Introduction
The G6PD fluorescent spot test (G6PD test) is a rapid and reliable method for diagnosing G6PD deficiency. The test involves measuring the activity of G6PD in a blood sample. A low level of G6PD activity indicates a deficiency.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a common inherited red blood cell disorder that affects approximately 400 million people worldwide.
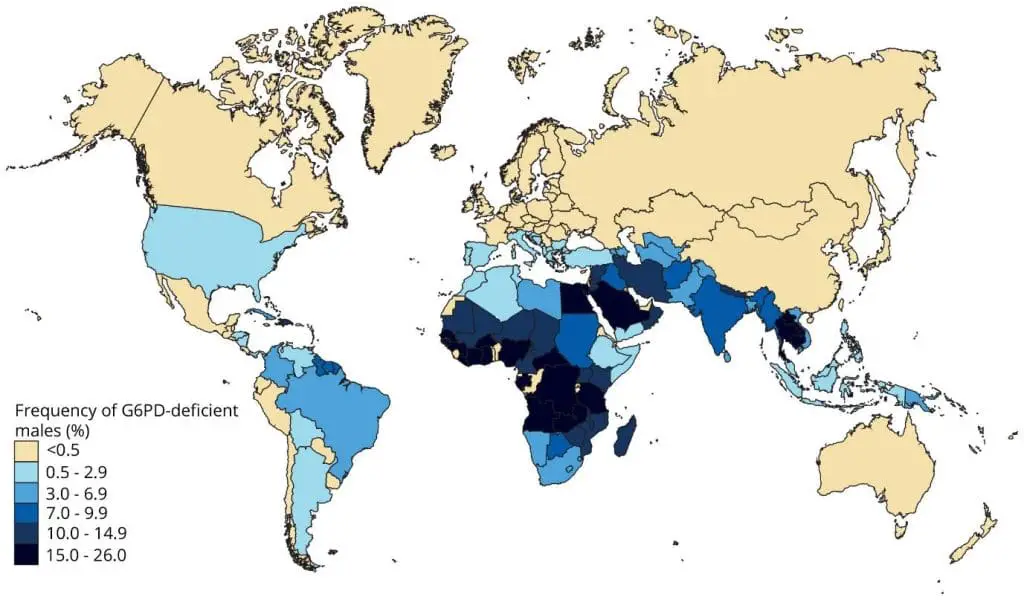
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme that plays a crucial role in protecting red blood cells from damage caused by oxidative stress. When glucose-6-phosphate dehydrogenase (G6PD) is deficient, red blood cells become more susceptible to hemolysis, a condition in which red blood cells rupture and release hemoglobin, the protein that carries oxygen. This will subsequently lead to anemia if the exposure to oxidative stress is prolonged.

G6PD deficiency is an X-linked recessive genetic disorder. This means that the gene responsible for glucose-6-phosphate dehydrogenase (G6PD) production is located on the X chromosome, one of the two sex chromosomes (X and Y). Females have two X chromosomes, while males have one X chromosome and one Y chromosome. In order for a female to have G6PD deficiency, she must inherit a defective glucose-6-phosphate dehydrogenase (G6PD) gene from both of her parents (carrier mother and affected father). Males, on the other hand, only need to inherit one defective glucose-6-phosphate dehydrogenase (G6PD) gene from their mother to be affected by the disorder.

In most cases, G6PD deficiency does not cause any symptoms. However, when red blood cells are exposed to certain triggers, such as certain medications, infections, or fava beans, it can lead to a sudden episode of hemolysis called a hemolytic crisis. Symptoms of a hemolytic crisis can include:
- Pale skin
- Jaundice (yellowing of the skin and eyes)
- Fatigue
- Weakness
- Dark urine
- Shortness of breath
- Abdominal pain
- Fever
There is no cure for G6PD deficiency. However, there are treatments that can help to prevent and manage hemolytic crises. The most important treatment is to avoid triggers that can cause hemolysis. This includes avoiding certain medications, infections, and fava beans. In some cases, individuals with G6PD deficiency may need to receive blood transfusions to treat severe anemia.
