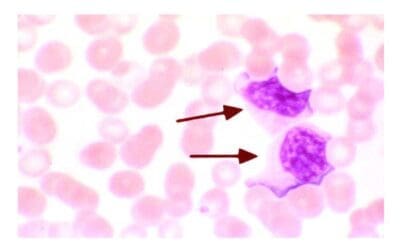
Introduction
Similar to the Leishman stain, May-Grünwald Giemsa (MGG) stain is part of the Romanowsky stains and adopts the same principle. May-Grünwald Giemsa (MGG) stain consists of May-Grünwald stain and Giemsa stain. MGG stain is used for morphology assessment of bone marrow aspirates especially in diseases like chronic lymphocytic leukemia, chronic myeloid leukemia, aplastic anemia.
Principle of May-Grünwald Giemsa (MGG) Stain
May-Grünwald Giemsa (MGG) stain works by differentially staining different cellular components based on their acidic or basic properties. Acidic components, such as DNA and chromatin, stain blue or purple, while basic components, such as cytoplasm and proteins, stain pink or red. The stain also highlights nuclear structures, such as nucleoli and nuclear membranes.
May-Grünwald Giemsa (MGG) stain is used to visualize cells in a variety of tissues, including blood, bone marrow, and lymph nodes. It is particularly useful for identifying and classifying different types of white blood cells, such as lymphocytes, granulocytes, and monocytes. It can also be used to detect abnormalities in cells, such as the presence of cancer cells or infection.
Method differs slightly according to the manufacturer’s protocol.
Materials
- May-Grünwald dye
- Absolute methanol
- Giemsa dye
- Glycerol
- Phosphate buffer pH 6.8
Protocol
Preparation of May-Grünwald stain
- Dissolve 0.3 g of May-Grünwald dye in 100 mL absolute methanol in a 250 mL conical flask.
- Warm the mixture to 50°C in a water bath for a few hours and allow it to cool to room temperature.
- Stir the mixture on a magnetic stirrer and leave it stirring for 24 hours.
- Filter the mixture and stain is ready for use.
Preparation of Giemsa stain
- Add 1.0 g of Giemsa dye into 66 mL of glycerol and warm the mixture in a conical flask for 1-2 hours at 50°C.
- Cool the mixture to room temperature and add 66 mL of absolute methanol.
- Leave the mixture to dissolve for 2-3 days, mixing it at intervals.
- The stain is then ready for use after filtering.
Staining
- Prepare a solution of May-Grünwald stain and phosphate buffer at 1:1 ratio and mix well.
- Prepare a 1:10 dilution of Giemsa stain with phosphate buffer pH 6.8 and mix well.
- Fix bone marrow aspirate smear in absolute methanol for 10 -15 minutes.
- Fully cover the slide with May-Grünwald-phosphate mixture and incubate for 10 minutes.
- Decant the May Grunwald-phosphate mixture from the slide and fully cover the slide with the Giemsa-phosphate mixture.
- Incubate for 15 minutes.
- Decant the mixture and rinse the slide with slow running tap water.
- Wipe the back of the slide and edges with Kim wipes. Be careful not to touch the smear.
- Dry the slide using a hair dryer on the lowest speed (not more than 10 seconds at a time) or air dry in a tilted position.
- Mount the slide with Depex and cover the zone of morphology with a long cover slip.
- This slide is now ready for viewing.
