TL;DR
Polycythemia is an abnormally high increase in red blood cells, leading to elevated hemoglobin and hematocrit. It can be absolute (true increase in RBC mass) or relative (due to decreased plasma volume).
- Classification ▾: Absolute polycythemia includes primary (Polycythemia Vera – often JAK2 mutation-related) and secondary (due to increased EPO from hypoxia or tumors). Relative polycythemia (pseudopolycythemia) results from hemoconcentration.
- Causes ▾: PV is due to clonal hematopoiesis from mutations like JAK2. Secondary polycythemia is driven by increased EPO from various conditions. Relative polycythemia is caused by fluid loss or decreased plasma volume.
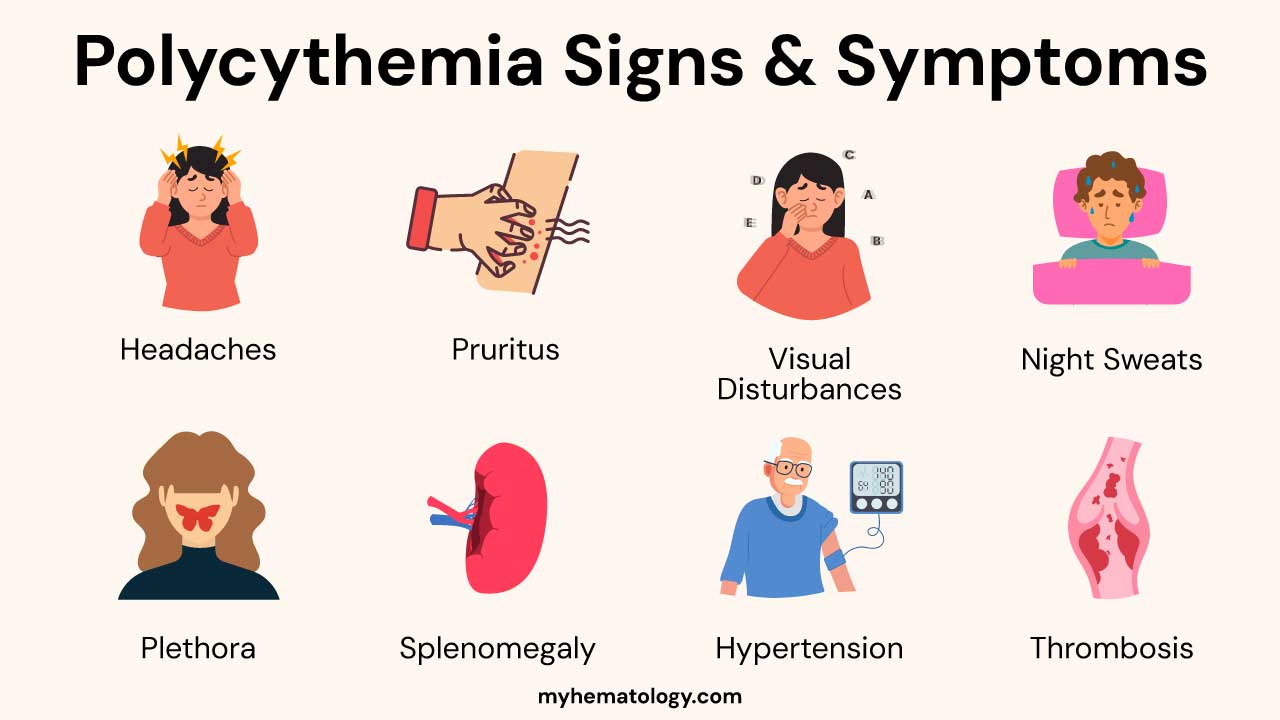
- Symptoms & Signs ▾: These include headache, dizziness, fatigue, pruritus, plethora, splenomegaly, and increased risk of thrombosis and hemorrhage. Secondary polycythemia may have symptoms of the underlying cause.
- Laboratory Investigations ▾: Diagnosis involves CBC (high Hb, Hct, RBC), EPO levels (low in PV, high in secondary), JAK2 mutation testing (positive in PV), and bone marrow biopsy. Other tests help identify underlying causes.
- Treatment & Management ▾: PV management involves phlebotomy, low-dose aspirin, and sometimes cytoreductive therapy. Secondary polycythemia treatment focuses on the underlying cause; phlebotomy may offer symptomatic relief. Relative polycythemia is managed by addressing the cause of fluid imbalance.
- Complications ▾: Complications of PV include thrombosis, hemorrhage, myelofibrosis, and leukemia transformation. Secondary polycythemia complications relate to the cause and hyperviscosity.
*Click ▾ for more information
Introduction
Polycythemia, also known as erythrocytosis, is a condition characterized by an abnormally high increase in the number of red blood cells (erythrocytes) in the blood. This increase leads to a higher-than-normal concentration of hemoglobin and consequently, an elevated hematocrit (the percentage of blood volume occupied by red blood cells).
