TL;DR
Key Function of Fibrinolysis: Dissolves fibrin clots after wound healing, restoring blood flow and preventing unwanted thrombus formation.
Essential Players ▾
- Plasminogen: The inactive precursor protein, waiting to be activated.
- Plasmin: The active enzyme responsible for cleaving fibrin and dismantling clots.
- Activators:
- tPA (tissue plasminogen activator): Primarily activates plasmin at the clot site.
- uPA (urokinase plasminogen activator): Regulates broader fibrinolysis in tissues and blood vessels.
- Inhibitors:
- Alpha2-antiplasmin: Quickly inactivates plasmin, preventing uncontrolled proteolysis.
- PAIs (plasminogen activator inhibitors): Regulate the activity of tPA and uPA.
Pathways ▾
- Intrinsic (uPA): uPA activates plasminogen directly or through other proteases, primarily in tissues and vasculature.
- Extrinsic (tPA): Tissue plasminogen activator (tPA) binds directly to fibrin and activates plasminogen, mainly at the site of a clot.
Significance ▾
- Maintains balance: Prevents excessive clotting (thrombosis) and bleeding.
- Wound healing: Dissolves clots after repair, allowing tissue regeneration.
- Medical applications: Fibrinolytic agents like tissue plasminogen activator (tPA) can dissolve clots in strokes and heart attacks.
Understanding fibrinolysis is crucial for diagnosing and treating various diseases:
- Bleeding disorders: Deficiencies in key players can lead to excessive bleeding.
- Thrombosis: Impaired fibrinolysis can worsen clot formation and increase risk of blood clots.
- DIC (disseminated intravascular coagulation): Can involve both excessive clot formation and breakdown.
*Click ▾ for more information
Introduction
Hemostasis ensures our blood flows effortlessly while also sealing leaks at the slightest injury. This intricate process balances clot formation (coagulation) and clot breakdown (fibrinolysis), maintaining a delicate equilibrium critical for both healthy blood flow and efficient wound healing.
Coagulation: Building the Plug
First, platelets like tiny bricks rush to the scene, adhering to the injury and each other, forming a temporary plug in primary hemostasis. Then, the coagulation cascade, a finely tuned sequence of enzymatic reactions, kicks in. This cascade orchestrates the conversion of fibrinogen into fibrin, a mesh-like web that reinforces the platelet plug, creating a sturdy clot. Calcium ions act as essential scaffolding, ensuring the fibrin mesh holds strong.
Fibrinolysis: The Cleanup Crew
Once the wound starts healing, another team, the fibrinolytic system, takes over. Plasminogen, another circulating protein, gets activated into plasmin, the demolition expert. Plasmin carefully snips apart the fibrin mesh, gradually dismantling the clot and restoring blood flow to the repaired tissue.
The Importance of Balance
This exquisite equilibrium between coagulation and fibrinolysis is vital.
- Excessive clot formation (thrombosis): If the brakes on coagulation fail, clots can form within intact blood vessels, blocking vital arteries and veins. This can lead to potentially life-threatening conditions like deep vein thrombosis, pulmonary embolism, and stroke.
- Excessive clot breakdown (bleeding): Conversely, if fibrinolysis goes into overdrive, the clot dissolves too quickly, leading to uncontrolled bleeding and delayed wound healing. This can pose significant risks in conditions like hemophilia and traumatic injuries.
Key Players of Fibrinolysis
Fibrinolysis plays a crucial role in dismantling clots after wound healing, maintaining healthy blood flow, and preventing unwanted thrombus formation. Let’s dive into the key players involved:
Precursor and Active Enzyme
Plasminogen plays a pivotal role in fibrinolysis, acting as the inert precursor protein to the active enzyme plasmin (inactive zymogen). It’s the key player waiting in the wings, ready to take center stage and dismantle fibrin clots when needed. Here’s a deeper dive into its functions:
- Source of Plasmin
- Plasminogen circulates abundantly in the blood, accounting for 1-2% of total plasma protein. This ensures a readily available pool for plasmin generation whenever needed.
- Its specific structure includes five distinct domains, each with crucial functions.
- Upon activation by specific enzymes called plasminogen activators, plasminogen undergoes a conformational change, exposing the active site of the resulting plasmin enzyme.
- Localized Activation
- Plasminogen activation isn’t a haphazard process. It’s tightly regulated and primarily occurs at the site of a clot through specific mechanisms:
- Binding to fibrin: Plasminogen has a high affinity for fibrin within the clot, allowing it to be concentrated where its proteolytic activity is needed.