Principle of G6PD fluorescent spot test
The glucose-6-phosphate dehydrogenase (G6PD) fluorescent spot test (G6PD test) is a rapid and reliable method for diagnosing glucose-6-phosphate dehydrogenase (G6PD) deficiency. The test is based on the principle that glucose-6-phosphate dehydrogenase (G6PD) catalyzes the reduction of nicotinamide adenine dinucleotide phosphate (NADP+) to nicotinamide adenine dinucleotide phosphate hydrogen (NADPH). NADPH is a fluorescent molecule that can be visualized under ultraviolet (UV) light.
The chemical reaction that underlies the glucose-6-phosphate dehydrogenase (G6PD) fluorescent spot test (G6PD test) is as follows:
G6P + NADP+ + H2O → 6-phosphogluconate + NADPH + H+
The G6PD fluorescent spot test (G6PD test) procedure involves incubating a sample of blood with glucose-6-phosphate (G6P) and NADP+ in a substrate reagent. If glucose-6-phosphate dehydrogenase (G6PD) is present, it will catalyze the reduction of NADP+ to NADPH. The amount of NADPH produced is directly proportional to the glucose-6-phosphate dehydrogenase (G6PD) activity in the sample.
After incubation, the sample is spotted onto a filter paper and allowed to dry. The filter paper is then viewed under long-wave UV light. If glucose-6-phosphate dehydrogenase (G6PD) activity is normal, the spot will fluoresce bright green, indicating the presence of NADPH. If glucose-6-phosphate dehydrogenase (G6PD) activity is deficient, the spot will either have reduced fluorescence or no fluorescence at all.
Method differs slightly according to the manufacturer’s protocol.
Materials
- 5 uL cord blood sample with sodium citrate anticoagulant (BLUE TOP).
- Dilution buffer
- Glucose-6-phosphate dehydrogenase (G6PD) reagent
- Distilled water
- QC vials: normal control, intermediate control, deficiency control
- Filter paper (“Guthrie Test Paper”, Schleicher & Schuell No. 2992)
- Microcentrifuge tubes
- Long Wave UV box
Protocol
- Prepare glucose-6-phosphate dehydrogenase (G6PD) reagent stock substrate solution: Add 5 mL dilution into G6PD reagent freeze-dried powder vial. Leave for 30 minutes until completely dissolved and mixed properly. This reagent is stable for four weeks at +4°C and two months at -20°C.
- Prepare quality control (QC) solution: Add 50 uL distilled water into each QC freeze-dried powder vial. Leave for 30 minutes until completely dissolved and mixed properly.
- Normal Control
- Intermediate Control
- Deficiency Control
- Label 4 microcentrifuge tube with the following, respectively:
- Normal Control
- Intermediate Control
- Deficiency Control
- Patient
- Add 100 uL glucose-6-phosphate dehydrogenase (G6PD) reagent into each of the microcentrifuge tubes, respectively.
- Add 5 uL of QC material and patient sample into their respective labeled tube.
- Mix the solution properly.
- Allow to incubate for 10 minutes at room temperature.
- After 10 minutes, take 10 uL of the Normal Control mixture and place it on a labeled filter paper.
- Repeat with the rest of the tubes on their own labeled filter paper or space.
- Let the filter papers dry completely at room temperature.
- After the filter papers are completely dry (approximately 1 hour), view the results under a long-wave UV lamp in a darkened room.
- Compare the results of the patient sample to the QCs for reporting purposes.