Understanding polycythemia is crucial in clinical practice because it serves as a key indicator of underlying conditions ranging from benign physiological adaptations to serious hematological disorders or even occult malignancies. Recognizing polycythemia early allows for timely investigation into its etiology, enabling clinicians to differentiate between primary bone marrow disorders like polycythemia vera, secondary causes stemming from hypoxia or EPO-secreting tumors, and relative polycythemia due to dehydration. Accurate diagnosis is paramount as it dictates appropriate management strategies, which may include phlebotomy, cytoreductive therapies, or addressing the primary cause, ultimately preventing potentially severe complications such as thrombosis, hemorrhage, and disease progression, thereby significantly impacting patient outcomes and quality of life.
Classification of Polycythemia
The classification of polycythemias is primarily divided into two main categories based on the underlying cause and the absolute red blood cell mass.
Absolute (True) Polycythemia
This category involves a genuine increase in the total red blood cell mass in the body. It is further sub-classified into:
Primary Polycythemia: This arises from an intrinsic defect in the bone marrow stem cells, leading to the overproduction of red blood cells independent of normal regulatory mechanisms like erythropoietin (EPO). The most common type is Polycythemia Vera (PV), a myeloproliferative neoplasm often associated with a mutation in the JAK2 gene. Other rare primary familial and congenital polycythemias also exist due to genetic abnormalities affecting erythroid progenitors or EPO regulation.
Secondary Polycythemia: This occurs due to an increased production of EPO, which in turn stimulates the bone marrow to produce more red blood cells. This can be a physiological response to chronic hypoxia (e.g., high altitude, chronic lung disease, cyanotic heart disease, obstructive sleep apnea), or an inappropriate production of EPO by tumors (e.g., renal cell carcinoma, hepatocellular carcinoma, cerebellar hemangioblastoma), or due to exogenous EPO administration. Certain medications like androgens can also stimulate red blood cell production.
Relative (Pseudopolycythemia or Spurious) Polycythemia
In this condition, the red blood cell count, hemoglobin, and hematocrit appear elevated, but this is due to a decrease in plasma volume rather than an actual increase in the red blood cell mass. Common causes include dehydration (due to reduced fluid intake, vomiting, diarrhea, or excessive sweating), diuretic use, and stress erythrocytosis (Gaisböck syndrome), often seen in obese, hypertensive smokers. Once the underlying cause of plasma volume depletion is addressed, the blood counts typically return to normal.
Causes and Pathophysiology
Absolute (True) Polycythemia
Primary Polycythemia Vera (PV)
- Causes: The primary cause of PV is a somatic mutation in the Janus kinase 2 (JAK2) gene, most commonly the JAK2 V617F mutation. This mutation leads to a constitutively active JAK2 protein, which is a key signaling molecule in the EPO receptor pathway and other cytokine receptors involved in hematopoiesis. The persistent activation of JAK2 results in erythropoietin-independent proliferation and survival of erythroid progenitor cells, leading to excessive red blood cell production. Other less common JAK2 exon 12 mutations and mutations in the MPL (myeloproliferative leukemia virus oncogene) gene, which encodes the thrombopoietin receptor, can also cause PV.
- Pathophysiology: The hallmark of PV is clonal hematopoiesis, where a mutated hematopoietic stem cell gives rise to a population of abnormal blood cells, predominantly red blood cells but also often including increased white blood cells and platelets. The increased red blood cell mass leads to hyperviscosity of the blood, contributing to many of the clinical manifestations. While EPO levels are typically low or normal in PV due to negative feedback from the increased red blood cell mass, the erythroid progenitors have become hypersensitive to even low levels of EPO.
Secondary Polycythemia
- Causes: Secondary polycythemia arises from an increase in erythropoietin (EPO) production or other factors that stimulate erythropoiesis.
- Hypoxia-induced Increased EPO
- High Altitude: Lower atmospheric oxygen tension stimulates EPO production by the kidneys.
- Chronic Lung Diseases: Conditions like COPD and interstitial lung disease can lead to chronic hypoxemia.
- Obstructive Sleep Apnea (OSA): Intermittent hypoxia during sleep triggers EPO release.
- Cyanotic Heart Disease: Right-to-left shunts result in poorly oxygenated blood circulating systemically.
- Hemoglobinopathies with High Oxygen Affinity: Rare conditions for example Hemoglobin Chesapeake, where the hemoglobin releases oxygen poorly to tissues, leading to perceived hypoxia and increased EPO. Others include Hemoglobin Olympia, Hemoglobin Rainier and others.
- Inappropriate Increased EPO Production
- EPO-Secreting Tumors: Certain tumors, most notably renal cell carcinoma, but also hepatocellular carcinoma, pheochromocytoma, and cerebellar hemangioblastoma, can secrete EPO autonomously.
- Exogenous EPO Administration: Use of recombinant EPO for conditions like anemia of chronic kidney disease or as a performance-enhancing drug.
