TL;DR
Myeloproliferative neoplasms (MPNs) are a group of chronic myeloid blood cancers characterized by overproduction of one or more types of blood cells in the bone marrow.
- Classifications ▾:
- Essential Thrombocythemia (ET): Increased platelet count
- Polycythemia Vera (PV): Increased red blood cell count
- Primary Myelofibrosis (PMF): Bone marrow fibrosis leading to abnormal blood cell production
- Risk Factors ▾:
- Age (increased risk with age)
- Family history of myeloproliferative neoplasms (MPNs)
- Certain genetic mutations (JAK2, CALR, MPL)
- Exposure to radiation or specific chemicals (benzene)
- Symptoms ▾:
- General: Fatigue, weakness, weight loss (may be absent)
- Specific:
- ET: Easy bruising, bleeding
- PV: Headache, dizziness, redness of the face
- PMF: Splenomegaly, night sweats, bone pain
- Diagnosis ▾:
- Blood tests (CBC, peripheral blood smear)
- Bone marrow aspiration and biopsy
- Genetic testing for mutations
- Treatment ▾:
- Reduce risk of blood clots
- Control symptoms
- Improve blood cell counts
- Achieve remission (in some cases)
- Prognosis ▾:
- Generally, ET has the best prognosis, followed by PV and PMF.
- Risk of transformation to acute leukemia exists, especially in PMF.
*Click ▾ for more information
Introduction
Myeloproliferative neoplasms (MPNs) are a group of rare blood cancers that originate in the bone marrow. They are characterized by the overproduction of one or more types of mature blood cells: red blood cells, white blood cells, or platelets. This uncontrolled growth can lead to various complications affecting blood flow, organ function, and overall health.
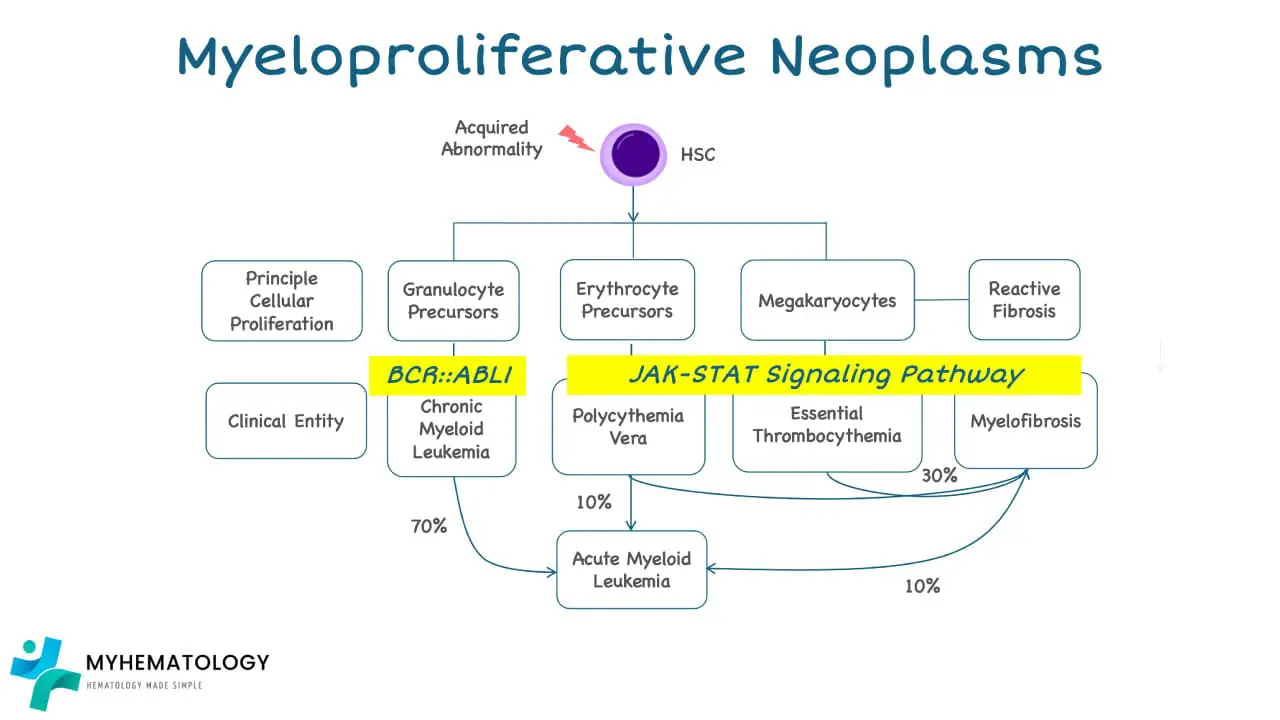
Classification
Myeloproliferative neoplasms (MPNs) are classified into four main types based on the specific blood cell line primarily affected:
- Essential Thrombocythemia (ET): Characterized by an increased platelet count in the blood.
- Polycythemia Vera (PV): Characterized by an increased red blood cell count and often elevated hematocrit (percentage of red blood cells in the blood).
- Primary Myelofibrosis (PMF): Characterized by bone marrow fibrosis (scarring) alongside abnormal blood cell production, often affecting all three cell lines.
- Chronic Myeloid Leukemia (CML): While also classified as an myeloproliferative neoplasm (MPN) , it differs from the others by harboring a specific genetic abnormality (Philadelphia chromosome) and having a distinct clinical course requiring different treatment approaches.
Epidemiology
Myeloproliferative neoplasms (MPNs) are considered relatively rare blood cancers.
Compared to other types of cancers, myeloproliferative neoplasms (MPNs) represent a smaller portion of overall cancer cases. However, they still pose significant challenges for patients due to their potential impact on health and quality of life.
The risk of developing myeloproliferative neoplasms (MPNs) increases with age, with the majority of cases diagnosed in individuals over 50 years old. However, younger individuals can also be affected, although less commonly.
Due to their chronic nature, myeloproliferative neoplasms (MPNs) contribute to a higher prevalence compared to their annual incidence. Estimates suggest a global prevalence of 35-50 cases per 100,000 individuals.
Distribution by MPN Type
- Essential Thrombocythemia (ET): Most common myeloproliferative neoplasm (MPN), accounting for approximately 50-60% of all cases.
- Polycythemia Vera (PV): Around 30-40% of myeloproliferative neoplasm (MPN) cases.
- Primary Myelofibrosis (PMF): Less common, representing 10-20% of myeloproliferative neoplasms (MPNs).
- Chronic Myeloid Leukemia (CML): Although classified as an myeloproliferative neoplasm (MPN), it is considered a distinct entity due to its unique genetic abnormality and clinical course. CML has a separate incidence and prevalence profile with a slightly higher incidence compared to PMF.
Pathogenesis and Pathophysiology
Myeloproliferative neoplasms (MPNs) originate in the bone marrow, specifically within hematopoietic stem cells (HSCs). These stem cells serve as the foundation for blood cell production, constantly dividing and maturing into various types of red blood cells, white blood cells, and platelets.
Normal hematopoiesis
Hematopoiesis, the process of blood cell production, begins in the bone marrow with hematopoietic stem cells (HSCs). These versatile cells can either self-renew or differentiate into common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs).
CLPs mature further into various types of lymphoid cells involved in the immune response, including B cells and T cells. Meanwhile, CMPs diversify into several myeloid cell types, responsible for diverse functions like oxygen transport (red blood cells), blood clotting (platelets), and fighting infections (white blood cells like neutrophils and monocytes).