Interpretation
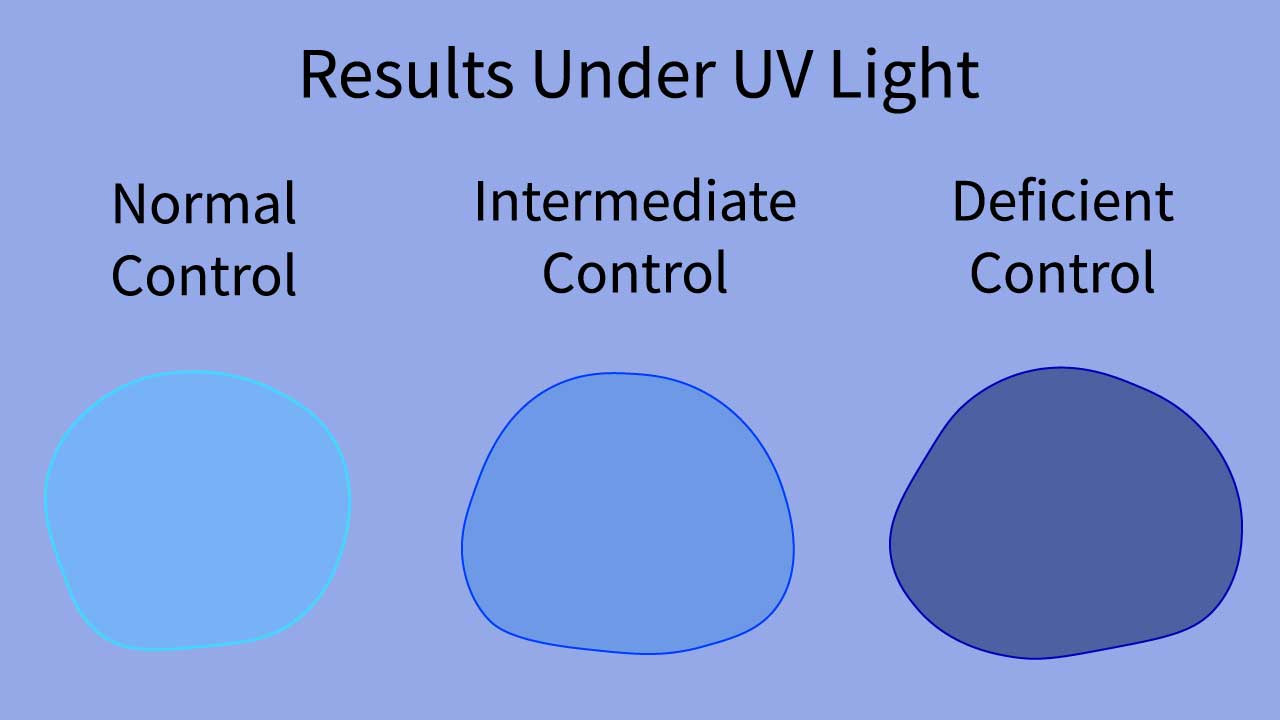
The glucose-6-phosphate dehydrogenase (G6PD) fluorescent spot test (G6PD test) is a qualitative test, meaning that it provides a visual indication of glucose-6-phosphate dehydrogenase (G6PD) activity rather than a quantitative measurement. The interpretation of the test results is based on the intensity of fluorescence observed under long-wave UV light.
- Normal glucose-6-phosphate dehydrogenase (G6PD) activity: A sample with normal glucose-6-phosphate dehydrogenase (G6PD) activity will exhibit bright green fluorescence, indicating the presence of a sufficient amount of NADPH in the G6PD fluorescent spot test (G6PD test).
- Intermediate glucose-6-phosphate dehydrogenase (G6PD) activity: A sample with intermediate glucose-6-phosphate dehydrogenase (G6PD) activity may show reduced fluorescence compared to a normal sample. The fluorescence may be dim or have a yellowish hue in the G6PD fluorescent spot test (G6PD test).
- Deficient glucose-6-phosphate dehydrogenase (G6PD) activity: A sample with deficient glucose-6-phosphate dehydrogenase (G6PD) activity will show no or very weak fluorescence, indicating a lack of NADPH production in the G6PD fluorescent spot test (G6PD test).
Troubleshooting
The accuracy of the glucose-6-phosphate dehydrogenase (G6PD) fluorescent spot test (G6PD test) can be affected by several factors, including:
- Sample preparation: Proper sample collection, storage, and handling are crucial to ensure accurate results.
- Reagent quality: The quality of the reagents used in the test can impact the sensitivity and specificity of the results.
- Incubation time: The incubation time of the blood sample with the substrate reagent should be controlled to ensure optimal NADPH production.
- UV light source: The intensity and wavelength of the UV light source can affect the visibility of fluorescence.