- Other Mechanisms
- Androgen Use: Androgens can stimulate erythropoiesis.
- Congenital Erythrocytosis: A group of rare inherited disorders caused by mutations in genes involved in oxygen sensing (e.g., VHL, EGLN1, EPOR) or hemoglobin function, leading to increased EPO production or increased sensitivity of erythroid progenitors to EPO.
- Hypoxia-induced Increased EPO
- Pathophysiology: The underlying condition triggers an increase in EPO levels (either appropriately due to hypoxia or inappropriately by a tumor). EPO then binds to its receptors on erythroid progenitor cells in the bone marrow, stimulating their proliferation, differentiation, and maturation into red blood cells. This leads to an increase in the red blood cell mass, and the resulting hyperviscosity can contribute to clinical symptoms.
Relative (Pseudopolycythemia or Spurious) Polycythemia
- Causes: Relative polycythemia is not due to an overproduction of red blood cells but rather a reduction in plasma volume, concentrating the red blood cells.
- Dehydration: Inadequate fluid intake, excessive fluid loss through vomiting, diarrhea, or sweating.
- Diuretic Use: Medications that increase fluid excretion.
- Stress Erythrocytosis (Gaisböck Syndrome): Often seen in obese, hypertensive smokers, the exact mechanism is not fully understood but is thought to involve a combination of reduced plasma volume and potentially increased red blood cell count due to chronic stress and sympathetic nervous system activation.
- Pathophysiology: The total red blood cell mass remains normal. However, the decreased plasma volume leads to hemoconcentration, resulting in elevated hemoglobin, hematocrit, and red blood cell count. The blood viscosity may be mildly increased due to the higher concentration of red blood cells within a smaller volume of plasma. Addressing the underlying cause of fluid loss or plasma volume reduction typically resolves the polycythemia.
Signs and Symptoms
The signs and symptoms can vary depending on the underlying cause, the degree of red blood cell elevation, and the presence of complications. Some individuals, especially in the early stages or in mild cases, may be asymptomatic. However, as the red blood cell count and blood viscosity increase, a range of manifestations can occur.
General Symptoms
These symptoms are often non-specific and can be present in various conditions, but they are common in polycythemia due to the increased blood viscosity and impaired blood flow.
- Headache: Can range from mild to severe and may be due to increased intracranial pressure or impaired cerebral blood flow.
- Dizziness and Vertigo: Feelings of lightheadedness, unsteadiness, or a spinning sensation.
- Fatigue and Weakness: Unexplained tiredness and a lack of energy.
- Pruritus (Itching): Often described as aquagenic pruritus, meaning it is triggered or worsened by contact with warm water (e.g., showering or bathing). This is particularly common in Polycythemia Vera.
- Visual Disturbances: Blurred vision, double vision (diplopia), or temporary vision loss due to impaired blood flow to the eyes.
- Night Sweats: Excessive sweating during sleep, which can be a feature of myeloproliferative neoplasms like Polycythemia Vera.
- Weight Loss: Unintentional loss of weight, although less common as a primary symptom of polycythemia itself, it can occur in underlying conditions like malignancies.
Signs Related to Increased Blood Viscosity and Volume
These are more directly related to the physical effects of having an excess of red blood cells.
- Plethora: A characteristic ruddy or flushed complexion, particularly noticeable in the face, lips, and mucous membranes. This is due to the increased volume of red blood cells in the superficial blood vessels.
- Engorged Retinal Veins: On fundoscopic examination (examination of the back of the eye), the retinal veins may appear dilated and tortuous due to increased blood volume and viscosity.
- Splenomegaly: Enlargement of the spleen, often palpable on physical examination. This is more common in Polycythemia Vera as the spleen can become involved in extramedullary hematopoiesis (blood cell production outside the bone marrow).
- Hepatomegaly: Enlargement of the liver, less common than splenomegaly but can occur in some cases of polycythemia.
- Hypertension: High blood pressure, which can be exacerbated by the increased blood volume and viscosity.
Thrombotic and Hemorrhagic Complications
The increased blood viscosity and, in some cases, platelet abnormalities can lead to both blood clots and bleeding.
- Thrombosis (Blood Clots): Increased risk of arterial and venous thromboembolic events, including:
- Stroke and Transient Ischemic Attacks (TIAs): Due to reduced cerebral blood flow or clot formation in the brain.
- Myocardial Infarction (Heart Attack): Due to clots in the coronary arteries.
- Deep Vein Thrombosis (DVT): Blood clots in the deep veins, usually in the legs.
- Pulmonary Embolism (PE): A blood clot that travels to the lungs.
- Budd-Chiari Syndrome: Thrombosis of the hepatic veins.