Interpretation
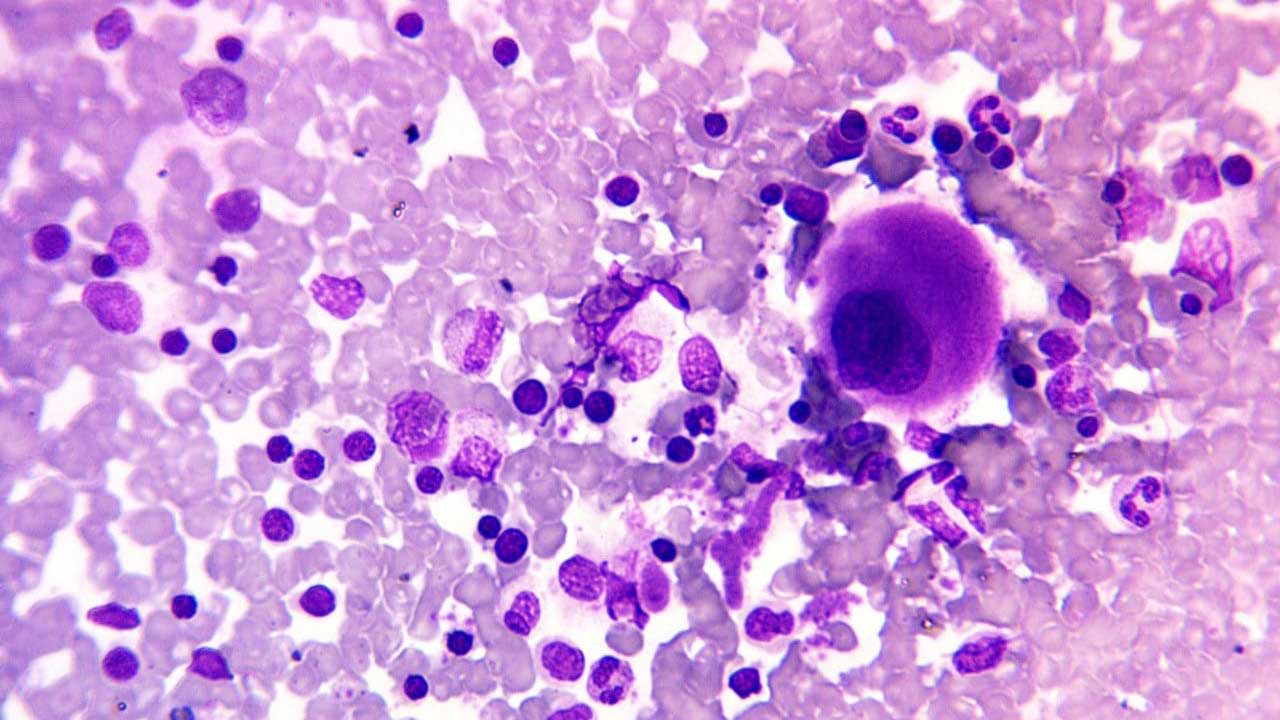
To assess the bone marrow aspirate morphology accurately, prior knowledge of the patient’s history and also clinical indication for the procedure is necessary.
Microscopy examination usually covers 10X magnification, 40X magnification as well as 100X (oil immersion) magnification assessment.
Under 10X objective magnification, things to note include:
- Hypercellular or hypocellular bone marrow fragment
- Normal or diluted or hypercellular cell trails
- Number and morphology of megakaryocytes available
- Presence of abnormal cell clumps are suggestive of marrow infiltration
Under 40X objective magnification, things to note include:
- Whether the erythropoiesis are normoblastic, megaloblastic or dysplastic in nature
- Presences of all stages of normal maturation for the myeloid and lymphoid lineage cells including myeloblasts and lymphoblasts
- Presence of normal or abnormal plasma cells and macrophages and whether their number is increased.
- Presence of any metastatic cells.
