TL;DR
Disseminated intravascular coagulation (DIC) is a condition with widespread activation of coagulation and fibrinolytic pathways as a secondary complication causing generalized hemorrhage and thrombosis.
- Hypersensitivity reactions
- Incompatible transfusion reaction
- Incompatible organ transplantation
- Infections
- Gram-negative and meningococcal sepsis
- Severe malaria
- Viral infections
- Neoplasms
- Acute promyelocytic leukemia
- Adenocarcinoma
- Obstetric complications
- Abruptio placentae
- Amniotic fluid embolism
- Eclampsia & toxemia
- Retained fetus / placenta
- Septic abortion
- Vascular disorders
- Aortic aneurysm
- Cardiac bypass surgery
- Giant hemangioma
- Widespread tissue injury
- Burns
- Surgery
- Trauma
- Miscellaneous
- Heat stroke
- Hypothermia
- Liver failure
- Pancreatitis
- Shock
- Snake bites
- Bleeding
- Thrombosis
- Tissue damage and necrosis due to thrombosis
- Peripheral blood characteristics: Microangiopathic hemolytic anemia, thrombocytopenia
- ↑: APTT, PT, TT, bleeding time, FDP, D-dimer
- ↓: platelet count, fibrinogen
- Treat the underlying cause
- Bleeding: supportive therapy with fresh frozen plasma, platelet concentrates and cryoprecipitate
- Thrombosis: anticoagulant therapy i.e. heparin
- Protein C concentrate and antithrombin in selected patients
*Click ▾ for more information
What is disseminated intravascular coagulation (DIC)?
Disseminated intravascular coagulation (DIC) also known as disseminated intravascular coagulopathy or DIC syndrome is a complex and often life-threatening disorder in which widespread blood clotting occurs throughout the body. This excessive clotting can lead to the consumption of clotting factors and platelets, resulting in a bleeding diathesis.
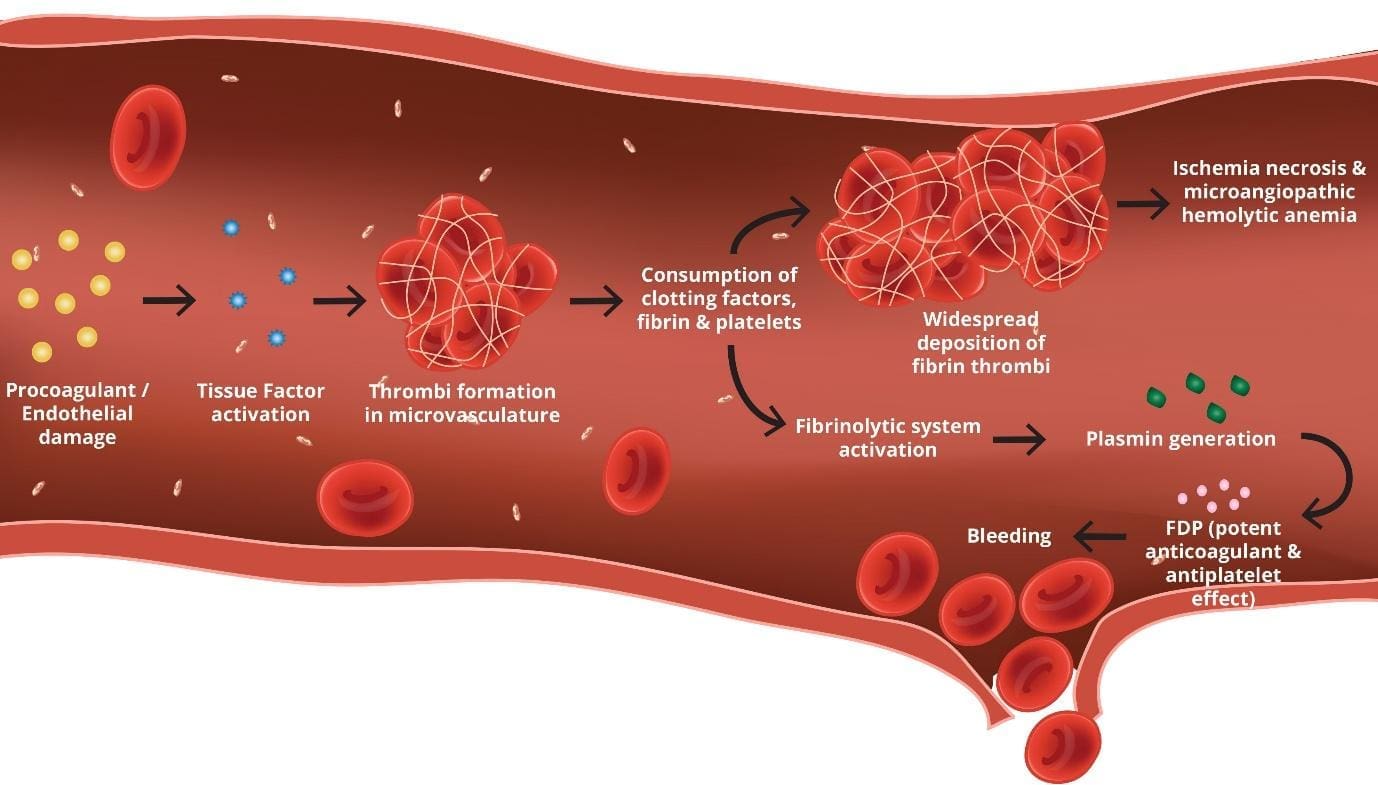
The hallmark of disseminated intravascular coagulation is the activation of both the coagulation and fibrinolysis. This activation leads to the formation of microthrombi (small blood clots) in the microvasculature.
The microthrombi can obstruct blood flow and damage small blood vessels causing microangiopathy. The consumption of clotting factors and platelets can lead to bleeding, which can manifest as bruises, petechiae (small red spots on the skin), or even major hemorrhage.
Causes of disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) can be triggered by a variety of underlying conditions. Some of the most common causes include:
- Sepsis: Sepsis is a life-threatening condition that occurs when the body’s immune system overreacts to an infection. Sepsis is a major cause of DIC (disseminated intravascular coagulation), as the inflammatory response can trigger the activation of both the coagulation and fibrinolytic systems.