Mutations in Myeloproliferative Neoplasms (MPNs)
In myeloproliferative neoplasms (MPNs), several key mutations have been identified in different genes that play a role in the uncontrolled growth of blood cells.
JAK2 V617F
This is the most common mutation found in myeloproliferative neoplasms (MPNs), occurring in approximately:
- 95% of patients with Polycythemia Vera (PV)
- 50-60% of patients with Essential Thrombocythemia (ET)
- 50-65% of patients with Primary Myelofibrosis (PMF)
The mutation occurs in the JAK2 gene, specifically at codon 617, where a valine (V) is replaced by a phenylalanine (F).
This alteration leads to constitutive activation of the JAK2 protein, constantly sending growth signals and promoting uncontrolled cell proliferation.
CALR
Mutations in the CALR gene are the second most common in myeloproliferative neoplasms (MPNs), found in approximately:
- 25% of patients with ET and PMF
These mutations are insertions or deletions within exon 9 of the CALR gene, leading to an altered protein structure and function.
Similar to JAK2 V617F, the altered CALR protein also promotes uncontrolled cell growth through various signaling pathways.
JAK2 Exon 12
Mutations in exon 12 of the JAK2 gene are less common than JAK2 V617F, typically found in 5-10% of myeloproliferative neoplasm (MPN) patients who are negative for both JAK2 V617F and CALR mutations.
These mutations involve various deletions or insertions within exon 12, leading to similar consequences as JAK2 V617F, albeit with potentially different signaling pathways involved.
The JAK2p.V617F mutation can produce both erythrocytosis in PV and thrombocytosis in myeloproliferative neoplasms (MPNs), while JAK2 exon 12 mutations cause only erythrocytosis.
MPL
Mutations in the MPL gene are the least common, typically found in 3-5% of myeloproliferative neoplasm (MPN) patients, predominantly in ET and PMF.
The mutations typically involve substitutions within the exon 10 of the MPL gene, leading to an altered MPL protein and subsequent activation of signaling pathways promoting uncontrolled cell growth.
These mutations are mutually exclusive in most cases, meaning a patient will typically have either JAK2 V617F, CALR, JAK2 Exon 12, or MPL mutation, not a combination of them. However, rare cases of patients with combined mutations have been reported.
Understanding the specific mutation present in an myeloproliferative neoplasm (MPN) patient helps guide treatment decisions and potentially provides insights into the prognosis.
What is the JAK-STAT Pathway?
The JAK-STAT pathway, short for Janus kinase-signal transducer and activator of transcription, is a crucial communication system within cells, acting as a cellular messenger that translates signals from outside the cell to actions inside the nucleus. This pathway plays a vital role in regulating various critical cellular processes, including:
- Cell growth and proliferation
- Differentiation (specialization)
- Cell survival
- Immune response
- Metabolism
How the JAK-STAT Pathway works
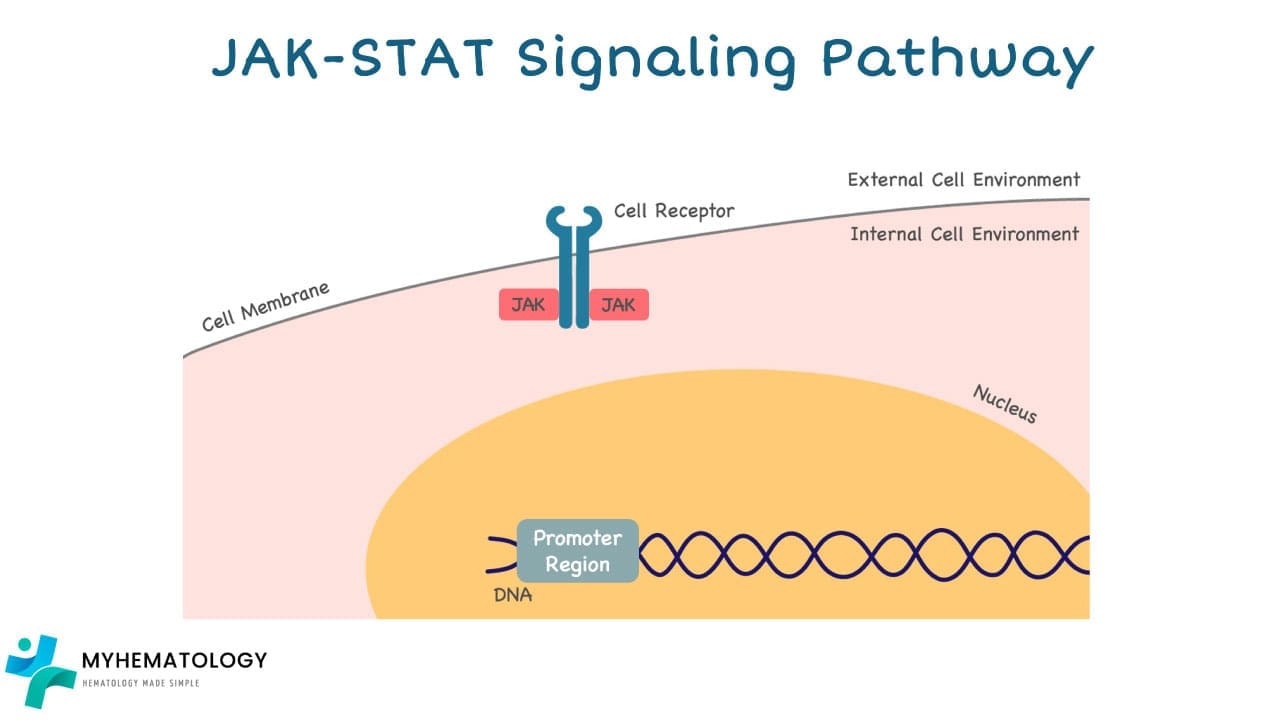
1. External Signal: The pathway begins with external signals such as hormones, growth factors, or cytokines binding to specific receptor proteins on the cell surface.