- Hemorrhage (Bleeding): Paradoxically, some patients with polycythemia, particularly PV, can experience increased bleeding, such as:
- Epistaxis (Nosebleeds)
- Gingival Bleeding (Bleeding Gums)
- Easy Bruising
- Gastrointestinal Bleeding: This can occur due to engorged blood vessels in the gastrointestinal tract or associated conditions like peptic ulcers. The increased risk of bleeding is often related to abnormal platelet function, despite a potentially elevated platelet count in some cases of PV.
Symptoms Related to Underlying Causes (in Secondary Polycythemia)
In cases of secondary polycythemia, patients may also exhibit symptoms related to the underlying condition causing the increased EPO production or hypoxia.
- Chronic Lung Disease: Cough, shortness of breath, wheezing.
- Cyanotic Heart Disease: Bluish discoloration of the skin and mucous membranes (cyanosis), shortness of breath, fatigue.
- Obstructive Sleep Apnea: Loud snoring, daytime sleepiness, witnessed apneas.
- Symptoms of EPO-Secreting Tumors: Flank pain, hematuria (blood in urine) in renal cell carcinoma; abdominal pain in hepatocellular carcinoma; neurological symptoms in cerebellar hemangioblastoma.
Other Potential Findings
- Gout: Elevated uric acid levels, particularly in PV due to increased cell turnover, can lead to gouty arthritis.
- Peptic Ulcer Disease: Increased risk in PV, possibly due to abnormal histamine release from basophils.
Laboratory Investigations
Laboratory investigations are crucial for diagnosing polycythemia, differentiating between its various types (absolute vs. relative, primary vs. secondary), and identifying the underlying cause.
Complete Blood Count (CBC)
This is the cornerstone of initial evaluation. Expected findings include:
- Elevated Hemoglobin (Hb): Diagnostic thresholds typically vary by sex and altitude but are generally >16.5 g/dL in men and >16.0 g/dL in women (WHO criteria).
- Elevated Hematocrit (Hct): Generally >49% in men and >48% in women (WHO criteria).
- Elevated Red Blood Cell (RBC) Count: An absolute increase in the number of red blood cells per microliter of blood.
- Red Cell Indices (MCV, MCH, MCHC): In most cases of primary and secondary polycythemia, they are normocytic and normochromic (normal size and hemoglobin content). However, they can provide clues to underlying conditions (e.g., microcytic anemia suggesting iron deficiency, which can sometimes mask an underlying polycythemia).
- White Blood Cell (WBC) Count and Platelet Count: These may also be elevated in primary polycythemia vera (PV), aiding in its diagnosis as a myeloproliferative neoplasm.
Differentiating Absolute from Relative Polycythemia
- Clinical Context and Repeat CBC after Adequate Hydration: If relative polycythemia due to dehydration is suspected, a repeat CBC after ensuring adequate fluid intake may show a normalization of hemoglobin and hematocrit.
- Plasma Volume Assessment (Less Commonly Done in Routine Practice): Direct measurement of plasma volume using isotope dilution techniques can definitively distinguish between a true increase in red cell mass (absolute) and a decrease in plasma volume (relative). However, this is not routinely performed due to its complexity and cost. The clinical picture and other laboratory findings usually suffice.
Investigating Absolute Polycythemia
- Serum Erythropoietin (EPO) Level: This is a key test to differentiate primary from secondary polycythemia.
- Low or Normal EPO: Suggests primary polycythemia (e.g., PV), where the bone marrow is producing red blood cells autonomously, independent of EPO stimulation.
- Elevated EPO: Indicates secondary polycythemia, where the increased red blood cell production is driven by increased EPO levels. Further investigation is needed to determine if the EPO elevation is appropriate (due to hypoxia) or inappropriate (due to EPO-secreting tumors or other causes).
- JAK2 Mutation Testing: Essential for diagnosing PV.
- JAK2 V617F Mutation: Found in the majority (around 95%) of patients with PV.
- JAK2 Exon 12 Mutations: Found in a smaller percentage (around 3-5%) of PV patients who are negative for the V617F mutation. Testing for both is crucial. A positive JAK2 mutation strongly supports a diagnosis of PV.
- Bone Marrow Biopsy and Aspirate:
- Typically hypercellular with erythroid, granulocytic, and megakaryocytic hyperplasia in PV.
- In PV, megakaryocytes are often large and pleomorphic.
- Presence of reticulin fibrosis can indicate progression to myelofibrosis
- Arterial Blood Gas (ABG) or Oxygen Saturation: Low partial pressure of oxygen (PaO2) or low oxygen saturation suggests hypoxia-induced polycythemia.
- Imaging Studies (e.g., Renal Ultrasound, CT Scan of Abdomen/Pelvis): To evaluate for EPO-secreting tumors, particularly renal cell carcinoma, which is a common cause of inappropriate secondary erythrocytosis. Other imaging modalities may be used depending on clinical suspicion of other tumor locations.
- Genetic Testing for Congenital Erythrocytosis: If secondary polycythemia is suspected but no obvious cause is identified, and there is a family history of erythrocytosis, genetic testing for mutations in genes involved in oxygen sensing (e.g., VHL, EGLN1, EPOR) or hemoglobin function (e.g., high-affinity hemoglobin variants) may be indicated.