- Trauma: Trauma can cause DIC (disseminated intravascular coagulation) by releasing tissue factors that activate the coagulation cascade. Disseminated intravascular coagulation is a common complication of major trauma, such as car accidents, falls, and gunshot wounds.
- Cancer: Cancer can cause DIC (disseminated intravascular coagulation) by releasing substances that activate the coagulation cascade. Certain types of cancer, such as leukemia, are more likely to be associated with DIC (disseminated intravascular coagulation).
- Obstetric complications: Obstetric complications, such as abruptio placentae (premature separation of the placenta from the uterus) and amniotic fluid embolism (a rare but serious condition in which amniotic fluid enters the bloodstream), can cause DIC (disseminated intravascular coagulation).
- Medications: Disseminated intravascular coagulation can also be caused by a number of medications including Asparaginase, L-asparaginase, Alteplase, Steptokinase, Urokinase.
- A number of other medical conditions can cause DIC (disseminated intravascular coagulation), including:
- Liver disease
- Hemolytic uremic syndrome (HUS)
- Thrombotic thrombocytopenic purpura (TTP)
- Snakebites
In some cases, the cause is unknown. This is known as idiopathic DIC.
Pathophysiology of DIC (disseminated intravascular coagulation)
The pathophysiology of DIC (disseminated intravascular coagulation) is complex and not fully understood. However, it is believed that DIC (disseminated intravascular coagulation) is caused by the activation of both the coagulation and fibrinolytic systems. This activation can be triggered by a variety of underlying conditions, such as sepsis, trauma, cancer, and obstetric complications.
The activation of the coagulation system leads to the formation of thrombin. Thrombin is an enzyme that converts fibrinogen into fibrin. Fibrin is a protein that forms the structural basis of blood clots. The formation of fibrin can lead to the development of microthrombi throughout the body.
The activation of the fibrinolytic system leads to the breakdown of fibrin. This can help to prevent the growth of microthrombi. However, the excessive activation of fibrinolysis can lead to the degradation of fibrinogen and other clotting factors, which can further exacerbate the bleeding diathesis.
Key events
- Initiation: The coagulation cascade is activated by a variety of triggers, such as tissue factor, thrombin, and activated protein C.
- Propagation: The activated coagulation factors generate thrombin. Thrombin converts fibrinogen into fibrin.
- Clot formation: Fibrin forms a mesh that traps red blood cells, platelets, and other blood cells. This forms a blood clot.
- Fibrinolysis: The fibrinolytic system is activated. Plasmin is generated from plasminogen. Plasmin breaks down fibrin.
- Consumption: Clotting factors and platelets are consumed in the process of clot formation. This can lead to a deficiency of clotting factors and platelets.
- Bleeding: The deficiency of clotting factors and platelets can lead to bleeding.

Fibrinolysis in disseminated intravascular coagulopathy (DIC)
Fibrinolysis is the process by which blood clots are broken down. In DIC (disseminated intravascular coagulation), fibrinolysis is activated in an attempt to remove the microthrombi that are forming throughout the body.
However, the excessive activation of fibrinolysis can lead to the degradation of fibrinogen and other clotting factors, which can further exacerbate the bleeding diathesis.
Microangiopathy in DIC (disseminated intravascular coagulation)
Microangiopathy is the damage to small blood vessels. In DIC, microangiopathy is caused by the formation of microthrombi in the microvasculature. The microthrombi can obstruct blood flow and damage the vessel walls. This can lead to a number of complications, such as organ dysfunction, bleeding, and death.
Inflammatory Activation in DIC (disseminated intravascular coagulation)
Inflammatory activation is a core feature of DIC (disseminated intravascular coagulation) and plays a crucial role in its development and progression.
DIC (disseminated intravascular coagulation) often arises due to underlying inflammatory conditions like severe infections (sepsis), trauma, cancer, or autoimmune diseases. These conditions trigger the release of various inflammatory mediators, including cytokines, chemokines, and adhesion molecules. These mediators, in turn, activate the coagulation system, leading to the characteristic blood clotting abnormalities in DIC (disseminated intravascular coagulation).
Mechanisms of Inflammatory Activation
- Direct activation of coagulation pathways: Cytokines like TNF-α and IL-1β directly activate various components of the coagulation cascade, promoting clot formation.
- Endothelial dysfunction: Inflammatory mediators damage the endothelium, the lining of blood vessels, making them prone to clot formation and leakage (bleeding).
- Activation of immune cells: Inflammatory stimuli trigger immune cells to release additional procoagulant factors, further amplifying the clotting response.
Consequences of Inflammatory Activation
- Microvascular thrombosis: Small clots form in various organs, leading to tissue damage and organ dysfunction.
- Bleeding: Depletion of clotting factors due to excessive consumption and endothelial damage leads to uncontrolled bleeding.
- Organ failure: Prolonged inflammation and vascular damage can lead to the failure of vital organs like the lungs, kidneys, and liver.
A concise overview of key etiologies and their primary mechanisms of DIC induction
| Etiology Category | Specific Examples | Primary Mechanism of DIC Induction |
| Sepsis/Infection | Gram-negative sepsis Severe malaria Viral infections Parasites | Systemic inflammatory response leading to widespread tissue factor expression; Release of bacterial endotoxins or components triggering inflammation |
| Malignancy | Acute promyelocytic leukemia, Adenocarcinoma | Release of procoagulant substances (e.g., tissue factor, cancer procoagulant) directly into the bloodstream |
| Trauma/Tissue Injury | Severe burns Major surgery Massive trauma | Release of large amounts of tissue factor from damaged cells, initiating widespread coagulation |
| Obstetric Complications | Abruptio placentae Amniotic fluid embolism Eclampsia & toxemia Retained fetus/placenta Septic abortion | Release of thromboplastin-rich material into the maternal circulation |
| Vascular Disorders | Aortic aneurysm Giant hemangioma Cardiac bypass surgery | Vascular stasis or local activation of the coagulation system |
| Miscellaneous Causes | Heat stroke Hypothermia Liver failure Pancreatitis Shock Snake bites Major transfusion reactions | Diverse mechanisms including systemic inflammation, release of exogenous toxins, or severe organ dysfunction |
Acute vs. Chronic DIC: Understanding the Differences
Disseminated intravascular coagulation comes in two distinct forms: acute and chronic. Recognizing these differences is crucial for understanding the disease and providing appropriate treatment.