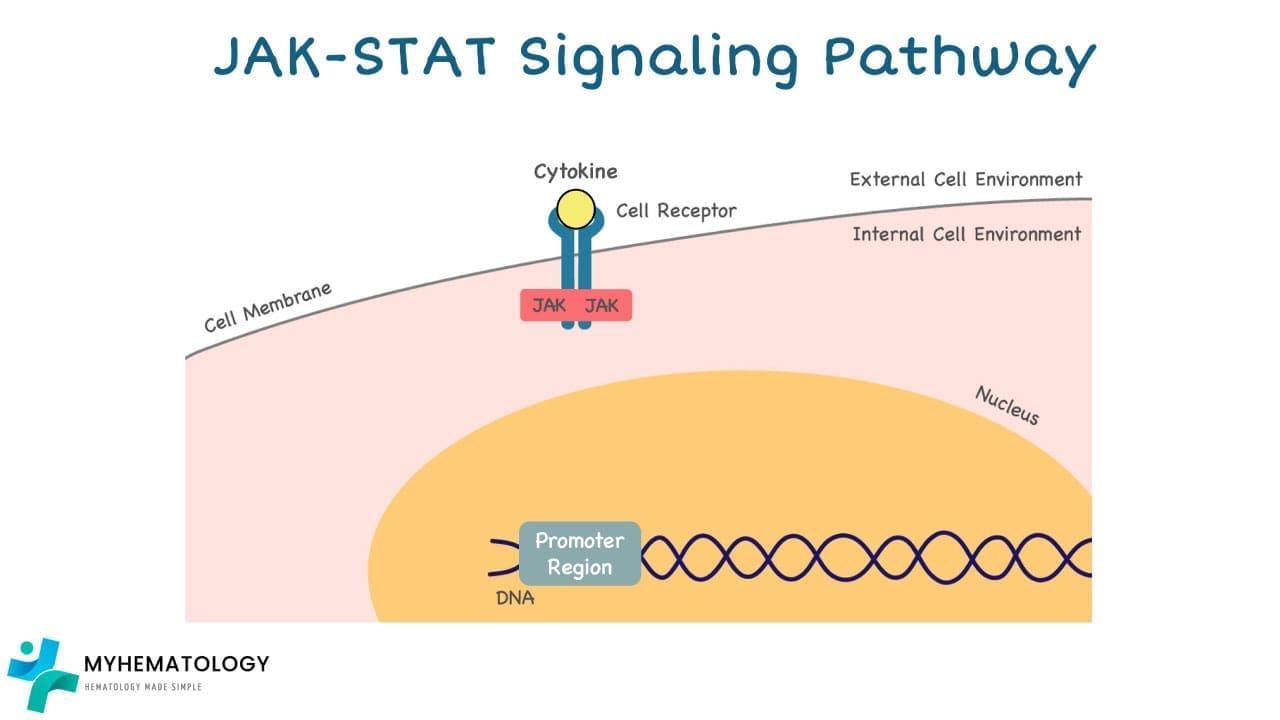
2. JAK Activation: Once bound, the receptor undergoes a conformational change, activating the Janus kinases (JAKs) associated with it.
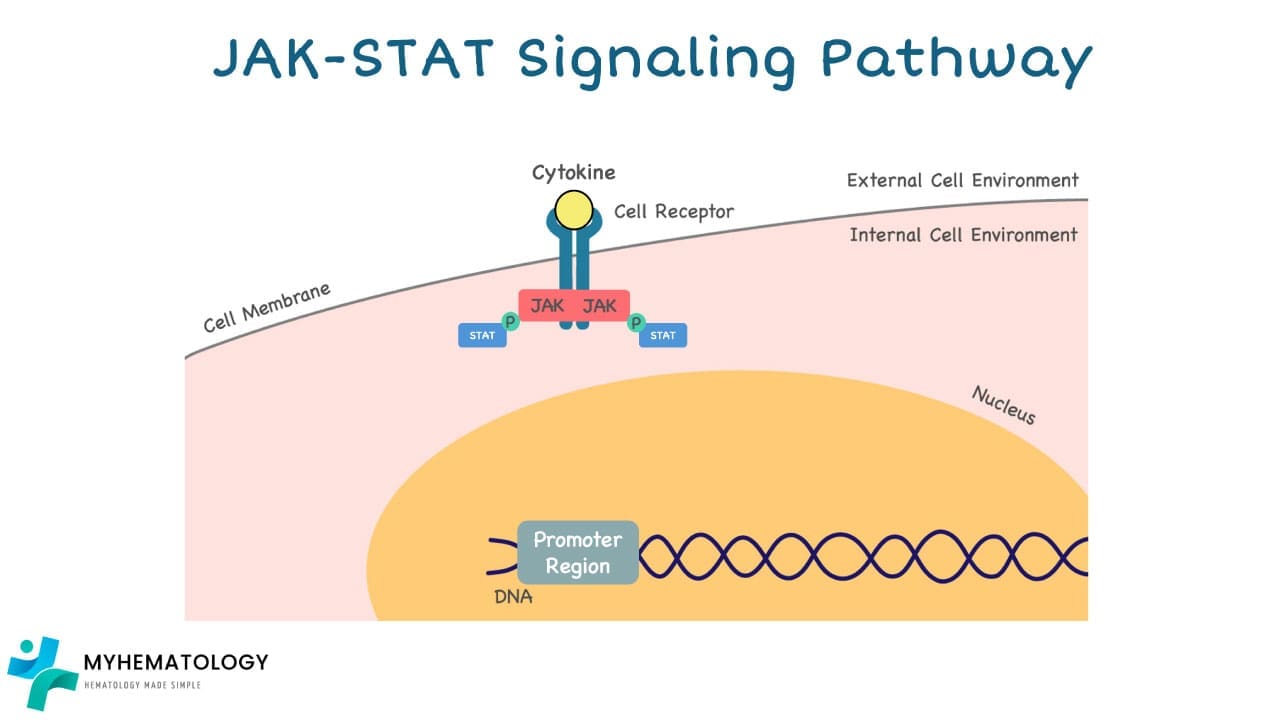
3. STAT Phosphorylation: Activated JAKs then phosphorylate (add a phosphate group) specific proteins called signal transducer and activator of transcription (STATs).
4. Nuclear Translocation: This phosphorylation activates the STATs, allowing them to dimerize (form pairs) and translocate (move) to the nucleus of the cell.
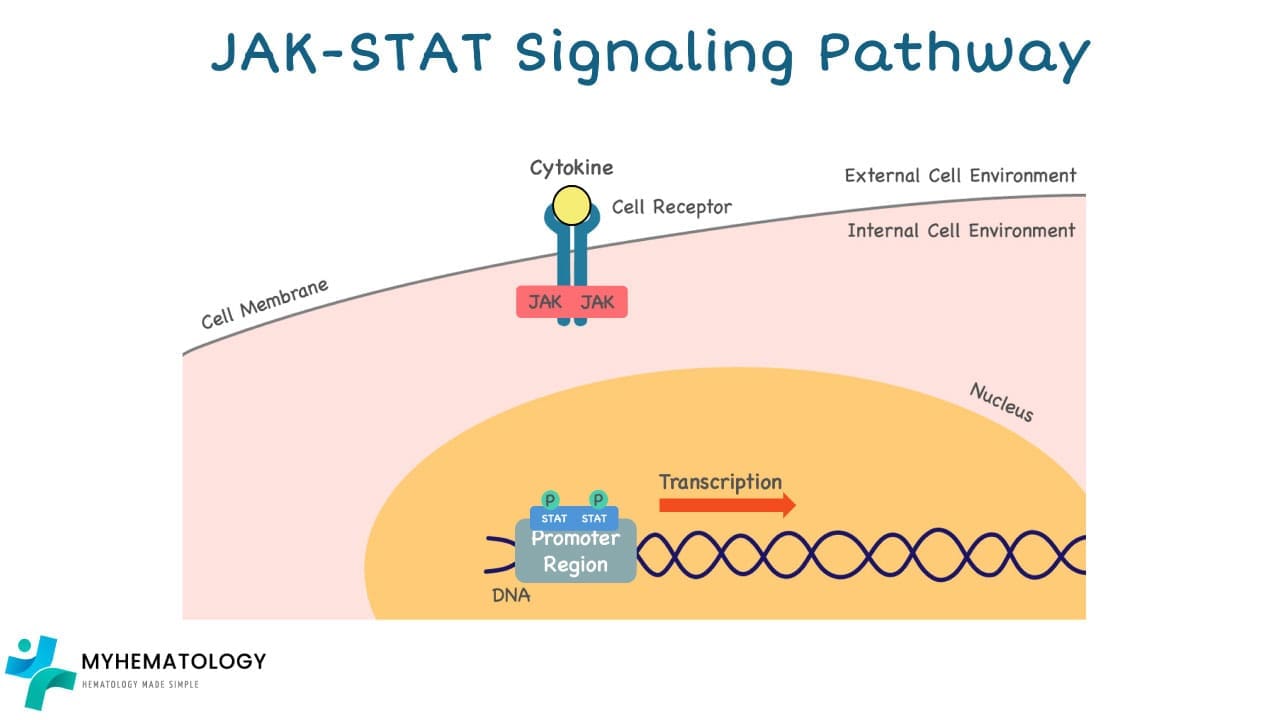
5. Gene Transcription: Inside the nucleus, the STAT dimers bind to specific DNA sequences in the promoters of target genes, activating their transcription (conversion of DNA to RNA).