- Activator targeting: Specific plasminogen activators like tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) bind to fibrin and plasminogen, facilitating their interaction and cleavage.
- Plasminogen activation isn’t a haphazard process. It’s tightly regulated and primarily occurs at the site of a clot through specific mechanisms:
- Controlled Proteolysis
- Once activated, plasmin becomes a potent serine protease, capable of cleaving fibrin at specific sites within its mesh-like structure. This targeted proteolysis gradually dismantles the clot, allowing blood flow to resume in the repaired tissue.
- The specificity of plasmin’s action minimizes damage to surrounding tissues and prevents uncontrolled proteolysis of other proteins in the blood.
Activators
- Tissue plasminogen activator (tPA)
- Plays a key role in localized fibrinolysis at the site of a clot by binding to fibrin within the clot, bringing plasminogen in close proximity and facilitating its activation by cleaving off a specific peptide bond.
- Primarily used in thrombolytic therapy to dissolve occlusive clots in conditions like ischemic stroke and myocardial infarction.
- Urokinase plasminogen activator (uPA)
- Contributes to broader fibrinolysis regulation beyond the immediate clot area.
- Primarily expressed in various tissues and at the blood vessel wall, activating plasminogen in the surrounding extracellular matrix.
- Plays a role in tissue remodeling and inflammatory processes.
- Other activators
- Streptokinase: A bacterial protein with broader plasminogen activating activity used in the past, but largely replaced by tissue plasminogen activator (tPA) due to its higher risk of bleeding complications.
- Factor XIIa: An enzyme in the coagulation cascade can indirectly activate plasminogen under certain conditions.
Inhibitors
- Alpha2-antiplasmin (α2-AP)
- This is the primary physiological inhibitor of plasmin. It circulates in the blood at high concentrations and rapidly forms a stable, irreversible complex with plasmin, effectively inactivating it.
- This prevents widespread proteolysis of other proteins beyond the targeted fibrin degradation in a clot.
- Deficiencies in α2-AP can lead to increased bleeding risk.
- Plasminogen activator inhibitor (PAI)
- This family of proteins primarily regulates the activity of the plasminogen activators, tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA), preventing their uncontrolled activation and subsequent plasmin generation.
- Two main subtypes exist:
- PAI-1: Specifically targets tissue plasminogen activator (tPA), and is the major regulator of fibrinolysis in the circulation.
- PAI-2: Primarily regulates urokinase plasminogen activator (uPA) activity in tissues and at the blood vessel wall.
- Elevated PAI levels can lead to impaired fibrinolysis and increased thrombosis risk.
Other Molecules
- Fibrin: The substrate for plasmin, forming the mesh-like structure of the clot.
- Calcium ions: Important for stabilizing the fibrin mesh and regulating plasmin activity.
- Annexin A2: A protein that binds to phosphatidylserine exposed on activated platelets and aids in plasminogen binding and clot lysis.
- Tissue inhibitors of metalloproteinases (TIMPs): Indirectly contribute to fibrinolysis by inhibiting enzymes that can degrade plasminogen activators.
The Process of Fibrinolysis
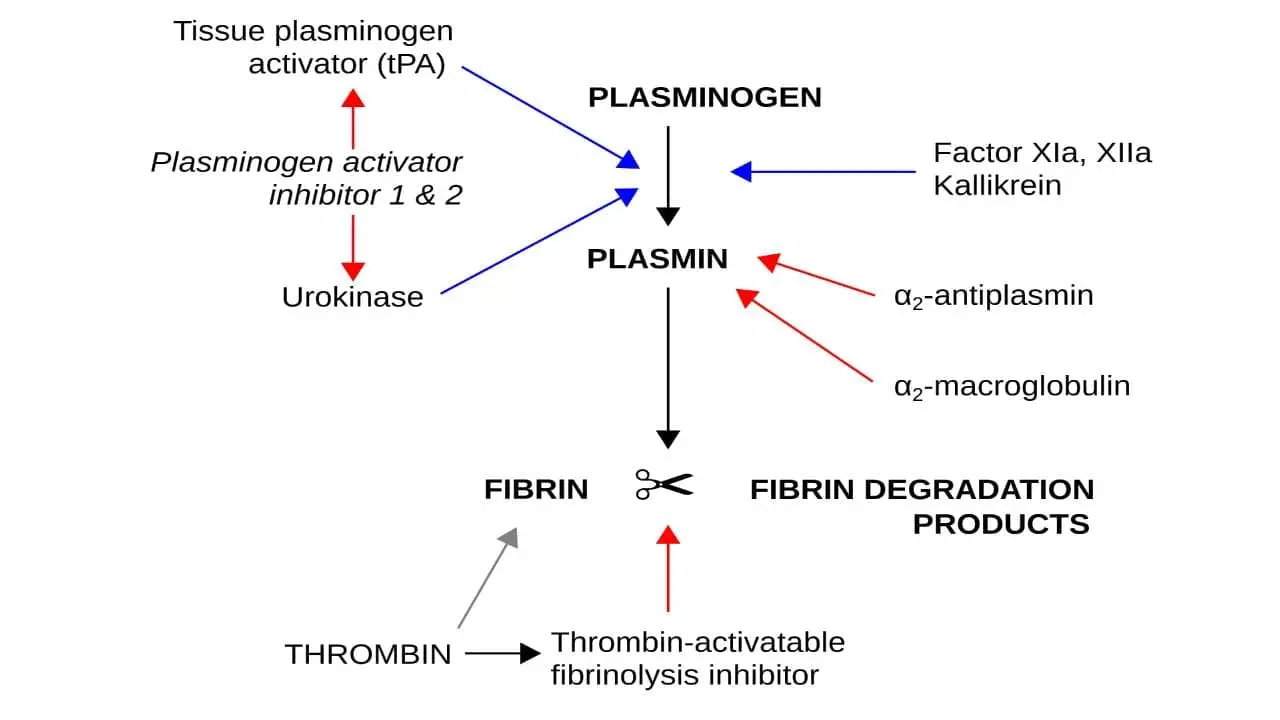