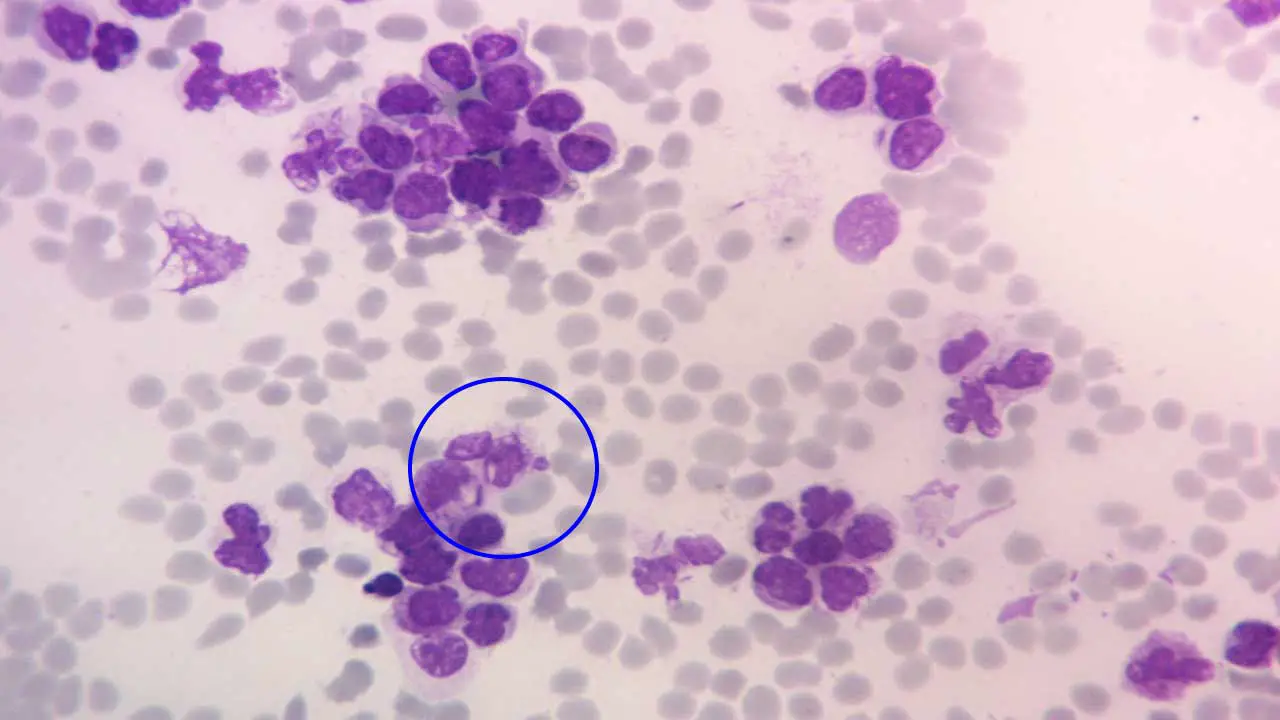
Troubleshooting
| Problem | Appearance on Slide | Likely Causes | Solutions |
| Pale or Under-stained | Cells are very light, poor detail, weak nuclear staining, RBCs may be too pink. | – Insufficient staining time (May-Grünwald or Giemsa) – Stain solutions too dilute – Buffer pH too acidic (inhibits basic dye uptake) – Expired or degraded stain solutions – Inadequate fixation (methanol) – Excessive washing after staining | – Increase staining time for specific step(s) – Check/adjust stain dilution (ensure correct working solution) – Adjust buffer pH to be more alkaline (closer to 6.8-7.2) – Use fresh, unexpired reagents; filter if necessary- Ensure adequate methanol fixation time – Reduce washing time or intensity |
| Too Dark or Over-stained | Cells are excessively dark, details obscured, poor differentiation (e.g., uniformly blue/purple), RBCs may be bluish. | – Excessive staining time (May-Grünwald or Giemsa) – Stain solutions too concentrated – Buffer pH too alkaline (enhances basic dye uptake) – Insufficient washing after staining | – Decrease staining time for specific step(s) – Check/adjust stain dilution (make more dilute) – Adjust buffer pH to be more acidic (closer to 6.8) – Increase washing time or intensity |
| Uneven Staining / Patchy | Some areas well-stained, others pale, dark, or show patchy coloration. | – Uneven smear preparation (thick/thin areas) – Incomplete drying of smear before fixation/staining – Inconsistent immersion in stain solutions (parts not covered) – Improper mixing of stain solutions – Contamination or precipitate on slides/reagents | – Improve smear technique (thin, even, no ridges) – Ensure smears are completely air-dried – Ensure slides are fully submerged in stain; use adequate volume – Gently mix working stain solutions before use – Clean all glassware/jars; filter stain solutions before use |
| Poor Cellular Morphology / Artifacts | Cells distorted, lysed, shrunken, or contain unusual deposits/crystals. | – Improper or delayed fixation – Over-drying of smears before fixation – Contaminated reagents (methanol, water, buffer) – Dye precipitation (especially Giemsa) – Rough handling of slides | – Fix smears promptly with fresh, absolute methanol – Ensure proper air-drying but avoid excessive drying – Use pure, filtered distilled/deionized water and reagents – Filter Giemsa working solution immediately before use; check pH – Handle slides gently throughout the process |
| Red Blood Cells Greenish or Bluish | RBCs appear abnormal color instead of pink-orange/buff. | – Buffer pH too alkaline (basic dyes bind to RBCs) – Insufficient May-Grünwald (eosin) staining | – Adjust buffer pH to be more acidic (closer to 6.8) – Increase May-Grünwald staining time or concentration |
Frequently Asked Questions (FAQs)
What is the May-Grünwald Giemsa (MGG) stain used for?
