfusions can be necessary to
- Increase oxygen-carrying capacity of the blood.
- Reduce fatigue and improve quality of life.
- Transfusions should be used judiciously to avoid iron overload, especially in patients requiring frequent transfusions.
Other Management Strategies
- Pain Management: For conditions like sickle cell disease, pain management strategies are crucial to improve quality of life.
- Genetic Counseling: If the patient has a family history of hereditary hemolytic anemia, genetic counseling can help them understand the risk of passing the condition to their children.
- Psychological Support: Chronic illness can take a toll on mental well-being. Psychological support can be valuable for patients and their families dealing with hemolytic anemia.
Prognosis
The prognosis for hemolytic anemia varies depending on the type and severity of the condition. Early diagnosis and proper management can significantly improve the quality of life and life expectancy for many patients with hemolytic anemia.
Frequently Asked Questions (FAQs)
What deficiency causes hemolytic anemia?
The most common enzyme deficiency that causes hemolytic anemia is glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Who is most at risk for hemolytic anemia?
The risk factors for hemolytic anemia can be categorized based on whether it’s hereditary or acquired.
Hereditary Hemolytic Anemia
- Family History: Having a family member with a hereditary hemolytic anemia significantly increases your risk. These conditions are passed down through genes, so if a parent has a specific type, their children have a higher chance of inheriting it.
- Ethnicity: Certain ethnicities are more prone to specific hereditary hemolytic anemia types. For example, sickle cell disease is more prevalent in people of African descent, while thalassemia is more common in people of Mediterranean, Middle Eastern, and Southeast Asian origin.
Acquired Hemolytic Anemia
- Age: While hemolytic anemia can occur at any age, some acquired forms are more frequent in specific age groups. For instance, autoimmune hemolytic anemia can develop at any time, while G6PD deficiency is typically present from birth.
- Underlying Medical Conditions: Certain medical conditions like autoimmune diseases (lupus, HIV), infections (malaria, babesiosis), and some blood cancers can increase the risk of acquiring hemolytic anemia.
- Medications: Certain medications can damage red blood cells or trigger an immune reaction leading to hemolysis. People taking these medications are at higher risk.
- Blood Transfusions: In rare cases, blood transfusions can lead to hemolytic transfusion reactions if incompatible blood types are mixed.
- Splenic dysfunction: A diseased or malfunctioning spleen can sometimes contribute to hemolytic anemia.
- Environmental factors: Exposure to certain toxins or chemicals can damage red blood cells, leading to hemolytic anemia.
Additional Considerations
- Blood Type: People with certain blood types (e.g., Rh-negative) may be more susceptible to hemolytic transfusion reactions.
- Autoimmune diseases: Women are more likely than men to develop autoimmune disorders that can cause autoimmune hemolytic anemia.
- Geographic location: The prevalence of certain infections that can trigger hemolytic anemia can vary depending on geographic location.
Can hemolytic anemia be cured?
Whether hemolytic anemia can be cured depends on the underlying cause:
| Type of Hemolytic Anemia | Can it be Cured? | Explanation |
| Hereditary | No (but manageable) | Caused by genetic mutations, so a true cure is difficult. However, management strategies can significantly improve symptoms. |
| Acquired (Curable Causes) | Yes (depending on cause) | If the cause is an infection, medication, or a temporary condition, addressing it can potentially cure the hemolytic anemia. |
| Acquired (Manageable Causes) | No (but manageable) | In cases like autoimmune hemolytic anemia, a complete cure might not be possible. However, long-term management with medications can control the condition. |
Is thalassemia a hemolytic anemia?
Yes, thalassemia is a type of hemolytic anemia. It’s actually one of the most common inherited forms of hemolytic anemia. There are different types of thalassemia based on which globin chain production is affected (alpha or beta) and the severity of the mutation.
What organ is affected by hemolytic anemia?
While no single organ is directly “affected” by hemolytic anemia, several organs are involved in the disease process. The breakdown of red blood cells with increased destruction of RBCs – the spleen has to work harder, and it might enlarge (splenomegaly); the body’s response to compensate – the bone marrow typically increases RBC production to compensate for the ongoing destruction; and the consequences of increased cell destruction all have an impact on various organs – with increased RBC breakdown, the liver may become overloaded, leading to elevated bilirubin levels and potentially causing jaundice (yellowing of the skin and eyes).