Acute
- Definition: It’s a rapidly developing and life-threatening form of disseminated intravascular coagulation. The clotting system activates excessively, leading to widespread clotting and bleeding within the blood vessels.
- Symptoms: Often dramatic and include widespread blood clots (thrombosis) in organs like lungs, kidneys, and skin, and uncontrolled bleeding from various sites (gums, nose, gastrointestinal tract).
- Causes: Typically triggered by sudden and severe events like sepsis, major trauma, burns, pancreatitis, or certain autoimmune diseases.
- Treatment: Requires urgent medical intervention with anticoagulants to control clotting and supportive measures to address underlying causes and bleeding complications.
Chronic
- Definition: It’s a slower-developing and often less severe form of disseminated intravascular coagulation. The clotting system remains mildly activated for an extended period, leading to gradual depletion of clotting factors and gradual build-up of fibrin degradation products.
- Symptoms: Often non-specific and can include mild fatigue, low-grade fever, and occasional bleeding from gums or nose.
- Causes: Associated with chronic underlying conditions like certain cancers (particularly adenocarcinoma), autoimmune diseases, liver cirrhosis, and chronic infections.
- Treatment: Depends on the severity and underlying cause. May involve low-dose anticoagulants, management of the underlying condition, and addressing any bleeding complications.
Key Differences
| Feature | Acute DIC | Chronic DIC |
| Development | Rapid | Slow |
| Severity | Life-threatening | Less severe |
| Clots and bleeding | Widespread and dramatic | Gradual and mild |
| Causes | Sudden, severe events | Chronic underlying conditions |
| Treatment | Urgent anticoagulation and supportive care | May require anticoagulation, but focuses on managing underlying cause |
Clinical features of DIC (disseminated intravascular coagulation)
The clinical features of disseminated intravascular coagulation (DIC) can vary depending on the severity of the condition. However, some of the most common clinical features of DIC (disseminated intravascular coagulation) include:
- Bleeding: This can manifest as bruises, petechiae (small red spots on the skin), gingival bleeding, epistaxis (nosebleeds), or even major hemorrhage.
- Thrombosis: This can manifest as deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, or myocardial infarction (heart attack).
- Microvascular dysfunction: Microvascular dysfunction can lead to a variety of symptoms, such as organ dysfunction, shock, and death.
- Fever: This is likely due to the release of inflammatory cytokines.
- Hypotension (low blood pressure): This is likely due to the loss of blood volume and the effects of inflammatory cytokines.
- Tachycardia (rapid heart rate): This is likely due to the body’s attempt to compensate for the loss of blood volume.
- Oliguria (decreased urine output): This is likely due to the effects of inflammatory cytokines on the kidneys.
- Confusion: This is likely due to the effects of inflammatory cytokines on the brain.
- Coma: Coma is a rare but serious complication of DIC (disseminated intravascular coagulation). This is likely due to the effects of inflammatory cytokines on the brain.
Laboratory investigations
The laboratory investigations for disseminated intravascular coagulation (DIC) are aimed at assessing the activation of both the coagulation and fibrinolytic systems. Some of the most common laboratory investigations for DIC include:
- Complete blood count: Anemia and thrombocytopenia. Thrombocytopenia, a decrease in platelet count, is a hallmark feature of DIC (disseminated intravascular coagulation) .
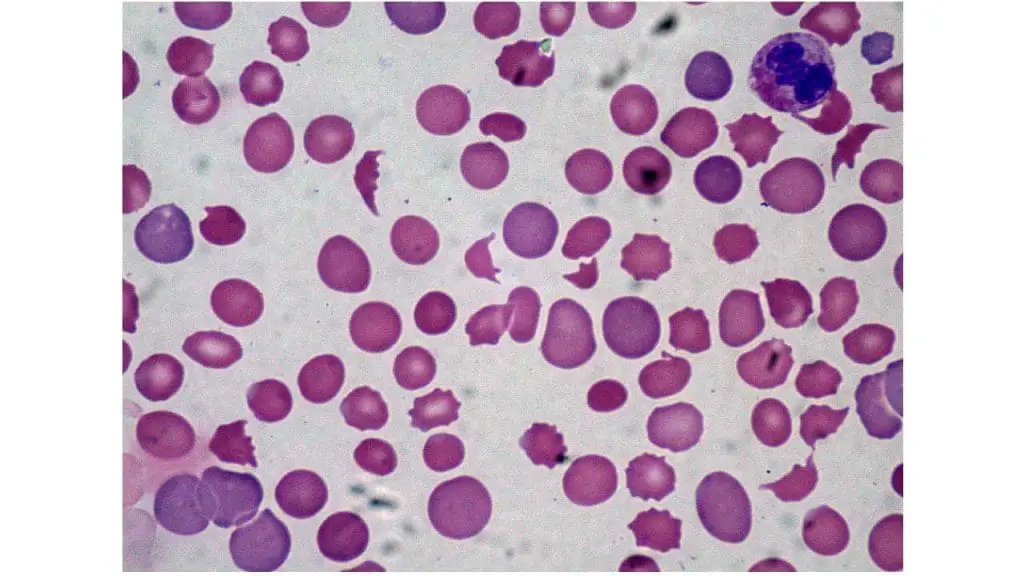
- Peripheral blood smear: Presence of schistocytes and thrombocytopenia. Schistocytes are fragmented red blood cells with a helmet-shaped appearance. They are a consequence of mechanical shearing forces caused by microthrombi formation in the microvasculature. The presence of schistocytes in the peripheral blood smear suggests DIC (disseminated intravascular coagulation) .