6. Cellular Response: The newly synthesized RNA molecules then leave the nucleus and travel to the cytoplasm, where they are eventually translated into proteins involved in various cellular functions.
Pathway Dysregulation
However, malfunctions in the JAK-STAT pathway can lead to various diseases, including:
- Cancers: Uncontrolled cell growth and proliferation can occur due to mutations in JAKs or STATs that lead to their constant activation.
- Autoimmune diseases: The immune system may wrongly attack healthy tissues due to abnormal activation of the pathway.
- Inflammatory diseases: Inappropriate immune responses and chronic inflammation can be triggered by dysregulation of the pathway.
What is the consequence of JAK2 V617F mutation in the JAK-STAT pathway?
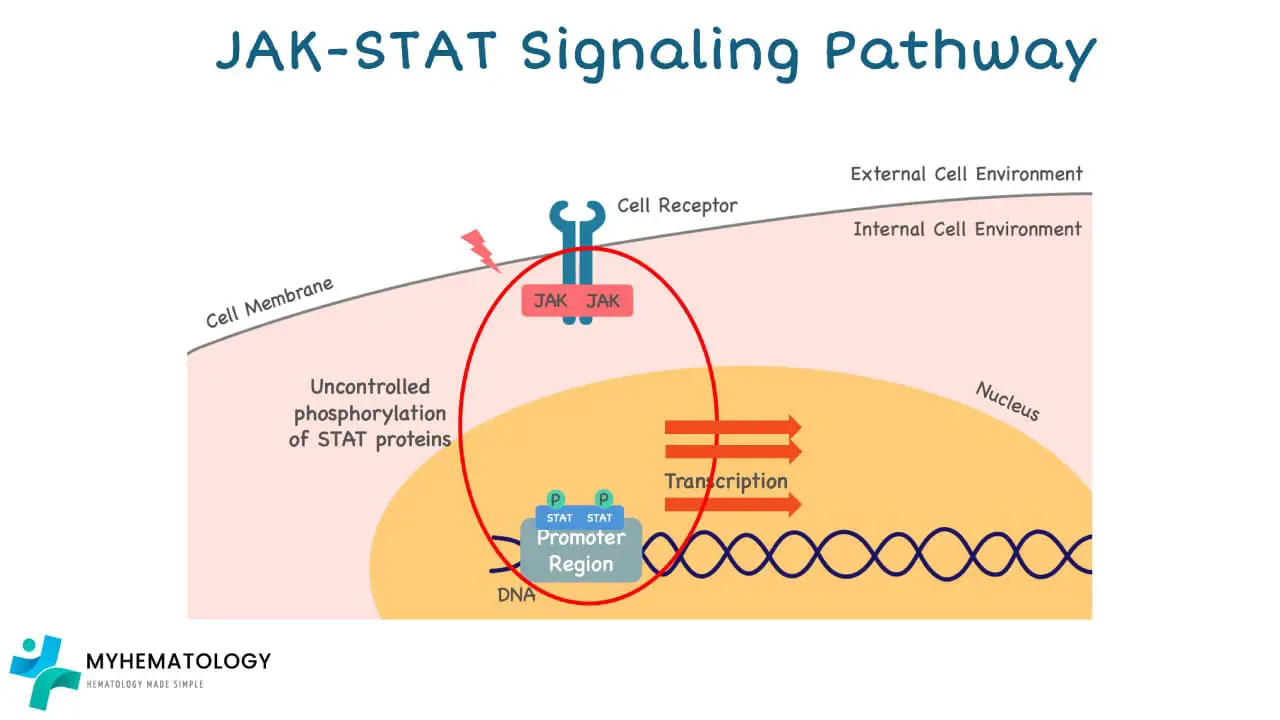
The JAK2 V617F mutation in Myeloproliferative Neoplasms (MPNs) has a profound impact on the JAK-STAT pathway, leading to its abnormal activation and ultimately, uncontrolled cell growth.
1. Disruption of Autoinhibition: Normally, the JAK2 protein has a built-in autoinhibitory domain that keeps it inactive until it receives an external signal. In the case of the JAK2 V617F mutation, the amino acid change disrupts the autoinhibitory domain, rendering JAK2 constitutively active even without external stimuli.
2. Constant Signaling: This constant activation of JAK2 leads to the continuous phosphorylation (activation) of STAT proteins. Activated STATs then translocate to the nucleus, where they bind to DNA and promote the transcription of genes involved in cell growth and proliferation.
3. Uncontrolled Cell Growth: The continuous activation of STATs and subsequent upregulation of growth-promoting genes leads to uncontrolled cell division and proliferation of blood cell progenitors in the bone marrow.
The abnormal JAK-STAT signaling can also lead to other consequences, including:
- Increased cell survival: Inhibiting apoptosis (programmed cell death) and promoting longer lifespans of mutated cells.
- Enhanced resistance to therapy: Making it harder for drugs to target and eliminate mutated cells.
Risk Factors
While the exact cause of myeloproliferative neoplasms (MPNs) remains unknown, several factors have been identified that increase the risk of developing these blood cancers.
Genetic Factors
- Family history: Having a first-degree relative (parent, sibling, child) diagnosed with an myeloproliferative neoplasm (MPN) increases your risk, although it remains relatively low.
- Specific gene mutations: Mutations in genes like JAK2, CALR, MPL are the driving force behind most myeloproliferative neoplasms (MPNs). These mutations can be inherited (rare) or acquired throughout life (more common).
Environmental Factors
- Exposure to radiation: High doses of radiation, such as from atomic bomb blasts or certain medical treatments, are known risk factors.
- Certain chemicals: Long-term exposure to benzene or other benzene-containing products has been linked to an increased risk of myeloproliferative neoplasms (MPNs).
- Smoking: Cigarette smoking has been associated with a higher risk, particularly for Polycythemia Vera (PV).
Other Potential Factors
- Age: The risk of myeloproliferative neoplasms (MPNs) increases significantly with age, with the majority of cases diagnosed in individuals over 50 years old.
- Sex: While not definitive, gender may play a role, with slight female predominance observed in Essential Thrombocythemia (ET) and a slight male predominance in PV.
General and Specific Symptoms of Myeloproliferative Neoplasms (MPNs)
Myeloproliferative neoplasms (MPNs) can present with a range of symptoms, some common across all types and others specific to each type.
General Symptoms
- Fatigue: A feeling of tiredness, exhaustion, and lack of energy is a common symptom experienced by individuals with different types of myeloproliferative neoplasms (MPNs). This fatigue can significantly impact daily activities and quality of life.