- In some glucose-6-phosphate dehydrogenase (G6PD) deficiencies, newly formed red blood cells exhibit normal enzyme activity, thus patients who have recently experiences a hemolytic crisis must have their older RBCs to be isolated from the young ones for proper interpretation.
- If the patient has received a blood transfusion, this test should only be considered clinically relevant after 30 days have passed. This is because donor red blood cells typically exhibit normal glucose-6-phosphate dehydrogenase (G6PD) activity, which can influence the test result before this time frame.
Laboratory Methods to Test for G6PD Deficiency (G6PD Test)
| Test Method | Principle | Pros | Cons |
| Fluorescent Spot Test (FST) | Measures NADPH production (fluorescence under UV light) by G6PD enzyme activity. Deficiency results in absent/faint fluorescence. | – Simple and inexpensive, suitable for mass screening and low-resource settings. – Rapid results (minutes). – Good sensitivity for severe deficiency. | – Qualitative/semi-quantitative; does not give a precise numerical value. – Cannot reliably detect mild/intermediate deficiency or all female carriers. – High risk of false negatives during/after hemolytic crisis or blood transfusion (due to presence of younger, more active red cells). – Subjective interpretation of fluorescence intensity. |
| Quantitative Spectrophotometric Assay | Directly measures the rate of NADPH formation (light absorbance) using a spectrophotometer, providing a precise numerical enzyme activity value. | – Gold standard for accuracy, providing precise quantitative enzyme activity (IU/gHb). – Reliably diagnoses severe, moderate, and mild deficiencies. – Objective results. | – More complex and expensive; requires specialized lab equipment and trained personnel. – Slower turnaround time (hours to days). – Susceptible to false negatives during/after hemolytic crisis or blood transfusion (similar to FST, but less pronounced). |
| Point-of-Care (POC) Quantitative Assays / Biosensors | Utilizes biosensor technology to provide a rapid, quantitative (or semi-quantitative) measure of G6PD activity, often with simultaneous hemoglobin measurement. | – Rapid results (minutes), suitable for bedside or remote clinics. – Provides numerical values, an improvement over FST. – Useful for rapid screening before administering G6PD-triggering drugs (e.g., primaquine). | – Can be more expensive than FST. – Performance can vary between devices. – May still miss some female carriers. – Susceptible to false negatives during/after hemolytic crisis or blood transfusion. – Some devices have specific temperature requirements. |
| Molecular Genetic Testing (DNA Analysis/Gene Sequencing) | Directly identifies specific mutations in the G6PD gene on the X chromosome. | – Definitive genetic diagnosis, unaffected by hemolytic status, recent transfusions, or age of red blood cells. – Most reliable for accurately identifying female carriers. – Can classify specific G6PD variants (useful for research/genetic counseling). – Valuable for neonates where enzyme assays can be unreliable. | – Very high cost and complexity; not feasible for routine screening. – Very slow turnaround time (days to weeks), impractical for acute management. – Identifies the genetic mutation, but doesn’t directly measure enzyme activity or predict clinical severity with absolute certainty (due to factors like X-inactivation). |
Frequently Asked Questions (FAQs)
What is the G6PD blood spot test and how does it work?
The G6PD fluorescent spot test (G6PD test), also known as FST, is a simple and quick way to screen for glucose-6-phosphate dehydrogenase (G6PD) deficiency, a common genetic condition affecting red blood cells.
Principle
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme in red blood cells that helps protect them from damage.
The test relies on the natural fluorescence of a molecule called NADPH, which is produced by the glucose-6-phosphate dehydrogenase (G6PD) enzyme.
In individuals with normal glucose-6-phosphate dehydrogenase (G6PD) activity, enough NADPH is produced, resulting in a bright green fluorescence under ultraviolet (UV) light. In individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency, less or no NADPH is produced, leading to little or no fluorescence.
The methodology and interpretation of the G6PD test has been described in the protocol above.
What is the normal fluorescence of G6PD?
In the glucose-6-phosphate dehydrogenase (G6PD) fluorescent spot test (G6PD test), “normal fluorescence” refers to a bright green color observed under UV light when the test is performed on a sample with normal glucose-6-phosphate dehydrogenase (G6PD) activity.