- Prothrombin time (PT) & INR: The PT measures the time it takes for blood to clot. A prolonged PT can be indicative of a deficiency in clotting factors II, V, VII, or X. The INR is a standardized measure of the PT. The INR is used to monitor the effects of warfarin therapy.
- Activated partial thromboplastin time (APTT): The APTT measures the time it takes for blood to clot. A prolonged APTT can be indicative of a deficiency in clotting factors VIII, IX, XI, or XII.
- D-dimer: D-dimer is a fragment of fibrin that is produced during the breakdown of blood clots. An elevated D-dimer level can be indicative of increased fibrinolytic activity.
- Fibrinogen: Fibrinogen is a clotting factor that is essential for the formation of blood clots. A decreased fibrinogen level can be indicative of consumption of clotting factors.
- Platelet count: Platelets are blood cells that are involved in the clotting process. A decreased platelet count can be indicative of consumption of platelets.
- Fibrin degradation products (FDPs): FDPs are fragments of fibrin that are produced during the breakdown of blood clots. An elevated FDP level can be indicative of increased fibrinolytic activity.
In addition to these laboratory investigations, other tests may be ordered to assess the underlying cause of DIC (disseminated intravascular coagulation). For example, a blood culture may be ordered to rule out sepsis.
Key laboratory tests and their expected findings in DIC
| Laboratory Test | Expected Finding |
| Platelet Count | Decreased (<100 x 109/L, often <50 x 109/L) |
| Prothrombin Time (PT) | Prolonged |
| Activated Partial Thromboplastin Time (aPTT) | Prolonged |
| Fibrinogen Level | Decreased (<1 g/L) |
| D-dimer / Fibrin Degradation Products (FDP) | Elevated |
| Peripheral Blood Smear | Presence of Schistocytes (fragmented red blood cells) |
The interpretation of laboratory investigations for DIC (disseminated intravascular coagulation) can be complex. A combination of abnormal test results is typically needed to make a diagnosis of DIC (disseminated intravascular coagulation). In some cases, a scoring system may be used to assess the likelihood of DIC (disseminated intravascular coagulation).
Scoring
There are several scoring systems used to diagnose DIC (disseminated intravascular coagulation), each with its own strengths and limitations. Here’s a breakdown of the most common ones:
ISTH DIC Score
The ISTH DIC score, developed by the International Society of Thrombosis and Hemostasis, is a scoring system used to support the diagnosis of Disseminated Intravascular Coagulation (DIC). It uses laboratory parameters and clinical features to categorize patients into different levels of DIC (disseminated intravascular coagulation) severity.
However, the score is not a definitive diagnostic tool and should be considered alongside clinical judgement and other information. A score of 5 or higher suggests probable overt DIC (disseminated intravascular coagulation) , while a lower score may indicate pre-DIC or other clotting disorders.
- Advantages: Simple and widely available, good diagnostic accuracy.
- Disadvantages: Subjective interpretation of some parameters, limited value in early DIC (disseminated intravascular coagulation) or specific causes like sepsis.
| Parameter | Value | Score |
| Platelet count (x 109/L) | > 100 50 – 100 < 50 | 0 1 2 |
| Prothrombin Time (PT) prolongation (seconds) | < 3 3 – 6 ≥ 6 | 0 1 2 |
| Fibrinogen level (g/L) | ≥ 1.0 < 1.0 | 0 1 |
| FDP/D-Dimer | No increase Moderate increase Severe increase | 0 2 3 |
Scoring and Interpretation
- Score 0-4: Unlikely for overt DIC. Needs further evaluation for alternative diagnoses.
- Score 5: Suggests possible overt DIC. Requires clinical correlation and confirmation with other tests.
- Score ≥ 5: Indicates probable overt DIC. Requires further investigation and immediate treatment.
Japanese Association for Acute Medicine (JAAM) DIC Score
The Japanese Association for Acute Medicine (JAAM) DIC Score is another scoring system used to diagnose DIC (disseminated intravascular coagulation) , specifically developed for critically ill patients in Japan.
While similar to the ISTH score, it focuses on parameters directly related to organ damage caused by DIC, (disseminated intravascular coagulation) offering potential advantages in certain situations.
- Advantages: More sensitive for early DIC and DIC in sepsis, considers organ damage.
- Disadvantages: More complex, not widely used outside of Japan.
| Parameter | Value | Score |
| Platelet count (x 109/L) | ≥ 120 ≥ 80 – < 120 or > 30% decrease within 24 hr ≥ 50 – < 80 or > 50% decrease within 24 hr < 50 | 0 1 2 3 |
| Prothrombin Time (PT) (value of patient/normal value) | < 1.25 ≥ 1.25 – < 1.67 ≥ 1.67 | 0 1 2 |
| Fibrin/ FDP (µg/mL) | < 10 ≥ 10 – < 20 ≥ 20 – < 40 ≥ 40 | 0 1 2 3 |
| Antithrombin activity (%) | ≤ 70 | 1 |
| Thrombin-antithrombin (TAT) complex, soluble fibrin, Prothrombin fragment (PF)1+2 | ≥ 2-fold of upper limit in normal range | 1 |
| Hepatic failure | If present | -3 |
| Diagnosis of DIC | ≥ 6 | |
Sepsis-Induced Coagulopathy (SIC) Score
The Sepsis-Induced Coagulopathy (SIC) score is a three-variable scoring system designed to identify septic patients likely to have SIC and inform clinicians of mortality values associated with each score increment.
It was developed following the Sepsis-3 definitions and incorporates new data from the Sepsis III datasets.
- Advantages: Specifically tailored for sepsis, good accuracy in this context.
- Disadvantages: Limited utility in non-sepsis DIC (disseminated intravascular coagulation) .
| Parameter | Value | Score |
| Platelet count (x 109/L) | ≥ 150 100 – 149 < 100 | 0 1 2 |
| International Normalized Ratio (INR) | ≤ 1.2 > 1.2 – 1.4 > 1.4 | 0 1 2 |
| Sequential Organ Failure Assessment (SOFA) score | 0 – 1 2 – 3 ≥ 4 | 0 1 2 |
Interpretation
- Score < 4: Unlikely for SIC.