Other Relevant Investigations
- Iron Studies (Serum Ferritin, Transferrin Saturation): Iron deficiency can sometimes coexist with or even mask polycythemia. In PV, iron deficiency can occur due to phlebotomy treatment or increased iron utilization.
- Vitamin B12 and Folate Levels: To rule out deficiencies that can affect red blood cell production and morphology.
- Liver and Kidney Function Tests: To assess overall organ function and look for potential underlying causes of secondary polycythemia (e.g., renal disease affecting EPO production).
- Uric Acid Levels: Often elevated in PV due to increased cell turnover.
- Lactate Dehydrogenase (LDH) Levels: Can be elevated in PV, reflecting increased cell turnover.
- Erythroid Colony Forming Unit (CFU-E) Assays: In research settings, these assays can demonstrate EPO-independent growth of erythroid progenitors in PV.
The specific sequence and choice of these investigations will be guided by the patient’s clinical presentation, initial laboratory findings, and the physician’s clinical judgment. A thorough and logical approach is essential to arrive at an accurate diagnosis and guide appropriate management.
Laboratory Findings in Different Types of Polycythemias
| Laboratory Investigation | Polycythemia Vera (Primary Absolute) | Secondary Polycythemia (Absolute) | Relative Polycythemia (Pseudopolycythemia) |
| Hemoglobin (Hb) | Elevated | Elevated | Elevated |
| Hematocrit (Hct) | Elevated | Elevated | Elevated |
| Red Blood Cell (RBC) Count | Elevated | Elevated | Elevated |
| Red Cell Indices (MCV, MCH, MCHC) | Usually Normal (Normocytic, Normochromic) | Usually Normal (Normocytic, Normochromic) | Variable (Often within normal limits) |
| White Blood Cell (WBC) Count | Normal to Elevated | Normal to Mildly Elevated | Normal |
| Platelet Count | Normal to Elevated | Normal | Normal |
| Serum Erythropoietin (EPO) | Low or Normal | Elevated | Normal |
| JAK2 Mutation (V617F/Exon 12) | Positive in most cases | Negative | Negative |
| Bone Marrow Biopsy | Hypercellular (Trilineage Hyperplasia), Increased Megakaryocytes (often large, pleomorphic) | Erythroid Hyperplasia | Normal Cellularity |
| Arterial Blood Gas (ABG) | Normal | Low PaO2 (in hypoxia-induced) | Normal |
| Plasma Volume | Normal to Slightly Decreased | Normal | Decreased |
| Iron Studies (Ferritin) | Normal to Decreased (if iron deficient due to phlebotomy or increased utilization) | Normal to Elevated (in some chronic conditions) | Normal |
| Uric Acid | Often Elevated | Normal to Elevated (in some underlying conditions) | Normal |
Treatment and Management
The treatment and management of polycythemias depend significantly on the underlying cause, the type of polycythemia (primary, secondary, or relative), the patient’s overall health, and their risk factors for complications. The primary goals of management are to reduce the risk of thrombotic and hemorrhagic events, alleviate symptoms, and address the underlying condition when possible.
General Principles of Management
- Risk Stratification: Patients with polycythemia, particularly PV, are often stratified into low-risk and high-risk categories based on factors such as age (>60 years), history of thrombosis, presence of cardiovascular risk factors (hypertension, diabetes, smoking), and significantly elevated blood counts. Risk stratification guides the intensity of treatment.
- Addressing the Underlying Cause: This is the most crucial aspect of managing secondary and relative polycythemia. Treating the primary condition often resolves or significantly improves the polycythemia.
Treatment of Polycythemia Vera (PV)
The management of PV focuses on reducing the risk of thrombosis and managing symptoms.
- Phlebotomy (Venesection): This is the cornerstone of initial and maintenance therapy for most patients with PV. Regular removal of blood (typically 300-500 mL at a time) helps to reduce the red blood cell mass and lower the hematocrit to target levels (usually <45% for men and women). The frequency of phlebotomy varies depending on the individual patient and their response.
- Low-Dose Aspirin: Daily low-dose aspirin (e.g., 81 mg) is commonly recommended for patients with PV to reduce the risk of thrombotic events, unless there are contraindications (e.g., history of bleeding).
- Cytoreductive Therapy: Medications that suppress bone marrow production of blood cells are used for high-risk patients or those with symptomatic splenomegaly, uncontrolled thrombocytosis, or frequent phlebotomy requirements.
- Hydroxyurea: This is a commonly used first-line cytoreductive agent. It inhibits DNA synthesis and reduces blood cell counts. Dosage is adjusted based on response and side effects.
- Interferon-alpha (IFN-α): This can be an alternative to hydroxyurea, particularly for younger patients, pregnant women (in some cases), or those with significant pruritus. It can help control blood counts and may have disease-modifying effects. Pegylated forms (peginterferon-alpha) are often preferred for their longer half-life and less frequent administration.