Plasminogen, the key to clot breakdown, sits in the wings, waiting for the right cue. This cue comes in the form of two distinct activation pathways: the intrinsic (urokinase plasminogen activator (uPA)) and the extrinsic (tissue plasminogen activator (tPA)). Let’s delve deeper into their mechanisms and appreciate the importance of localized fibrinolysis.
Intrinsic Pathway (uPA/uPAR System)
This pathway operates primarily within tissues and at the blood vessel wall. The urokinase plasminogen activator (uPA), primarily expressed in tissues and at the blood vessel wall. Urokinase plasminogen activator (uPA) binds to a specific receptor on the cell surface called uPAR (urokinase plasminogen activator receptor). Urokinase plasminogen activator (uPA) forms complexes with its specific inhibitor, PAI-1, keeping it in check. Tissue damage or inflammation disrupts this complex, freeing urokinase plasminogen activator (uPA). Once bound, urokinase plasminogen activator (uPA) undergoes a conformational change, increasing its affinity for plasminogen. Urokinase plasminogen activator (uPA) then cleaves a specific peptide bond in plasminogen, transforming it into the active enzyme, plasmin. The uPA/uPAR system ensures localized fibrinolysis, confining plasmin activity to the immediate vicinity of the clot. This minimizes damage to surrounding tissues and prevents unwanted systemic activation. The newly formed plasmin further activates urokinase plasminogen activator (uPA) in a positive feedback loop, amplifying the localized clot breakdown.
Extrinsic Pathway (tissue plasminogen activator (tPA)/Fibrin System)
This systemic pathway features tissue plasminogen activator (tPA), primarily circulating in the blood. Produced by endothelial cells lining blood vessels, tissue plasminogen activator (tPA) circulates in the blood in an inactive form. Upon encountering fibrin within a clot, tissue plasminogen activator (tPA) undergoes a conformational change, activating its plasminogen-cleaving ability. Tissue plasminogen activator (tPA) preferentially activates plasminogen bound to fibrin, further restricting its activity to the clot area. Unlike uPA, tissue plasminogen activator (tPA) cleaves plasminogen more efficiently, but its systemic presence requires tighter control.
Fibrin dismantling
Once activated, plasmin gets to work dismantling the fibrin mesh. It does this by meticulously cleaving specific peptide bonds within the fibrin molecule, effectively weakening its structure and causing the clot to unravel. This targeted action minimizes damage to surrounding proteins and tissues.
Localization Matters
Both pathways emphasize the importance of localized fibrinolysis. This ensures that clot breakdown occurs only where needed, preventing excessive bleeding and maintaining the delicate balance between hemostasis and fibrinolysis. Tight regulation by inhibitors and clearance mechanisms further ensures plasmin’s activity is kept in check.
Clinical Significance
Fibrinolysis plays a complex role in various cardiovascular and inflammatory conditions. While it aids in dissolving unwanted clots, excessive or inappropriate activation can lead to detrimental bleeding. Let’s explore its involvement in some key situations:
Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE)
- Impaired fibrinolysis: In DVT, insufficient fibrinolysis within the clot can worsen the initial thrombus formation and prevent its dissolution. This can lead to PE if the clot breaks off and travels to the lungs.