- Score >= 4 and Platelet/INR sum >= 3: Possible SIC, requires further investigation and monitoring.
- Score >= 4 and Platelet/INR sum < 3: Unlikely for SIC despite SOFA dysfunction.
Benefits of the SIC Score
- Simple and easy to calculate.
- Focuses on sepsis-specific parameters.
- Good discrimination for mortality risk stratification.
Limitations
- Relatively new, requires further validation in various settings.
- May not be as accurate in non-sepsis contexts.
- Doesn’t replace clinical judgment and needs individual patient assessment.
Additional Information
- While the SIC score provides valuable insights, it’s not a definitive diagnosis for SIC.
- Management of SIC involves addressing the underlying infection, considering anticoagulation therapy, and managing organ dysfunction based on the SOFA score.
Sequential Organ Failure Assessment (SOFA) Score
The Sequential Organ Failure Assessment (SOFA) score is a widely used scoring system in critical care to assess the degree of organ dysfunction in critically ill patients. While not specifically designed for DIC (disseminated intravascular coagulation), it plays a crucial role in understanding a patient’s overall condition and contributes to the diagnosis and management of DIC (disseminated intravascular coagulation) alongside other scoring systems.
The score assigns points to six different organ systems based on specific parameters:
- Respiratory: PaO2/FiO2 ratio (oxygenation)
- Cardiovascular: Mean arterial pressure and need for vasopressors (blood pressure)
- Hepatic: Bilirubin level (liver function)
- Coagulation: Platelet count (blood clotting)
- Renal: Creatinine level and urine output (kidney function)
- Neurological: Glasgow Coma Scale score (mental state)
| Organ System | Parameter | Value | Score |
| Respiratory | PaO2/FiO2 ratio | ≥ 400 300 – 399 200 – 299 100 – 199 ≤ 99 | 0 1 2 3* 4* |
| Cardiovascular | Mean arterial pressure (MAP) (mmHg) and Vasopressors | No hypotension or MAP ≥ 70 MAP < 70 Dopamine ≤ 5 or dobutamine (any dose) Dopamine > 5, epinephrine ≤ 0.1, or norepinephrine ≤0.1 Dopamine > 15, epinephrine > 0.1, or norepinephrine > 0.1 | 0 1 2 3 4 |
| Hepatic | Bilirubin (mg/dL) | ≤ 1.2 1.3 – 1.9 2.0 – 5.9 6.0 – 11.9 ≥ 12 | 0 1 2 3 4 |
| Coagulation | Platelet count (x 109/L) | ≥ 150 100 – 149 50 – 99 21 – 49 ≤ 20 | 0 1 2 3 4 |
| Renal | Creatinine (mg/dL) & Urine Output (ml/day) | ≤ 1.2 1.3 – 1.9 / < 500 2.0 – 3.4 / < 300 3.5 – 4.9 / < 150 ≥ 5.0 or anuria | 0 1 2 3 4 |
| Neurological | Glasgow Coma Scale (GCS) | 15 13 – 14 10 -12 6 – 9 ≤ 6 | 0 1 2 3 4 |
*with ventilatory support
Interpretation
- Each organ system receives a score between 0 and 4, with higher scores indicating greater dysfunction.
- The total SOFA score is calculated by adding up the scores from all six systems.
- A higher total score indicates more severe organ dysfunction and generally predicts a worse prognosis.
Example:
Consider a patient with the following values:
- PaO2/FiO2 ratio: 250
- MAP: 55 mmHg with low-dose vasopressors
- Bilirubin: 3.5 mg/dL
- Platelet count: 75,000/μL
- Creatinine: 2.2 mg/dL, Urine Output: 400 mL/day
- GCS: 11
Calculating the individual scores:
- Respiratory: Score 2
- Cardiovascular: Score 2
- Hepatic: Score 2
- Coagulation: Score 2
- Renal: Score 1
- Neurological: Score 1
Total SOFA score: 2 + 2 + 2 + 2 + 1 + 1 = 10
In this case, a SOFA score of 10 suggests significant organ dysfunction and a potentially critical condition.
Clinical Usefulness
- Risk stratification: SOFA score helps predict mortality risk in critically ill patients, including those with DIC (disseminated intravascular coagulation).
- Monitoring response to treatment: Serial SOFA scores can track a patient’s response to treatments and their recovery from organ dysfunction.
- Supporting DIC diagnosis: In conjunction with other DIC (disseminated intravascular coagulation) scoring systems like ISTH or JAAM, SOFA score can provide further context about organ damage from DIC (disseminated intravascular coagulation) and its severity.
Limitations
- SOFA score doesn’t diagnose specific conditions like DIC (disseminated intravascular coagulation), it measures overall organ dysfunction.
- Requires regular monitoring and data collection, increasing workload for healthcare professionals.
- Cut-off values for defining severity may vary depending on the clinical context and specific disease processes.
Additional Notes
- SOFA score is just one tool and should be interpreted in conjunction with other clinical information and diagnostic tests.
- Cut-off values for defining severity may vary slightly depending on the specific setting and patient population.
- Serial SOFA scores over time can be helpful in monitoring the response to treatment and patient’s overall progress.