- Ruxolitinib: This is a JAK1/JAK2 inhibitor approved for PV patients who are resistant or intolerant to hydroxyurea. It can effectively control blood counts, reduce spleen size, and alleviate constitutional symptoms like pruritus and fatigue.
- Busulfan and Pipobroman: These are older alkylating agents that may be used in some cases, particularly in older patients or those refractory to other therapies, but they have a higher risk of long-term side effects, including leukemogenic potential.
- Management of Pruritus: Aquagenic pruritus can be challenging to treat. Strategies include:
- Antihistamines (though often not very effective).
- Selective serotonin reuptake inhibitors (SSRIs) like paroxetine.
- PUVA (psoralen plus ultraviolet A) therapy.
- Interferon-alpha.
- Management of Hyperuricemia: Allopurinol may be prescribed if uric acid levels are high and the patient has a history of gout or is at risk.
- Management of Myelofibrosis Transformation: If PV progresses to myelofibrosis (post-polycythemic myelofibrosis), treatment strategies are similar to those for primary myelofibrosis and may include JAK inhibitors, blood transfusions, and potentially stem cell transplantation in selected cases.
Management of Secondary Polycythemia
The primary focus is on treating the underlying condition causing the increased EPO production or hypoxia.
- Hypoxia-Related Polycythemia:
- Smoking Cessation: Crucial for patients with chronic lung disease exacerbated by smoking.
- Oxygen Therapy: For patients with chronic hypoxemia due to lung or heart disease.
- Continuous Positive Airway Pressure (CPAP): For obstructive sleep apnea.
- Management of Underlying Cardiac or Pulmonary Conditions: Optimizing treatment for conditions like COPD or cyanotic heart disease.
- Avoidance of High Altitude: For individuals with pre-existing conditions that worsen with hypoxia.
- EPO-Secreting Tumors: Surgical resection of the tumor is the definitive treatment. Polycythemia usually resolves after successful removal.
- Exogenous EPO Use: Discontinuation of EPO is necessary unless medically indicated and carefully monitored.
- Androgen Use: Discontinuation of androgens may lead to resolution of polycythemia.
- Congenital Erythrocytosis: Management depends on the specific genetic defect and may involve phlebotomy in symptomatic individuals.
In some cases of symptomatic secondary polycythemia with significantly elevated hematocrit, phlebotomy may be considered to rapidly reduce blood viscosity while the underlying condition is being addressed. However, it is generally not a long-term solution without treating the primary cause.
Management of Relative Polycythemia
The primary treatment is to address the underlying cause of plasma volume depletion.
- Fluid Replacement: Increasing oral fluid intake or administering intravenous fluids to correct dehydration.
- Discontinuation of Diuretics (if appropriate): Under medical supervision, stopping or adjusting diuretic medications may be necessary.
- Management of Stress Erythrocytosis (Gaisböck Syndrome): Weight loss, smoking cessation, and management of hypertension are often recommended. Phlebotomy is generally not indicated unless the patient is significantly symptomatic or has other cardiovascular risk factors.
Regular Monitoring
Regardless of the type of polycythemia, regular monitoring of blood counts (hemoglobin, hematocrit, platelets, WBCs), assessment of symptoms, and evaluation for complications are essential to guide treatment adjustments and ensure optimal patient outcomes.
Potential Complications
The potential complications polycythemia vary significantly depending on the type (primary, secondary, or relative), the underlying cause, the effectiveness of management, and individual patient factors.
Complications of Polycythemia Vera (PV)
- Thrombosis (Arterial and Venous): The increased blood viscosity and, in some cases, platelet abnormalities significantly elevate the risk of blood clots in arteries (leading to stroke, TIA, myocardial infarction, peripheral artery disease) and veins (leading to DVT, pulmonary embolism, Budd-Chiari syndrome, portal vein thrombosis). This is the most significant cause of morbidity and mortality in PV.
- Hemorrhage: Paradoxically, despite the increased blood thickness, some PV patients can experience bleeding (e.g., epistaxis, gastrointestinal bleeding, easy bruising) due to abnormal platelet function.
- Transformation to Myelofibrosis (Post-Polycythemic Myelofibrosis): Over time, a subset of PV patients can develop progressive bone marrow fibrosis, leading to cytopenias (anemia, thrombocytopenia), splenomegaly, and constitutional symptoms. This transformation can worsen prognosis.
- Transformation to Acute Leukemia: A small percentage of PV patients can transform to acute myeloid leukemia (AML) or, rarely, acute lymphoblastic leukemia (ALL). The risk may be slightly increased with certain cytoreductive therapies (e.g., alkylating agents).
- Gout: Increased cell turnover in PV can lead to elevated uric acid levels, predisposing to gouty arthritis and kidney stones.