- Therapeutic applications: tissue plasminogen activator (tPA) can be used to dissolve DVTs and prevent PE, particularly in high-risk cases. However, the risk of bleeding increases with tissue plasminogen activator (tPA) use.
Myocardial Infarction (Heart Attack)
- Occluded coronary arteries: Fibrin deposition within the coronary arteries leads to clot formation and blockage, causing myocardial infarction.
- Early intervention: Prompt administration of tissue plasminogen activator (tPA) can break down the clot and restore blood flow to the heart, reducing tissue damage and improving prognosis. Time is crucial, and benefits of tPA diminish significantly after a certain delay.
Stroke
- Ischemic stroke: Similar to heart attack, ischemic stroke involves a blocked artery supplying blood to the brain. Fibrinolysis with tissue plasminogen activator (tPA) can be effective in certain types of ischemic stroke, depending on the location and timing of the occlusion.
- Hemorrhagic stroke: tissue plasminogen activator (tPA) is contraindicated in hemorrhagic stroke, as it can worsen bleeding within the brain.
Disseminated Intravascular Coagulation (DIC)
- Excessive clot formation and breakdown: DIC triggers widespread activation of both coagulation and fibrinolysis, leading to the formation and subsequent destruction of small clots throughout the circulation.
- Limited role of fibrinolysis: Due to the complex and often unpredictable nature of DIC, the use of fibrinolytic agents is generally not recommended, as it can further exacerbate bleeding.
Therapeutic Applications of Fibrinolytic Agents (tPA)
- Tissue plasminogen activator (tPA) is a powerful tool for dissolving clots in conditions like DVT, PE, and certain types of ischemic stroke, but its use requires careful consideration of:
- Time of occlusion: Early intervention is crucial for optimal benefit.
- Stroke type: Contraindicated in hemorrhagic stroke.
- Bleeding risk: Increased risk of bleeding with tPA use.
- Limitations of tissue plasminogen activator (tPA)
- Bleeding risk: The main limitation is the increased risk of potentially life-threatening hemorrhage, especially in older patients or those with pre-existing bleeding tendencies.
- Limited efficacy: Not effective in all types of clots or strokes.
- Time-sensitive: Efficacy rapidly decreases with time after the clot formation.
Frequently Asked Questions (FAQs)
What is the difference between fibrinolysis and thrombolysis?
Fibrinolysis is the natural process by which the body breaks down blood clots. It’s a normal physiological process that occurs to prevent clots from growing too large or blocking blood vessels.
Thrombolysis is the medical intervention to dissolve blood clots. It’s a therapeutic process that uses drugs like tPA to accelerate fibrinolysis and break down clots that are causing problems, such as those in a heart attack or stroke.
Essentially, fibrinolysis is the body’s natural clot-busting system, while thrombolysis is the medical treatment to enhance or mimic that process.
What is fibrinolysis for STEMI?
Fibrinolysis is a crucial treatment for STEMI (ST-segment elevation myocardial infarction), which is a heart attack caused by a complete blockage of a coronary artery.
- How it works: Fibrinolytic drugs, like tPA, break down the blood clot blocking the coronary artery, restoring blood flow to the heart muscle.
- Time is critical: To be most effective, fibrinolysis should be administered within the first few hours of symptom onset.
- Benefits: It can significantly reduce heart damage and improve survival rates when given promptly.
Challenges and Considerations
While fibrinolysis is a lifesaving treatment, it’s important to note:
- Risk of bleeding: These drugs can increase the risk of bleeding. Patients are carefully monitored for this complication.
- Alternative treatment: Primary percutaneous coronary intervention (PCI) is generally preferred over fibrinolysis when available, as it offers better outcomes. However, fibrinolysis remains a valuable option in areas without immediate access to PCI.
What are the two types of fibrinolysis?
There are two main types of fibrinolysis:
- Primary fibrinolysis: This is the natural process your body uses to dissolve blood clots after they’ve served their purpose. It’s a controlled process that prevents excessive clot formation.
- Secondary fibrinolysis: This occurs due to an external factor, such as:
- Medication (like tPA)
- A medical condition (like disseminated intravascular coagulation or DIC)
- Other causes (like severe infections)
Secondary fibrinolysis can sometimes be excessive, leading to bleeding problems.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Kaushansky K, Levi M. Williams Hematology Hemostasis and Thrombosis (McGraw-Hill). 2017.