Is hemolytic anemia painful?
In some cases, hemolytic anemia can be associated with pain:
- Splenomegaly (enlarged spleen): A severely enlarged spleen due to excessive red blood cell destruction can sometimes cause discomfort or a dull ache in the upper left abdomen.
- Gallstones: The breakdown of red blood cells increases the production of bilirubin, which can solidify and form gallstones in the gallbladder. Gallstones can cause sharp pain in the upper right abdomen, especially after eating fatty foods.
- Leg ulcers (sickle cell disease): In sickle cell disease, a specific type of hemolytic anemia, damaged red blood cells can block blood flow, leading to painful ulcers in the legs.
What is the difference between anemia and hemolytic anemia?
Anemia is a general term for a condition where your blood has a lower than normal number of red blood cells (RBCs) or not enough hemoglobin, the protein in RBCs that carries oxygen. This deficiency in healthy RBCs leads to reduced oxygen delivery throughout the body, causing symptoms like fatigue, weakness, and shortness of breath.
Hemolytic anemia is a specific type of anemia where red blood cells are destroyed prematurely. This excessive destruction disrupts the normal lifespan of RBCs, leading to a shortage of healthy oxygen carriers in the bloodstream and causing the symptoms of anemia.
Think of anemia as a broad category, and hemolytic anemia as a specific subtype within that category caused by a particular mechanism (excessive red blood cell destruction).
Is hemolytic anemia a form of cancer?
While some cancers, particularly cancers of the blood and bone marrow (like leukemia), can cause hemolytic anemia as a complication, hemolytic anemia itself is not a form of cancer. It’s a separate blood disorder with distinct causes and mechanisms.
Can hemolytic anemia cause death?
Hemolytic anemia itself is rarely fatal, especially if diagnosed early and treated properly. However, there are some situations where it can lead to serious complications that increase the risk of death.
Complications that can be life-threatening
- Severe anemia: Extremely low red blood cell count can significantly impair oxygen delivery to vital organs like the heart and brain. This can lead to heart failure, stroke, or coma in severe cases.
- Heart problems: Chronic hemolytic anemia forces the heart to work harder to pump enough oxygen-rich blood throughout the body. This can lead to heart enlargement (cardiomegaly) and eventually heart failure.
- Splenic rupture (rare): A severely enlarged spleen due to excessive red blood cell destruction can rarely rupture, causing internal bleeding and requiring emergency medical attention.
- Increased risk of infections: A chronic shortage of red blood cells can weaken the immune system, making you more susceptible to serious infections that can be life-threatening.
Additionally, the underlying cause of hemolytic anemia can also contribute to the risk of death. For example, severe cases of malaria or babesiosis (parasitic infections) that cause hemolytic anemia can be fatal if not treated promptly.
What is the life expectancy of a person with hemolytic anemia?
Hereditary hemolytic anemia: With proper management, some individuals with hereditary hemolytic anemia can lead full lifespans or close to it. However, conditions like severe sickle cell disease can have a significant impact on life expectancy.
Acquired hemolytic anemia: The life expectancy for acquired hemolytic anemia depends heavily on the underlying cause. If the cause is treatable or manageable, the life expectancy might not be significantly affected.
Can hemolytic anemia cause liver damage?
Hemolytic anemia doesn’t directly damage the liver, but it can cause a buildup of bilirubin and potentially contribute to complications like jaundice and gallstones. The severity of the impact on the liver depends on the degree of hemolytic anemia and the liver’s health beforehand.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Baldwin C, Pandey J, Olarewaju O. Hemolytic Anemia. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- https://www.msdmanuals.com/professional/hematology-and-oncology/anemias-caused-by-hemolysis/autoimmune-hemolytic-anemia
- Phillips J, Henderson AC. Hemolytic Anemia: Evaluation and Differential Diagnosis. Am Fam Physician. 2018 Sep 15;98(6):354-361. PMID: 30215915.
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.