MGG stain, or May-Grünwald Giemsa stain, has several important uses in various fields, primarily focusing on visualizing and analyzing cells.
Hematology
- Differential counting of blood cells (CBC with differential): This is the most common use of May-Grünwald Giemsa (MGG) stain. It effectively distinguishes and colors different types of white blood cells (neutrophils, eosinophils, basophils, lymphocytes, monocytes) and red blood cells, allowing for accurate assessment of their numbers and morphology.
- May-Grünwald Giemsa (MGG) stain highlights various cellular features such as nuclear size, shape, chromatin pattern, and cytoplasmic granules. This detailed visualization aids in identifying abnormal cell characteristics associated with different diseases like leukemia, anemia, and infections.
Cytology
- Examination of body fluids: May-Grünwald Giemsa (MGG) stain is used to analyze cells present in body fluids like cerebrospinal fluid, pleural fluid, and synovial fluid for the presence of abnormal cells, potentially indicating infections, inflammation, or malignancies.
- Fine-needle aspiration biopsies: The stain helps in examining cell samples obtained through minimally invasive procedures like fine needle aspirates, providing valuable information for diagnosing various tumors and other tissue abnormalities.
Histology
- Visualization of hematopoietic tissues: This stain can be used on tissue sections, particularly bone marrow biopsies, to assess the composition and morphology of blood-forming cells, aiding in diagnosing blood disorders.
- Detection of parasites and microorganisms: In some cases, this stain can help identify certain parasites and microorganisms within tissue samples.
Why is May-Grünwald Giemsa (MGG) stain better than Leishman stain for bone marrow analysis?
When examining bone marrow aspirate smears, May-Grünwald-Giemsa (MGG) stain is generally preferred over Leishman stain due to its superior ability to highlight cellular details and provide a more comprehensive picture of bone marrow morphology.
While Leishman stain is a widely used Romanowsky stain for peripheral blood smears and is valued for its simplicity and quick results, it may not offer the same level of detailed differentiation as MGG for the complex cellularity of bone marrow.
| Feature/Aspect | May-Grünwald-Giemsa (MGG) Stain | Leishman Stain |
| Stain Type | Two-step Romanowsky stain | Single-step Romanowsky stain |
| Components | May-Grünwald followed by Giemsa | Methylene blue, Eosin, and alcohol in one solution |
| Typical Use for Bone Marrow | Preferred for routine bone marrow aspirates | Less preferred, primarily for peripheral blood smears |
| Nuclear Detail | Excellent, distinct chromatin patterns | Good, but often less distinct chromatin patterns |
| Cytoplasmic Detail | Superior, clear differentiation of cytoplasm and inclusions | Good, but less nuanced differentiation |
| Granule Staining | Excellent, precise differentiation of various granule types (e.g., azurophilic, eosinophilic) | Good, but less consistent and distinct granule differentiation |
| Cell Lineage Differentiation | Enhanced, allows for precise identification of all hematopoietic cell lineages and maturation stages | Good, but may be less precise for subtle distinctions, especially in abnormal bone marrow |
| Identification of Subtle Abnormalities | Highly effective for detecting dysplastic features, subtle changes in leukemias/lymphomas | Less effective due to potentially less detailed morphology |
| Background Staining | Often cleaner and more informative | Can be prone to precipitation, potentially obscuring background |
| Complexity of Use | Slightly more involved (two steps) | Simpler (single step) |
| Overall Value for Bone Marrow | Provides a more comprehensive and detailed morphological picture, crucial for diagnosis. | Generally sufficient for less complex samples or when MGG is unavailable, but less ideal for full bone marrow assessment. |
What are the advantages and disadvantages of using May-Grünwald Giemsa (MGG) stain?