- Peptic Ulcer Disease: The incidence of peptic ulcers may be higher in PV, possibly due to increased histamine release from basophils.
- Myeloid Malignancies: There is a slightly increased risk of developing other myeloid neoplasms over the long term.
Complications of Secondary Polycythemia
Complications in secondary polycythemia are often related to both the increased red blood cell mass and the underlying condition causing it.
- Thrombosis: Increased blood viscosity can elevate the risk of blood clots, although generally lower than in PV.
- Complications of the Underlying Condition: These can vary widely depending on the cause (e.g., worsening hypoxemia in lung disease, complications of tumors, progression of cardiac disease).
- Pulmonary Hypertension: Chronic hypoxemia can lead to pulmonary vasoconstriction and eventually pulmonary hypertension.
- Hyperviscosity Syndrome: Severe elevation in red blood cell count can lead to symptoms like headache, visual disturbances, and neurological deficits due to sluggish blood flow.
Complications of Relative Polycythemia
Complications are usually related to the underlying cause of plasma volume depletion.
- Dehydration-related issues: Electrolyte imbalances, kidney injury, and cardiovascular stress.
- Increased risk of thrombotic events in the context of underlying risk factors like obesity, hypertension, and smoking (as seen in Gaisböck syndrome).
Prognosis of Polycythemia
Prognosis of Polycythemia Vera (PV)
The prognosis for patients with PV has significantly improved with current management strategies. With regular phlebotomy and, when indicated, cytoreductive therapy and low-dose aspirin, many patients can live for decades with a good quality of life.
Median survival for well-managed PV patients can be comparable to that of an age-matched general population in some studies, often exceeding 15-20 years or more.
However, the risk of complications, particularly thrombosis and transformation to myelofibrosis or leukemia, remains a concern and can impact long-term survival. Factors associated with a poorer prognosis include older age at diagnosis, a history of thrombosis, and the development of significant myelofibrosis or leukemic transformation.
Prognosis of Secondary Polycythemia
The prognosis of secondary polycythemia is primarily determined by the underlying condition.
- Polycythemia due to treatable causes (e.g., EPO-secreting tumors that can be resected, reversible hypoxia) may resolve with treatment of the primary condition.
- Polycythemia associated with chronic conditions (e.g., chronic lung disease, cyanotic heart disease) will have a prognosis largely dependent on the progression and management of the underlying disease. The increased red cell mass can contribute to morbidity and mortality associated with these conditions.
Prognosis of Relative Polycythemia
The prognosis of relative polycythemia is generally good once the underlying cause of plasma volume depletion is identified and corrected. For example, polycythemia due to dehydration resolves with rehydration. In cases like Gaisböck syndrome, managing associated risk factors (obesity, smoking, hypertension) is important for long-term cardiovascular health.
Frequently Asked Questions (FAQs)
Is polycythemia life threatening?
Yes, polycythemia can be life-threatening, especially if left untreated or not properly managed. The primary danger stems from the increased thickness of the blood (hyperviscosity) due to the elevated number of red blood cells which can lead to several complications like thrombosis and splenomegaly.
What organ does polycythemia affect?
Polycythemia primarily affects the bone marrow, where the overproduction of red blood cells (and sometimes white blood cells and platelets) originates. However, due to the increased number of blood cells and the resulting thicker blood, it can have significant effects on various other organs.
Can you donate blood with polycythemia?
Generally, individuals with polycythemia vera are not eligible for regular blood donation due to the underlying blood disorder. However, therapeutic phlebotomy, a procedure similar to blood donation, is a primary treatment for PV, where blood is removed to reduce the red blood cell count and manage the condition. This blood is typically discarded and not used for transfusion to others. Individuals with secondary polycythemia might be eligible in some specific circumstances if the underlying cause is not transmissible and their blood counts are well-controlled, but this would require strict medical evaluation and approval.
What is the last stage of polycythemia?
The “last stage” of polycythemia vera (PV) is often referred to as the “spent phase” or post-polycythemic myelofibrosis (PPV-MF). In this stage, the bone marrow, which initially overproduced blood cells, becomes progressively scarred (fibrotic) and loses its ability to produce sufficient healthy blood cells, leading to anemia (low red blood cell count), thrombocytopenia (low platelet count), and often significant splenomegaly (enlarged spleen) with extramedullary hematopoiesis (blood cell production outside the bone marrow). This phase is also associated with an increased risk of developing acute myeloid leukemia (AML).
Who is most at risk for polycythemia?
Individuals most at risk for PV typically include:
- Older adults: The condition is most commonly diagnosed in people over the age of 60.
- Men: Men are slightly more likely to develop polycythemia vera than women.
- Individuals with a JAK2 gene mutation: This genetic mutation is found in the vast majority of people with PV. While usually acquired rather than inherited, its presence is a major risk factor.
- People with a family history: Although most cases are not hereditary, having a family member with PV may slightly increase the risk.