This green fluorescence arises because the glucose-6-phosphate dehydrogenase (G6PD) enzyme converts a specific molecule called glucose-6-phosphate (G6P) into another molecule called NADPH. NADPH has the natural property of fluorescing green when exposed to UV light.
Can the G6PD deficiency test be wrong?
Yes, the G6PD test can be wrong in a few different ways.
False-positives
- Borderline cases: The G6PD test is qualitative, meaning it only indicates presence or absence of G6PD activity, not the exact level. Sometimes, individuals with mild G6PD deficiency might have an intermediate level of enzyme activity that falls near the threshold between normal and deficient. This can lead to a false-positive result.
- Interfering substances: In rare cases, substances in the blood, such as certain medications or vitamins, might interfere with the G6PD test and cause a false-positive result.
- Technical errors: Mistakes during sample collection, handling, or processing can also lead to inaccurate results in the G6PD test.
False-negatives
- Timing of the test: If the G6PD test is done during or shortly after an episode of hemolytic anemia (where red blood cells are destroyed), most of the affected cells might already be gone. This can lead to a false-negative G6PD test result, as the remaining cells might have normal glucose-6-phosphate dehydrogenase (G6PD) activity.
- Severity of deficiency: Individuals with very mild glucose-6-phosphate dehydrogenase (G6PD) deficiency might have enough enzyme activity to pass the G6PD test, even though they have the condition.
Here are some additional factors to consider:
- Different test methods: There are several types of G6PD tests available, each with its own limitations. Some are more specific and accurate than others.
- Experience of the healthcare professional: Interpreting the G6PD test results requires expertise and experience. A healthcare professional with a good understanding of G6PD deficiency is better equipped to interpret borderline results and consider other factors that might influence the accuracy.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Beutler E. Drug-induced hemolytic anemia and non-spherocytic hemolytic anemia. In Glucose-6-Phosphate Dehydrogenase (Yoshida A. and Beutler E., Eds) pp. 3-12, 1986. Academic, Orlando.
- Beutler E. A series of new screening procedures for pyruvate kinase deficiency, glucose – 6 – phosphate dehydrogenase deficiency and glutathione reductase deficiency. Blood 1966;28:553-562.
- Dow PA, Petteway MB, Alperin JB. Simplified method for G6PD screening using blood collected on filter paper. Am J Pathol 1974;61:333-336.
- Luzzatto, L., Mehta, A. and Vulliamy, T. (2001) Glucose 6-Phosphate Dehydrogenase Deficiency. In: Scriver, C.R., Beaudet, A.L., Sly, W.S. and Valle, D., Eds., The Metabolic and Molecular Bases of Inherited Disease, 8th Edition, McGraw-Hill, New York, 4517-4533.
- Sharma, U., Mishra, S., Gautam, N., & Gupta, B. K. (2020). Qualitative and quantitative assay of glucose 6 phosphate dehydrogenase in patients attending tertiary care center. BMC research notes, 13(1), 298. https://doi.org/10.1186/s13104-020-05145-8
- Zailani, M. A. H., Raja Sabudin, R. Z. A., Abdul Jalil, D., Ithnin, A., Ayub, N. A., Alauddin, H., Jalil, N., Md Fauzi, A., Cheah, F. C., Lim, L. S., Safian, N., Yusuf, M. M., & Othman, A. (2023). Evaluation of quantitative point-of-care test for measurement of glucose-6-phosphate dehydrogenase enzyme activity in Malaysia. The Malaysian journal of pathology, 45(1), 31–41.
- Richardson SR, O’Malley GF. Glucose-6-Phosphate Dehydrogenase Deficiency. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470315/
- Morris, S. A., Crews, K. R., Hayden, R. T., Takemoto, C. M., Yang, W., Baker, D. K., Broeckel, U., Relling, M. V., & Haidar, C. E. (2022). Incorporating G6PD genotyping to identify patients with G6PD deficiency. Pharmacogenetics and genomics, 32(3), 87–93. https://doi.org/10.1097/FPC.0000000000000456