Differential Diagnosis of Disseminated Intravascular Coagulation
| Condition | Key Clinical Features | Typical Lab Findings | Distinguishing Points from DIC |
| DIC | Bleeding, thrombosis, multiorgan dysfunction, severe underlying condition (sepsis, trauma, malignancy, obstetric) | Decreased Platelets Prolonged PT/aPTT Decreased Fibrinogen Elevated D-dimer/FDP Schistocytes | Systemic activation of coagulation and fibrinolysis, consumptive coagulopathy |
| Thrombotic Thrombocytopenic Purpura (TTP) / Hemolytic Uremic Syndrome (HUS) | Microangiopathic hemolytic anemia (MAHA), thrombocytopenia, renal failure, neurological symptoms (TTP), fever | Decreased Platelets NORMAL PT/aPTT Elevated LDH Schistocytes Normal Fibrinogen | Absence of widespread coagulation factor consumption ADAMTS13 deficiency (TTP) |
| Severe Liver Disease | Jaundice, ascites, encephalopathy, bleeding | Decreased Platelets Prolonged PT/aPTT Normal/Elevated Fibrinogen (unless very severe) Variable D-dimer | Primary defect in synthesis of clotting factors, not consumption Liver biopsy/function tests help |
| Heparin-Induced Thrombocytopenia (HIT) | Thrombocytopenia (often severe), new or worsening thrombosis (arterial or venous) after heparin exposure | Decreased Platelets Normal PT/aPTT Normal Fibrinogen Normal D-dimer (unless thrombosis present) | History of heparin exposure, presence of HIT antibodies |
| Massive Blood Loss | Hypovolemic shock, severe bleeding | Decreased Platelets Prolonged PT/aPTT Decreased Fibrinogen (due to dilution/consumption) | Primary cause is volume depletion and dilution, not systemic coagulation activation |
| Vitamin K Deficiency | Bleeding, often associated with malnutrition, malabsorption, or anticoagulant use (warfarin) | Normal Platelets Prolonged PT (more than aPTT) Normal Fibrinogen Normal D-dimer | Responds to Vitamin K administration |
How is disseminated intravascular coagulation treated and managed?
The treatment and management of disseminated intravascular coagulation are aimed at addressing the underlying cause and preventing further clotting and bleeding. The specific treatment approach will vary depending on the severity of the condition and the underlying cause.
- Treatment of the underlying cause: The most important step in the treatment of DIC (disseminated intravascular coagulation) is to identify and address the underlying cause. This may involve treating an infection, removing a tumor, or controlling bleeding.
- Replacement of clotting factors and platelets: If clotting factors or platelets are consumed, they may need to be replaced. This can be done with transfusions of fresh frozen plasma, cryoprecipitate, or platelets.
- Anticoagulation therapy: Anticoagulation therapy can be used to prevent further clotting. This may involve the use of heparin, warfarin, or other anticoagulant medications.
- Fibrinolytic therapy: Fibrinolytic therapy can be used to break down blood clots. This may involve the use of alteplase, streptokinase, or other fibrinolytic medications.
In addition to these specific treatments, supportive care is also important. This may include:
- Fluid resuscitation: Fluid resuscitation may be necessary to maintain blood volume and blood pressure.
- Organ support: Organ support may be necessary if DIC (disseminated intravascular coagulation) has caused damage to organs such as the kidneys, liver, or lungs.
- Nutritional support: Nutritional support may be necessary to ensure that the body is getting the nutrients it needs to heal.
Current treatment modalities for DIC, their primary indications, and key considerations
| Treatment Modality | Primary Indication(s) | Key Considerations/Caveats |
| Treatment of Underlying Cause | All cases of DIC | Cornerstone of DIC management; addressing the precipitating illness is paramount for resolution |
| Platelet Transfusion | Bleeding patients or high risk of bleeding (e.g., pre-procedure) with platelet count <50 x 10^9/L | Generally reserved for active bleeding or high-risk situations; prophylactic transfusion in non-bleeding patients is not recommended |
| Fresh Frozen Plasma (FFP) | Bleeding patients with prolonged PT/aPTT, especially if invasive procedures are planned | Should be considered in active bleeding; not to be instituted based on laboratory tests alone |
| Cryoprecipitate | Hypofibrinogenemia (fibrinogen <1 g/L) | Replaces fibrinogen and Factor VIII; used when fibrinogen levels are critically low |
| Anticoagulant Therapy (Heparin) | DIC where thrombosis predominates (e.g., arterial/venous thromboembolism, purpura fulminans) | Continuous infusion UFH may be preferred due to short half-life/reversibility if bleeding risk is high; prophylactic doses for VTE prophylaxis in non-bleeding critically ill patients |
| Antifibrinolytic Agents (e.g., Tranexamic Acid) | Patients with a primary hyperfibrinolytic state and severe bleeding | Generally not recommended in DIC due to risk of exacerbating thrombosis; use only in highly selected cases |
The prognosis of disseminated intravascular coagulation depends on the severity of the condition and the underlying cause. With early diagnosis and appropriate treatment, the outcome can be improved. However, DIC (disseminated intravascular coagulation) is a serious condition with a high mortality rate.
Frequently Asked Questions (FAQs)
How serious is DIC?
DIC (Disseminated Intravascular Coagulation) is a serious condition where your body’s blood clotting system goes haywire. Normally, clotting helps seal wounds and prevent excessive bleeding. But in DIC (disseminated intravascular coagulation), the clotting gets out of control, leading to widespread clot formation and potential bleeding complications.
DIC (disseminated intravascular coagulation) can be life-threatening if not identified and treated promptly. It often occurs alongside other critical conditions like sepsis, trauma, or cancer.
What are the 3 most common conditions associated with DIC (disseminated intravascular coagulation)?
While the specific ranking might vary depending on the clinical setting and population, the 3 most common conditions associated with DIC (disseminated intravascular coagulation) are generally considered to be:
- Sepsis: This severe systemic inflammatory response syndrome (SIRS) caused by infection is the most frequent trigger for DIC (disseminated intravascular coagulation), often leading to rapid and widespread activation of the coagulation system.
- Cancer: Particularly disseminated cancers and certain types like leukemia can significantly increase the risk of DIC (disseminated intravascular coagulation) due to various mechanisms, including tissue damage, tumor procoagulant activity, and vascular complications.
- Pregnancy complications: Conditions like preeclampsia, HELLP syndrome, and placental abruption can disrupt the delicate balance of coagulation in pregnancy, leading to DIC (disseminated intravascular coagulation) and potentially life-threatening complications for both mother and baby.
What happens if DIC (disseminated intravascular coagulation) is left untreated?
Left untreated, disseminated intravascular coagulation unfolds like a devastating cascade, wreaking havoc throughout the body. The uncontrolled clotting continues, forming tiny blockages in vital organs like lungs, kidneys, and brain. These blockages starve them of oxygen and nutrients, leading to progressive dysfunction and potentially organ failure.