- Weakness: A general feeling of lack of strength and reduced ability to perform physical activities can occur due to various factors, including anemia (low red blood cell count) and the impact of the disease on overall health.
- Weight loss: Unintended weight loss can be a symptom in some myeloproliferative neoplasm (MPN) patients, though it’s not as common as the other two. It can be caused by decreased appetite, increased metabolic rate, or underlying inflammatory processes.
Specific Symptoms by Myeloproliferative Neoplasm (MPN) Type
- Essential Thrombocythemia (ET)
- Thrombosis: Due to the increased number of platelets, ET can increase the risk of blood clots forming in various locations.
- Bleeding: Individuals with ET may experience more frequent or prolonged bleeding from cuts, nosebleeds, or heavy menstrual periods.
- Polycythemia Vera (PV):
- Headache: The increased blood viscosity caused by a high red blood cell count can lead to headaches due to increased pressure in the blood vessels.
- Dizziness: Similar to headaches, the thickened blood flow can lead to dizziness or lightheadedness due to reduced oxygen delivery to the brain.
- Redness of the face (plethora): This can occur due to increased blood flow and vasodilation (widening) of blood vessels in the face, especially in the cheeks.
- Primary Myelofibrosis (PMF):
- Splenomegaly: An enlarged spleen due to increased workload in filtering and removing abnormal blood cells.
- Night sweats: Excessive sweating at night can occur due to unknown reasons, potentially related to the body’s response to inflammation or increased metabolic activity.
- Bone pain: This can be caused by changes in the bone marrow due to fibrosis (scarring) and expansion, impacting surrounding nerves and tissues.
Laboratory Tests for MPN Investigations
Investigating suspected Myeloproliferative Neoplasms (MPNs) involves a combination of clinical evaluation and various laboratory tests.
Complete Blood Count (CBC)
- Increased red blood cell count (RBC) in Polycythemia Vera (PV)
- Increased white blood cell count (WBC) (may be variable)
- Elevated platelet count in Essential Thrombocythemia (ET)
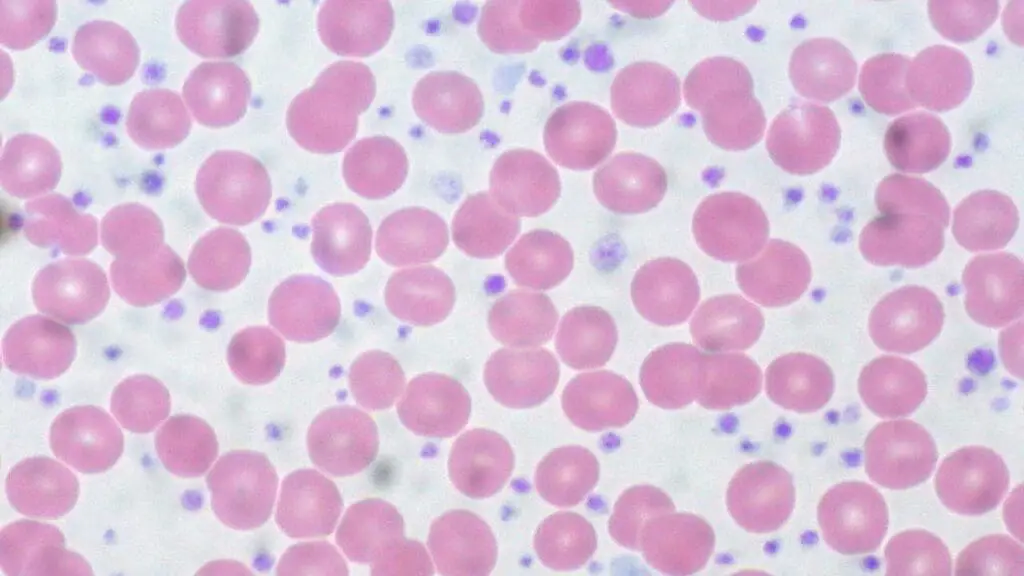
Peripheral Blood Smear
- May reveal abnormal red blood cell shapes, such as teardrops or ovalocytes (in PV)
- Increased number of platelets (in ET) may be visualized
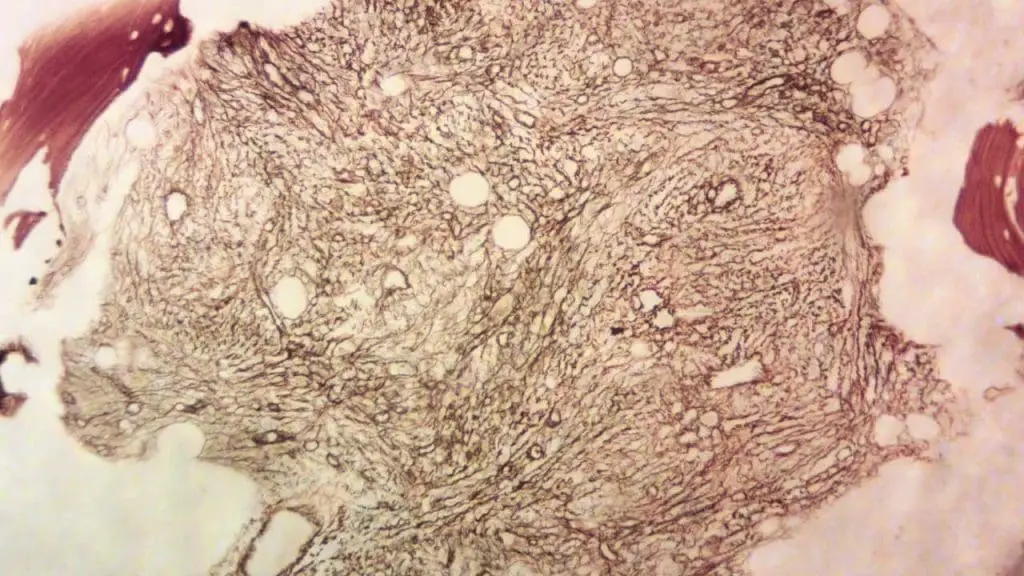
Bone Marrow Aspiration and Biopsy
- Increased cellularity (cellular density) in the bone marrow
- Presence of abnormal cells, such as megakaryocytes (platelet-producing cells) in ET or atypical granulocyte precursors in PMF
Blood Tests for Genetic Mutations
- Polymerase Chain Reaction (PCR): Amplifies specific DNA sequences to detect mutations, including:
- JAK2 V617F mutation (most common)
- CALR mutations
- MPL mutations
- Next-generation sequencing (NGS): A more comprehensive approach that can identify a broader range of potential mutations.
Other Tests
- Serum uric acid: May be elevated in some myeloproliferative neoplasms (MPNs) due to increased cell breakdown.
- Liver function tests: May be abnormal if the disease affects the liver.
- Imaging tests (X-ray, CT scan, MRI): May be used to assess for potential complications, such as splenomegaly or bone marrow fibrosis.
The specific tests ordered will depend on the individual’s clinical presentation and suspected type of myeloproliferative neoplasm (MPN). Abnormal laboratory findings alone are not diagnostic and need to be interpreted in the context of clinical history and physical examination.
What is the treatment and management for MPNs?