- Exposure to high levels of radiation or certain toxic substances: Some studies suggest a possible link, but this is not as well-established as age and the JAK2 mutation.
For secondary polycythemia, the risk factors depend on the underlying cause, such as chronic lung or heart disease leading to low oxygen levels, living at high altitudes, certain tumors, or the use of performance-enhancing drugs like anabolic steroids or erythropoietin.
Can stress cause polycythemia?
Yes, stress can cause a type of polycythemia known as stress erythrocytosis or Gaisböck syndrome. This is a relative polycythemia, meaning the increase in red blood cell concentration is due to a decrease in plasma volume rather than an absolute increase in red blood cell production. Chronic stress, particularly in combination with factors like obesity, hypertension, and diuretic use, can lead to a contracted plasma volume, resulting in a higher hematocrit even though the actual red blood cell mass is normal or only minimally increased.
Does drinking a lot of water help polycythemia?
Yes, drinking a lot of water can be beneficial for managing polycythemia, particularly relative polycythemia where dehydration contributes to the increased red blood cell concentration by reducing plasma volume. 1 Adequate hydration helps to increase plasma volume, thereby diluting the blood and potentially lowering the hematocrit. While it may offer some benefit in absolute polycythemia by slightly reducing blood viscosity, it is not a primary treatment and does not address the underlying overproduction of red blood cells; therefore, medical interventions like phlebotomy and cytoreductive therapy remain crucial for absolute polycythemia management.
What foods should you avoid if you have polycythemia?
In PV, the avoidance of foods high in purines and oxalates is primarily related to managing potential complications associated with the disease.
High Purine Foods and Gout
- Increased Uric Acid: The increased turnover of blood cells in PV can lead to higher levels of uric acid in the blood. Purines, found in foods like red meat, organ meats, and some seafood, are broken down into uric acid in the body.
- Risk of Gout: Elevated uric acid levels can crystallize in the joints, causing gout, a painful form of arthritis. While not everyone with PV develops gout, limiting high-purine foods can help manage uric acid levels and potentially reduce this risk, especially in individuals with a predisposition to gout or elevated uric acid.
High Oxalate Foods and Kidney Stones
- Increased Risk of Kidney Stones: PV can sometimes be associated with an increased risk of kidney problems, partly due to the higher blood cell turnover and potential for dehydration (thick blood can make kidneys work harder). Oxalates are natural compounds found in many foods, and in susceptible individuals, they can contribute to the formation of kidney stones, particularly calcium oxalate stones.
- Preventing Stone Formation: Limiting foods high in oxalates, such as spinach, rhubarb, beets, nuts, and berries, can help reduce the amount of oxalate in the urine and potentially lower the risk of developing kidney stones in individuals with PV. Maintaining good hydration is also crucial for preventing kidney stones.
While diet alone cannot treat PV, these dietary recommendations aim to reduce the risk of specific complications – gout (related to purine breakdown) and kidney stones (related to oxalate content) – that can occur in some individuals with the condition. It’s important for individuals with PV to discuss their dietary needs and potential restrictions with their healthcare provider or a registered dietitian for personalized advice.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Madden C. THE PROACTIVE APPROACH TO POLYCYTHEMIA VERA: Essential Strategies For Patients, Caregivers, And Healthcare Professionals – Navigating Diagnosis, Treatment, And Practical Coping Strategies
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Nagy ZF, Pfliegler G, Kósa J, Árvai K, Istenes I, Doros A, Timár B, Lakatos P, Demeter J. Case Report: Importance of high-throughput genetic investigations in the differential diagnosis of unexplained erythrocytosis. Pathol Oncol Res. 2025 Mar 10;31:1612037. doi: 10.3389/pore.2025.1612037. PMID: 40130200; PMCID: PMC11930660.
- Harrison CN, Ross DM, Fogliatto LM, Foltz L, Busque L, Xiao Z, Heidel FH, Koehler M, Palumbo GA, Breccia M, Komatsu N, Kirito K, Xicoy Cirici B, Martinez-Lopez J, Rovo A, Petruk C, Bobirca C, Mirams L, McMillan A, Harper G, Kiladjian JJ. Patient and physician perceptions regarding treatment expectations and symptomatology in polycythemia vera: Insights from the Landmark 2.0 global health survey. Hemasphere. 2025 Mar 24;9(3):e70106. doi: 10.1002/hem3.70106. PMID: 40130070; PMCID: PMC11931442.
- Mahdi A, Rampotas A, Roberts P, Stokes J, Mahdi E, Witherall R, Mannari D, Ibrahim N, Naylor G, Garg M, Manjra I, Glancy P, Katis G, Bhagat S, Coppell J, McGregor A, Frewin R, Butt NM. Safety and Efficacy of Busulphan Based on Dosing Patterns in the Real-World Management of Myeloproliferative Neoplasms. EJHaem. 2025 Mar 19;6(2):e1097. doi: 10.1002/jha2.1097. PMID: 40110073; PMCID: PMC11920812.