Simultaneously, depleted clotting factors and damaged blood vessels contribute to uncontrolled bleeding, further compounding the problem. This vicious cycle of clotting and bleeding escalates, leading to shock, coma, and potentially death. Early diagnosis and treatment are crucial to break this cycle, prevent organ damage, and improve chances of survival.
Can DIC (disseminated intravascular coagulation) be prevented?
Preventing disseminated intravascular coagulation entirely isn’t always possible, as it often arises from unpredictable events like severe infections or trauma. However, reducing the risk factors and addressing underlying conditions can go a long way in creating a less favorable environment for disseminated intravascular coagulation.
- Managing chronic conditions: Effectively controlling illnesses like diabetes, high blood pressure, or autoimmune diseases can minimize inflammatory triggers.
- Early treatment of infections: Prompt antibiotic use for infections and aggressive management of sepsis can prevent inflammation from escalating to disseminated intravascular coagulation.
- Careful monitoring during pregnancy: Regular prenatal care and prompt intervention in preeclampsia or HELLP syndrome can help maintain proper coagulation balance.
- Minimizing invasive procedures: Reducing unnecessary surgeries or invasive procedures when possible can lessen potential tissue damage and inflammatory responses.
While complete prevention might not be guaranteed, mitigating risk factors and seeking timely medical attention for underlying conditions are crucial steps in decreasing the likelihood of DIC and its potentially devastating consequences.
What is the difference between DIC (disseminated intravascular coagulation) and blood clots?
Both DIC (disseminated intravascular coagulation) and blood clots involve the formation of abnormal clots within the bloodstream, but they differ significantly in their nature and impact:
Blood Clots
- Localized: Blood clots typically form at specific sites of injury or damage to blood vessels, acting as a natural repair mechanism to stop bleeding.
- Isolated: They don’t necessarily affect other parts of the body and may resolve naturally over time.
- Varying Severity: Some blood clots, like deep vein thrombosis (DVT) or pulmonary embolism (PE), can be serious but generally have localized effects.
DIC (Disseminated Intravascular Coagulation)
- Widespread: In DIC (disseminated intravascular coagulation), numerous small clots form throughout the body, blocking blood flow in various organs and tissues.
- Systemic: This widespread clotting disrupts the entire blood clotting system, leading to both excessive clotting and uncontrolled bleeding.
- Life-Threatening: DIC (disseminated intravascular coagulation) can lead to organ damage, multi-organ failure, and even death if not treated promptly.
Are there any alternative or natural treatments for DIC (disseminated intravascular coagulation)?
Unfortunately, there are no proven alternative or natural treatments for DIC (disseminated intravascular coagulation). While some natural products might have potential anticoagulant or anti-inflammatory properties in general, their effectiveness and safety in treating DIC (disseminated intravascular coagulation) haven’t been established through rigorous scientific research. In fact, using such products could potentially interfere with conventional treatments and worsen the condition.
Is preeclampsia a risk factor for DIC (disseminated intravascular coagulation)?
Yes, preeclampsia can be a significant risk factor for DIC (disseminated intravascular coagulation), although the exact risk depends on the severity of the condition. Here’s why:
- Preeclampsia disrupts the normal function of the placenta, leading to widespread damage and inflammation. This inflammation triggers the release of substances that activate the clotting system, potentially leading to uncontrolled clotting and DIC (disseminated intravascular coagulation).
- The severity of preeclampsia plays a crucial role. Women with severe preeclampsia, characterized by high blood pressure, protein in the urine, and organ damage, are at much higher risk of developing DIC (disseminated intravascular coagulation) compared to those with mild preeclampsia.
- Additional complications arising from preeclampsia, such as placental abruption (premature separation of the placenta) or HELLP syndrome (a severe complication involving hemolysis, elevated liver enzymes, and low platelets), further increase the risk of DIC (disseminated intravascular coagulation).
Therefore, while preeclampsia itself poses a risk for DIC (disseminated intravascular coagulation), the severity of the condition and any accompanying complications significantly influence the individual risk level.
What predisposes a pregnant woman to DIC (disseminated intravascular coagulation)?
Several factors can predispose a pregnant woman to DIC (disseminated intravascular coagulation), increasing her risk of developing this potentially life-threatening condition. Here are some key ones:
Obstetric Complications
- Placental abruption: This occurs when the placenta prematurely detaches from the uterine wall, disrupting blood flow and triggering inflammation, potentially leading to DIC (disseminated intravascular coagulation).
- Retained placenta: When the placenta remains partially or entirely in the uterus after childbirth, it can cause bleeding and tissue breakdown, increasing the risk of DIC (disseminated intravascular coagulation).
- Amniotic fluid embolism: Although rare, this serious complication involves the entry of amniotic fluid and fetal debris into the mother’s bloodstream, causing a severe inflammatory response and potentially triggering DIC (disseminated intravascular coagulation).
- HELLP syndrome: This pregnancy-specific condition involving hemolysis (red blood cell destruction), elevated liver enzymes, and low platelets can progress to DIC (disseminated intravascular coagulation) if not managed promptly.
- Preeclampsia/eclampsia: This condition characterized by high blood pressure and protein in the urine can lead to widespread inflammation and damage in the mother’s body, increasing the risk of DIC (disseminated intravascular coagulation), especially in severe cases.
Underlying Medical Conditions
- Thrombophilia: Certain inherited or acquired conditions can make a woman more prone to blood clots, including DIC (disseminated intravascular coagulation), during pregnancy.
- Autoimmune diseases: Conditions like lupus or antiphospholipid syndrome can increase the risk of blood clotting complications like DIC (disseminated intravascular coagulation).
- Severe infections: Sepsis, a life-threatening infection spreading through the bloodstream, can trigger DIC (disseminated intravascular coagulation) in pregnant women.
- Liver or kidney disease: These pre-existing conditions can impair the body’s ability to regulate clotting, making pregnant women more susceptible to DIC (disseminated intravascular coagulation).
Other Factors
- Multiple pregnancy: Carrying twins, triplets, or more fetuses increases the risk of complications like preeclampsia and placental abruption, indirectly contributing to a higher risk of disseminated intravascular coagulation.
- Advanced maternal age: While the exact reason is unclear, older mothers may have a slightly higher risk of DIC (disseminated intravascular coagulation) compared to younger women.