Treatment and management plans for myeloproliferative neoplasms (MPNs) are tailored to the specific type of myeloproliferative neoplasm (MPN), individual patient characteristics, and disease stage. Here’s a general overview of the various approaches used:
Aims of Treatment
- Reduce the risk of thromboembolic events (blood clots): This is a primary focus, especially in high-risk patients, to prevent complications like stroke, heart attack, or deep vein thrombosis.
- Control symptoms: Alleviating symptoms like fatigue, bone pain, or splenomegaly can significantly improve quality of life.
- Improve blood cell counts: Bringing abnormal blood cell counts closer to normal ranges can help prevent further complications.
- Potentially achieve disease remission: This is aimed at specific circumstances, particularly in younger patients with high-risk features.
Treatment Options
- Cytoreductive therapy: These medications aim to reduce the overproduction of blood cells in the bone marrow. Common examples include:
- Hydroxyurea: The most commonly used cytoreductive agent in myeloproliferative neoplasms (MPNs), it suppresses DNA synthesis and reduces cell proliferation of blood white cells, red cells, platelets, and to reduce spleen size, and phlebotomy requirements.
- Interferon alpha: Stimulates the immune system and has anti-proliferative effects.
- JAK inhibitors: These newer targeted therapies block the JAK-STAT pathway, which is overactive in many MPNs due to specific mutations. Examples include:
- Ruxolitinib: Approved for all three major myeloproliferative neoplasms (MPNs) (PV, ET, PMF) and effective in managing symptoms and reducing spleen size.
- Fedratinib and Pacritinib: Approved for specific myeloproliferative neoplasms (MPNs) and offer alternative options for patients who do not respond well to other treatments.
- Low-dose aspirin: This helps prevent blood clots, especially in low-risk patients.
- Phlebotomy: Regularly removing blood is used in PV to reduce the red blood cell count and blood viscosity, lowering the risk of thrombosis.
- Stem cell transplantation (SCT): This is a potentially curative option for younger patients with high-risk myeloproliferative neoplasms (MPNs), but it carries significant risks and requires a suitable donor.
- Supportive care: This includes managing symptoms like pain, fatigue, and providing psychosocial support to improve the quality of life for patients.
Importance of Regular Monitoring
Patients diagnosed with myeloproliferative neoplasms (MPNs) require regular monitoring to assess their response to treatment, manage side effects, and detect any potential complications. This may involve:
- Regular blood tests: To monitor blood cell counts and assess treatment effectiveness.
- Imaging tests: To check for changes in spleen size or other potential complications.
- Bone marrow examinations: May be performed periodically to evaluate disease progression.
International Consensus Classification 2022 for MPNs
(taken from https://mpn-hub.com/medical-information/international-consensus-classification-2022-for-myeloproliferative-neoplasms)
| MPN subtypes | Criteria | Diagnosis |
| PV | Major criteria Increased Hb concentration hematocrit or RBC mass JAK2V617F or JAK2 exon 12 mutation Hypercellular BM biopsy (after age-adjusted) displaying proliferation of all lineages with prominent erythroid and granulocytic lineages and increase in pleomorphic, mature megakaryocytes without atypia Minor criterion Low serum EPO level | Requires either all 3 major criteria or first 2 major plus the minor criterion. |
| Post-PV MF | Required criteria Previous PV diagnosis Grade 2 or 3 BM fibrosis Additional criteria Anemia or ceasing of either phlebotomy or cytoreductive treatment of erythrocytosis Leukoerythroblastosis Enlargement of palpable splenomegaly of >5 cm from baseline or growth of a newly palpable splenomegaly Occurrence of any 2 (or all 3) of the following constitutional symptoms: >10% loss of weight in 6 months, night sweats, unexplained fever | All required criteria and 2 or more additional criteria |
| ET | Major criteria Platelet count ≥ 450 x 109/L Proliferation of the megakaryocytic lineage in the BM, with increased numbers of enlarged, mature megakaryocytes with hyperlobulated staghorn-like nuclei, infrequently dense clusters; no significant increase or left shift in neutrophil granulopoiesis or erythropoiesis; no relevant BM fibrosis Diagnostic criteria for BCR-ABL1-positive CML, PV, PMF or other myeloid neoplasms are not met JAK2, CALR, or MPL mutation Minor criterion Clonal marker or absence of evidence of reactive thrombocytosis | All major criteria or the first 3 major criteria plus the minor criterion |
| Post-ET MF | Required criteria Previous ET diagnosis Grade 2 or 3 BM fibrosis Additional criteria Anemia and >2 g/dL decrease from baseline Hb concentration Leukoerythroblastosis Enlargement of palpable splenomegaly of >5 cm from baseline or growth of a newly palpable splenomegaly Increased LDH level Occurrence of any 2 (or all 3) of the following constitutional symptoms: >10% loss of weight in 6 months, night sweats, unexplained fever | All required criteria and 2 or more additional criteria |
| PMF, early/ prefibrotic stage | Major criteria Megakaryocytic proliferation and atypia in BM biopsy, Grade <2 BM fibrosis, increased BM cellularity (age adjusted) with granulocytic proliferation and decreased erythropoiesis (often) JAK2, CALR, or MPL mutation or presence of another clonal marker or absence of reactive BM reticulin fibrosis Diagnostic criteria for BCR-ABL1-positive CML, PV, ET, MDS or other myeloid neoplasms are not met Minor criteria Anemia not due to a comorbid condition Leukocytosis ≥ 11 x 109/L Palpable splenomegaly Increased LDH level | All major criteria and 1 or more minor criteria |
| PMF, overt fibrotic stage | Major criteria Megakaryocytic proliferation and atypia in BM biopsy with reticulin and / or Grade 2 or 3 collagen fibrosis JAK2, CALR, or MPL mutation or presence of another clonal marker or absence of reactive MF Diagnostic criteria for BCR-ABL1-positive CML, PV, ET, MDS or other myeloid neoplasms are not met Minor criteria Anemia not due to a comorbid condition Leukocytosis ≥ 11 x 109/L Palpable splenomegaly Increased LDH level Leukoerythroblastosis | All major criteria and 1 or more minor criterion confirmed in 2 consecutive determinations |
| Chronic eosinophilic leukemia, NOS | Criteria Hypereosinophilia (≥1.5 x 109/L and 10% of WBCs)<20% blast cells in the BM and other diagnostic criteria for AML are not met No BCR-ABL1, ABL1, PDGFRA, PDGFRB, FGFR1, JAK2, FLT3 fusions or other tyrosine kinase gene fusion Other well-defined MPN, chronic myelomonocytic leukemia, or SM criteria are not met BM hypercellularity with dysplastic megakaryocytes with or without dysplastic features in other lineages and often significant fibrosis, associated with an eosinophilic infiltrate or increase blasts ≥ 5% in BM and / or ≥ 2% in peripheral blood Presence of clonal cytogenetic abnormality and / or somatic mutation(s) | All 6 criteria |
| Idiopathic hypereosinophilic syndrome | Criteria Persistent hypereosinophilia Organ damage and / or dysfunction due to tissue eosinophilic infiltrate Absence of reactive, well-defined autoimmune disease or neoplastic condition / disorder underlying the hypereosinophilia Exclusion of lymphocyte variant hypereosinophilic syndrome Normal BM morphology with increased eosinophils Absence of molecular genetic clonal abnormality, with the caveat of clonal hematopoiesis of indeterminate potential | All 6 criteria |
| Chronic neutrophilic leukemia | Criteria WBC ≥ 13 x 109/L with segmented and band neutrophils ≥ 80%. No significant dysgranulopoiesis. Neutrophil precursors <10% WBC. Rare circulating blasts. Monocyte count <10% WBC. Hypercellular BM with increased neutrophils associated with normal maturation CSF3RT618l or another activating CSF3R mutation or persistent neutrophilia (≥3 months), splenomegaly and no identifiable cause of reactive neutrophilia including absence of a plasma cell neoplasm or, if a plasma cell neoplasm is present, presence of clonality of myeloid cells by cytogenetic or molecular studies Diagnostic criteria for BCR-ABL1-positive CML, PV, ET, PMF or other myeloid/lymphoid neoplasm with eosinophilia and tyrosine kinase gene fusions are not met | |
| MPN, unclassifiable | Criteria Presence of clinical and hematologic myeloproliferative neoplasm (MPN) features JAK2, CALR, or MPL mutation or another clonal marker Diagnostic criteria for any other MPN, MDS, MDS/MPN or BCR-ABL1-positive CML are not met | All 3 criteria |
What is the prognosis for MPN?