| Advantages | Disadvantages |
| Excellent detail: Provides distinct nuclear and cytoplasmic staining, crucial for morphological assessment. Clear differentiation: Allows for subtle differentiation between various cell lines, including immature forms. Identifies subtle anomalies: Essential for diagnosing various hematological disorders by highlighting subtle morphological changes. Reliable for cell counting and classification: Enables accurate differential counts and classification of hematopoietic cells. Good background staining: Typically provides a clear background, facilitating better cell observation. | Two-step process: Requires more time and attention compared to single-step stains. Technique-sensitive: Proper dilution, timing, and pH are crucial for optimal results; variations can affect staining quality. Potential for precipitation: If not prepared or handled correctly, the Giemsa component can precipitate, creating artifacts on the slide. Can be over- or under-stained: Requires careful monitoring to prevent inadequate or excessive staining, which impacts interpretability. Reagent preparation and stability: The stain solutions need to be prepared correctly and have a limited shelf life once diluted. |
Compared to other methods
| Method | Advantages | Disadvantages |
| Wright stain: Uses a mixture of eosin (acidic) and methylene blue (basic) dyes to differentially color various components of blood and bone marrow cells, enabling their identification and morphological analysis under a light microscope. | Faster than May-Grünwald Giemsa (MGG), less expensive | Lower cytoplasmic detail, more prone to artifacts |
| Giemsa stain: Composed of methylene blue, eosin, and azure dyes, primarily used to visualize and differentiate cellular components (especially nuclei and cytoplasm) in blood and bone marrow smears, and to detect parasites like those causing malaria. | Simpler than May-Grünwald Giemsa (MGG), good for specific cell types | Less nuclear detail, not as versatile as May-Grünwald Giemsa (MGG) |
| Immunohistochemistry: Uses antibodies to detect specific antigens (e.g., proteins) in cells of a tissue section, visualizing their presence and localization through a colored reaction. | Highly specific for visualizing specific proteins | More complex and expensive, requires additional antibody preparation |
| Flow cytometry: A laser-based technology that rapidly analyzes and sorts individual cells or particles as they pass in a single file through a fluid stream, measuring their physical (e.g., size, granularity) and chemical (e.g., fluorescently-labeled protein expression) characteristics. | Can analyze large cell populations quickly | Limited morphological information, requires specialized equipment |
What are the components of May-Grünwald Giemsa stain?
May-Grünwald Giemsa (MGG) stain is actually a combination of two different stains: May-Grünwald stain and Giemsa stain. Both stains contain different components and work together to achieve the characteristic staining of various cell components.
May-Grünwald stain
- Eosin Y: This is an acidic dye that stains acidic components in cells, such as cytoplasm and some granules, in pink or orange tones.
- Methylene Blue: This is a basic dye that stains basic components in cells, such as nuclei and other granules, in blue or purple tones.
Giemsa stain
- Azures (Azure B and Azure II): These are oxidized methylene blue dyes that contribute to the characteristic purple-blue staining of nuclei.
- Eosin Y: This is the same component as in May-Grünwald stain, further enhancing cytoplasmic staining.
- Methylene Blue Chloride: This component interacts with the Azures to achieve the characteristic Romanowsky-Giemsa effect, resulting in the final purple coloration.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Bain BJ. A Practical Guide. 6th Edition (Wiley). 2022.
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- Carr JH. Clinical Hematology Atlas 6th Edition (Elsevier). 2021.
- Piaton, E., Fabre, M., Goubin-Versini, I., Bretz-Grenier, M. F., Courtade-Saïdi, M., Vincent, S., Belleannée, G., Thivolet, F., Boutonnat, J., Debaque, H., Fleury-Feith, J., Vielh, P., Egelé, C., Bellocq, J. P., Michiels, J. F., & Cochand-Priollet, B. (2016). Guidelines for May-Grünwald-Giemsa staining in haematology and non-gynaecological cytopathology: recommendations of the French Society of Clinical Cytology (SFCC) and of the French Association for Quality Assurance in Anatomic and Cytologic Pathology (AFAQAP). Cytopathology : official journal of the British Society for Clinical Cytology, 27(5), 359–368. https://doi.org/10.1111/cyt.12323
- Deepthi, B., Prayaga, A. K., & Rukmangadha, N. (2022). Comparison of Modified Ultrafast Giemsa Stain with the Standard May Grunwald Giemsa Stain in FNAC of Various Organs. Journal of cytology, 39(4), 174–179. https://doi.org/10.4103/joc.joc_43_22