However, not everyone with these risk factors will develop DIC (disseminated intravascular coagulation). Being aware of these predisposing factors and seeking proper prenatal care and monitoring can help identify potential risks and intervene promptly if needed.
Can cancer lead to DIC (disseminated intravascular coagulation)?
Yes, cancer can certainly lead to DIC (disseminated intravascular coagulation). In fact, cancer is one of the most common causes of DIC (disseminated intravascular coagulation), alongside severe infections and trauma.
Mechanisms of Cancer-Induced DIC (disseminated intravascular coagulation)
- Direct tumor effects: Certain cancers, particularly those with extensive tumor spread, can release substances that activate the clotting system, leading to uncontrolled clotting and DIC (disseminated intravascular coagulation).
- Tissue damage and inflammation: As tumors grow and invade surrounding tissues, they can cause significant damage and inflammation, triggering the release of clotting factors and contributing to DIC (disseminated intravascular coagulation).
- Treatment side effects: Chemotherapy and other cancer treatments can sometimes damage blood vessels or decrease platelets, increasing the risk of bleeding and potentially triggering DIC (disseminated intravascular coagulation).
Types of Cancers with Higher Risk
- Leukemias: Acute promyelocytic leukemia (APL) is particularly associated with DIC (disseminated intravascular coagulation).
- Solid tumors: Cancers of the prostate, lung, pancreas, breast, stomach, melanoma, gallbladder, and ovary are often linked to DIC (disseminated intravascular coagulation) risk.
What is the long-term prognosis for someone with DIC (disseminated intravascular coagulation)?
The long-term prognosis for someone with DIC (disseminated intravascular coagulation) depends on several factors, making it difficult to give a definitive answer.
Severity and Cause of DIC (disseminated intravascular coagulation)
- Mild DIC (disseminated intravascular coagulation) often resolves spontaneously or with minimal intervention, offering a good prognosis.
- Severe DIC (disseminated intravascular coagulation) with extensive organ damage or prolonged clotting dysfunction carries a higher risk of complications and worsens the prognosis.
- The underlying cause of DIC (disseminated intravascular coagulation) significantly affects the outlook. Conditions like sepsis or severe trauma with ongoing complications can have a poorer prognosis compared to DIC (disseminated intravascular coagulation) managed successfully with cancer treatment.
Timely Diagnosis and Treatment
- Early diagnosis and prompt intervention with appropriate treatment, like anticoagulants or blood transfusions, significantly improve the prognosis.
- Delays in diagnosis or inadequate treatment can lead to severe complications and worsen the outcome.
Individual Health Status
- Pre-existing health conditions like heart disease, kidney disease, or weakened immune system can impact the body’s ability to cope with DIC (disseminated intravascular coagulation) and its treatment, influencing the prognosis.
- Overall health and age also play a role, with younger, healthier individuals generally having a better prognosis than those with compromised health.
Complications of DIC (disseminated intravascular coagulation)
- The development of complications like organ failure, severe bleeding, or blood clots can significantly worsen the prognosis and decrease life expectancy.
Overall, the long-term prognosis for someone with DIC (disseminated intravascular coagulation) varies greatly depending on the individual circumstances. While it’s impossible to predict with certainty, understanding the influencing factors and seeking prompt medical attention can significantly improve the chances of a favorable outcome.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- Costello RA, Nehring SM. Disseminated Intravascular Coagulation. [Updated 2023 Jan 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- Unar A, Bertolino L, Patauner F, Gallo R, Durante-Mangoni E. Pathophysiology of Disseminated Intravascular Coagulation in Sepsis: A Clinically Focused Overview. Cells. 2023 Aug 22;12(17):2120. doi: 10.3390/cells12172120. PMID: 37681852; PMCID: PMC10486945.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007 Sep;35(9):2191-5. doi: 10.1097/01.ccm.0000281468.94108.4b. PMID: 17855836.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Delabranche X, Quenot JP, Lavigne T, Mercier E, François B, Severac F, Grunebaum L, Mehdi M, Zobairi F, Toti F, Meziani F, Boisramé-Helms J; on behalf to the Clinical Research in Intensive Care and Sepsis Network. Early Detection of Disseminated Intravascular Coagulation During Septic Shock: A Multicenter Prospective Study. Crit Care Med. 2016 Oct;44(10):e930-9. doi: 10.1097/CCM.0000000000001836. PMID: 27322364.
- Hoffman R, Benz EJ, Silberstein LE, Heslop H,Weitz J, Anastasi J. Hematology: Diagnosis and Treatment E-Book (Hematology Basic Principles and Practice) 6th Edition (Churchill Livingstone). 2013.
- Franchini, M., Lippi, G. & Manzato, F. Recent acquisitions in the pathophysiology, diagnosis and treatment of disseminated intravascular coagulation. Thrombosis J 4, 4 (2006). https://doi.org/10.1186/1477-9560-4-4
- Iba T, Di Nisio M, Thachil J, Wada H, Asakura H, Sato K, Saitoh D. A Proposal of the Modification of Japanese Society on Thrombosis and Hemostasis (JSTH) Disseminated Intravascular Coagulation (DIC) Diagnostic Criteria for Sepsis-Associated DIC. Clin Appl Thromb Hemost. 2018 Apr;24(3):439-445. doi: 10.1177/1076029617720069. Epub 2017 Jul 27. PMID: 28747069; PMCID: PMC6714647.
- ISTH Criteria for Disseminated Intravascular Coagulation (DIC): https://qxmd.com/calculate/calculator_649/dic-score
- https://qxmd.com/calculate/calculator_784/sepsis-induced-coagulopathy-sic-score
- Ghidini, Michele & Indini, Alice & Rijavec, Erika & Bareggi, Claudia & Cattaneo, Monica & Tomasello, Gianluca & Galassi, Barbara & Gambini, Donatella & Grossi, Francesco. (2021). The Appropriateness of Invasive Ventilation in COVID-19 Positive Cancer Patients: Proposal of a New Prognostic Score. Viruses. 13. 508. 10.3390/v13030508.