The prognosis for Myeloproliferative Neoplasms (MPNs) is highly variable and depends on several factors.
- Specific type of myeloproliferative neoplasm (MPN):
- Essential Thrombocythemia (ET): Generally has a favorable prognosis with a life expectancy close to the average population.
- Polycythemia Vera (PV): Prognosis is variable and depends on various factors, with a median survival ranging from 14-20 years.
- Primary Myelofibrosis (PMF): Has a worse prognosis compared to other myeloproliferative neoplasms (MPNs), with a median survival of 2-11 years depending on the risk category.
- Age at diagnosis: Younger patients generally have a better prognosis than older patients.
- Overall health: Patients with other health conditions may have a poorer prognosis.
- Presence of specific mutations: Mutations like JAK2 V617F are generally associated with a better prognosis than other mutations.
- Disease stage: Early-stage disease typically has a better prognosis than advanced stages.
- Response to treatment: Patients who respond well to treatment generally have a better long-term outlook.
- Risk of Transformation: Individuals with myeloproliferative neoplasms (MPNs), particularly Primary Myelofibrosis (PMF), have an increased risk of their disease transforming into a more aggressive form of blood cancer called acute myeloid leukemia (AML).
- Factors Affecting Risk: The risk of transformation varies depending on:
- MPN type: PMF carries the highest risk (estimated at 10-20% over 10 years), followed by PV (2-4%) and ET (around 1%).
- Individual factors: Certain genetic mutations, advanced age, abnormal blood counts, and specific disease features can increase an individual’s risk.
- Nature of Transformation: The acute leukemia arising from myeloproliferative neoplasms (MPNs) is often difficult to treat, with chemotherapy generally less effective than in de novo AML cases.
Frequently Asked Questions (FAQs)
Does MPN turn into leukemia?
Myeloproliferative neoplasms (MPNs) can, in some cases, transform into acute myeloid leukemia (AML), a more aggressive form of blood cancer. However, it’s important to understand that:
- Not all myeloproliferative neoplasms (MPNs) will transform into leukemia. The risk varies depending on several factors, including:
- Myeloproliferative neoplasm (MPN) type: Primary Myelofibrosis (PMF) carries the highest risk (around 10-20% over 10 years), followed by Polycythemia Vera (PV) (2-4%) and Essential Thrombocythemia (ET) (around 1%).
- Individual factors: Certain genetic mutations, advanced age, abnormal blood counts, and specific disease features can increase the risk for an individual.
- Transformation is not always predictable. Predicting which myeloproliferative neoplasm (MPN) patients will develop leukemia is challenging, and ongoing research aims to identify individuals at higher risk to allow for more proactive monitoring and potentially preventive strategies.
Can myeloproliferative neoplasm (MPN) be cured?
While treatments significantly improve the management and prognosis of myeloproliferative neoplasms (MPNs), the only potential cure currently available is allogeneic stem cell transplantation (SCT).
SCT involves replacing a patient’s unhealthy bone marrow with healthy stem cells from a donor, potentially providing a new blood and immune system. However, SCT carries significant risks and is only suitable for a subset of myeloproliferative neoplasm (MPN) patients, typically younger patients with high-risk forms of the disease.
While ongoing research is dedicated to finding additional curative options, current myeloproliferative neoplasm (MPN) therapies focus on controlling symptoms, reducing disease complications, and improving overall quality of life.
What are three 3 characteristics of myeloproliferative neoplasms (MPNs)?
Three key characteristics of myeloproliferative neoplasms (MPNs) are:
- Overproduction of one or more types of blood cells: This is the hallmark feature of myeloproliferative neoplasms (MPNs) and distinguishes them from other blood cancers. The affected cell type can be red blood cells (polycythemia vera), white blood cells (rare), or platelets (essential thrombocythemia).
- Presence of specific genetic mutations: While not always present, mutations in genes like JAK2, CALR, and MPL are frequently observed in myeloproliferative neoplasms (MPNs). These mutations contribute to the abnormal growth and proliferation of blood cells in the bone marrow.
- Increased risk of blood clots (thrombosis): Due to the abnormal blood cell production and potential changes in blood cell function, individuals with myeloproliferative neoplasms (MPNs) have a higher risk of developing blood clots in various parts of the body, which can lead to serious complications like stroke, heart attack, or deep vein thrombosis.
Does myeloproliferative neoplasm (MPN) cause pain?
Yes, myeloproliferative neoplasms (MPNs) can cause pain in some individuals, but the experience and severity of pain can vary depending on the specific type of myeloproliferative neoplasm (MPN) and other factors.
Types of Pain
- Bone pain: This is a common symptom in myeloproliferative neoplasms (MPNs), particularly primary myelofibrosis (PMF). It arises due to:
- Bone marrow fibrosis: The hallmark feature of PMF, where scar tissue forms in the bone marrow, putting pressure on nerves and causing pain.
- Increased activity of certain inflammatory pathways: This can contribute to bone and joint pain.
- Splenomegaly-related pain: An enlarged spleen, which can occur in some myeloproliferative neoplasms (MPNs), can put pressure on surrounding organs and cause discomfort or pain in the left upper abdomen.
- Headaches: More common in Polycythemia Vera (PV) due to increased blood flow and pressure within the head.
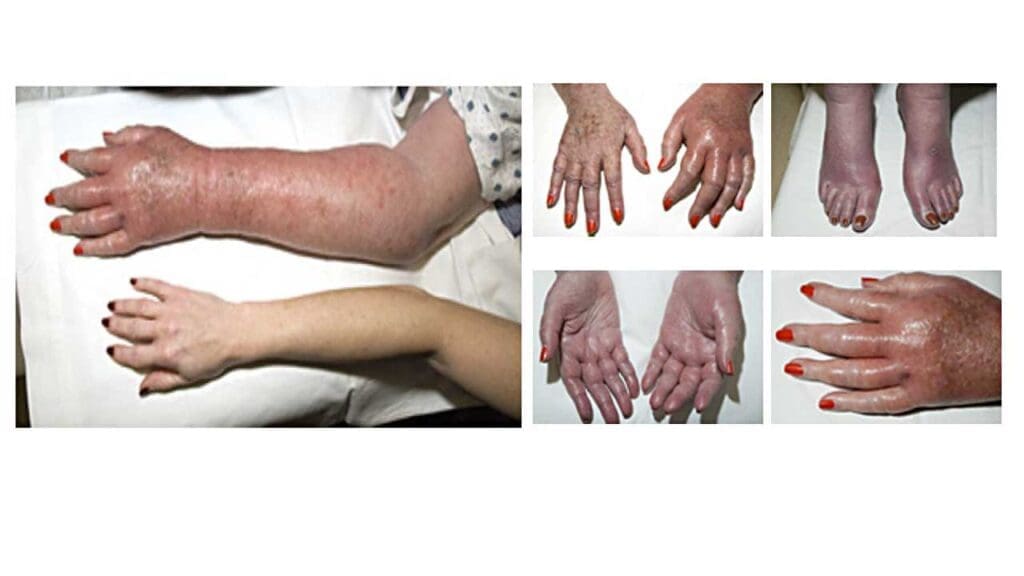
- Less common: In rare cases, specific types of myeloproliferative neoplasms (MPNs), like essential thrombocythemia (ET) with erythromelalgia, can cause burning pain, redness, and warmth in the hands and/or feet.
Factors Affecting Pain
- Type of myeloproliferative neoplasm (MPN): PMF is most likely to cause pain due to fibrosis.
- Disease stage and severity: Pain is generally worse in advanced stages with extensive bone marrow fibrosis.
- Individual factors: Age, overall health, and individual pain sensitivity can influence the experience of pain.
Management of Pain
- Pain management strategies aim to
- Control the underlying condition (e.g., with medication)
- Manage symptoms directly with pain relievers (e.g., over-the-counter pain medication or stronger pain medication for severe cases)
- Early intervention and addressing the underlying cause are crucial for effective pain management.
What is the difference between leukemia and myeloproliferative neoplasms (MPNs)?
Leukemia and myeloproliferative neoplasms (MPNs) are both blood cancers, but they have several key differences.
Cell Origin
- Leukemia: Arises from immature blood cells in the bone marrow. These immature cells cannot effectively perform their functions and crowd out the production of healthy blood cells.
- Myeloproliferative neoplasms (MPNs): Develops from mature blood cells in the bone marrow. These mature cells are often functional, but they are produced in excessive numbers.
Progression
- Leukemia: Generally considered a more aggressive and fast-growing cancer. The abnormal cells rapidly multiply and spread to other parts of the body if left untreated.
- Myeloproliferative neoplasms (MPNs): Typically slower-growing cancers. They can remain stable for years, but some may eventually transform into acute leukemia.
Symptoms
- Leukemia: Commonly presents with symptoms like fatigue, weakness, fever, frequent infections, and abnormal bleeding due to a lack of healthy blood cells.
- Myeloproliferative neoplasms (MPNs): Symptoms can be subtle or even absent in the early stages, especially for essential thrombocythemia (ET). Specific symptoms may arise depending on the type of myeloproliferative neoplasm (MPN).
- ET: Easy bruising and bleeding
- Polycythemia Vera (PV): Headache, dizziness, redness of the face
- Primary Myelofibrosis (PMF): Splenomegaly, night sweats, bone pain
Treatment
- Leukemia: Often involves intensive treatments like chemotherapy, radiation therapy, and stem cell transplantation to eliminate the abnormal cells.
- Myeloproliferative neoplasms (MPNs): Aims to control symptoms, reduce the risk of blood clots, and improve blood cell counts. Treatment options include cytoreductive therapy, JAK inhibitors, low-dose aspirin, and phlebotomy (for PV).
Prognosis
- Leukemia: Prognosis varies depending on the subtype and individual factors, but it can be challenging to treat, especially in advanced stages.
- Myeloproliferative neoplasms (MPNs): Prognosis also varies depending on the type and individual characteristics. Generally, ET has the best prognosis, followed by PV and PMF. The risk of transformation to leukemia exists, especially in PMF, which can worsen the prognosis.
| Feature | Leukemia | Myeloproliferative Neoplasms (MPNs) |
| Cell Origin | Immature blood cells | Mature blood cells |
| Progression | Rapidly growing | Slower growing; potential for transformation to leukemia |
| Symptoms | Fatigue, weakness, fever, infections, bleeding | Often subtle; specific symptoms depending on type |
| Treatment | Intensive therapies (chemotherapy, radiation, stem cell transplant) | Symptom control, reducing blood clot risk, improving blood cell counts |
| Prognosis | Variable; challenging in advanced stages | Variable; generally better than leukemia, but risk of transformation exists |
Is myeloproliferative neoplasm an autoimmune disease?
No, myeloproliferative neoplasms (MPNs) are not autoimmune diseases. Myeloproliferative neoplasms (MPNs) are cancers that arise from genetic mutations driving the overproduction of blood cells.
In contrast, autoimmune diseases occur when the immune system mistakenly attacks the body’s healthy tissues, triggered by a combination of genetic, environmental, and other unknown factors. While some individuals may have both an myeloproliferative neoplasm (MPN) and an autoimmune condition, these are separate diseases with distinct causes and mechanisms.
Are MPNs hereditary?
While the majority of Myeloproliferative Neoplasms (MPNs) are caused by acquired genetic mutations that develop during a person’s lifetime and are not inherited from parents, there is a recognized phenomenon of “familial MPN” or an inherited predisposition. MPNs are generally not directly inherited in the classic Mendelian sense (where a single gene mutation passed from parent to child causes the disease).
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- https://mpn-hub.com/medical-information/international-consensus-classification-2022-for-myeloproliferative-neoplasms
- Gianelli U, Thiele J, Orazi A, Gangat N, Vannucchi AM, Tefferi A, Kvasnicka HM. International Consensus Classification of myeloid and lymphoid neoplasms: myeloproliferative neoplasms. Virchows Arch. 2023 Jan;482(1):53-68. doi: 10.1007/s00428-022-03480-8. Epub 2022 Dec 29. PMID: 36580136; PMCID: PMC9852206.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391-405. doi: 10.1182/blood-2016-03-643544. Epub 2016 Apr 11. PMID: 27069254.
- Thapa B, Fazal S, Parsi M, et al. Myeloproliferative Neoplasms. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531464/
- Arana Yi C, Tam CS, Verstovsek S. Efficacy and safety of ruxolitinib in the treatment of patients with myelofibrosis. Future Oncol. 2015;11(5):719-33. doi: 10.2217/fon.14.272. PMID: 25757677; PMCID: PMC4920055.
- Madden C. THE PROACTIVE APPROACH TO POLYCYTHEMIA VERA: Essential Strategies For Patients, Caregivers, And Healthcare Professionals – Navigating Diagnosis, Treatment, And Practical Coping Strategies
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Mahmud, M., Vasireddy, S., Gowin, K., & Amaraneni, A. (2023). Myeloproliferative Neoplasms: Contemporary Review and Molecular Landscape. International Journal of Molecular Sciences, 24(24), 17383. https://doi.org/10.3390/ijms242417383
- Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood (2017) 129 (6): 680–692. https://doi.org/10.1182/blood-2016-10-695957
- Barbui, T., Ghirardi, A., Carobbio, A. et al. Thrombosis in myeloproliferative neoplasms: a viewpoint on its impact on myelofibrosis, mortality, and solid tumors. Blood Cancer J. 14, 188 (2024). https://doi.org/10.1038/s41408-024-01169-6
- Tefferi A, Pardanani A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015;1(1):97–105. doi:10.1001/jamaoncol.2015.89