TL;DR
Megaloblastic anemia is caused by defective DNA synthesis due to B12 deficiency or folate deficiency resulting in delayed red cell nucleus maturation in the bone marrow leading to macrocytic red cells (MCV > 95 fL in adults).
| General | Specific | Specific B12 deficiency symptoms |
| General features related to anemia Mild jaundice | Glossitis Angular stomatitis Sterility Fetal neural tube defects | Symmetrical neuropathy Tingling in feet Difficulty in gait Visual or psychiatric disorders |
| B12 deficiency | Folate deficiency |
| Lack of gastric intrinsic factor – Pernicious anemia (autoimmune gastritis) – Congenital or abnormality – Gastrectomy Intestinal malabsorption – Crohn’s disease – Ileal resection – Stagnant loop syndrome – Fish tapeworm Diet – Vegans | Diet Malabsorption – Celiac disease – Tropical sprue – Small bowel disease or resection – Genetics Increased requirement – Pregnancy – Hemolytic anemia – Myeloproliferative / malignancy / inflammatory Renal loss – Congestive cardiac failure – Dialysis Drugs – Anticonvulsants – Sulfasalazine |
- Complete blood count: ↓ hemoglobin, ↑ mean cell volume and normal or ↑ mean cell hemoglobin, ↓ reticulocytes. Moderate reduction of white blood cell and platelet counts in severe anemia.
- Peripheral blood smear: Oval macrocytes, anisopoikilocytosis and hypersegmented neutrophils.
- Bone marrow characteristics: Hypercellular with large erythroblasts. Giant and abnormally shaped metamyelocytes are characteristic.
- Vitamin B12 deficiency specific tests
- ↓ serum vitamin B12
- ↑ serum methylmalonic acid
- ↑ serum homocysteine
- Folate deficiency specific tests
- ↓ serum folate
- ↓ red cell folate
- Pernicious anemia specific tests
- Abnormal Schilling test for vitamin B12 absorption
- Severe deficiency of intrinsic factor
- Serum antibodies to intrinsic factor
Treatment and management of megaloblastic anemia ▾:
- Vitamin B12 deficiency treatment
- Hydroxocobalamin intramuscularly if not due to dietary intake
- Folate deficiency treatment
- Oral folic acid 5 mg once daily
*Click ▾ for more information
What is megaloblastic anemia?
Megaloblastic anemia is defined as a type of anemia caused by defective DNA synthesis due to Vitamin B12 (cobalamin) or folate deficiency. Anemia is defined as when a person’s hemoglobin level falls below the reference range or a reduction in the hemoglobin concentration of the blood according to gender and age. Megaloblastic anemia causes delayed red cell nucleus maturation in the bone marrow leading to macrocytic (abnormally large) red cells.
Importance of Vitamin B12 (cobalamin) and Folate
Hematopoiesis or production of blood cells depends on orderly cell division and differentiation for expansion and maturation of progenitor (early) cells into members of circulating blood cells. For this to happen, vitamin B12 and folate is needed for nucleic acid or DNA formation.
Vitamin B12 deficiency or folate deficiency in the body will lead to megaloblastic anemia in which DNA synthesis is impaired, resulting in slowing or arrest of cellular division during the DNA synthesis phase of the cell cycle which is the S phase. A high percentage of defective cells undergo apoptosis leading to ineffective erythropoiesis. However, RNA synthesis and cytoplasmic differentiation are relatively unaffected, thus surviving progenitors and progeny are enlarged or have the appearance of macrocytic cells which results in macrocytic or large red blood cells, hypersegmented neutrophils and thrombocytopenia (low production of platelets).
Sources of Vitamin B12 and Folate
Vitamin B12 is found in foods of animal origin such as liver, meat, fish and dairy products but not in fruit, cereals nor vegetables.
Folate is present primarily in fruits, vegetables, liver and certain meat products and can be inactivated by prolonged cooking.
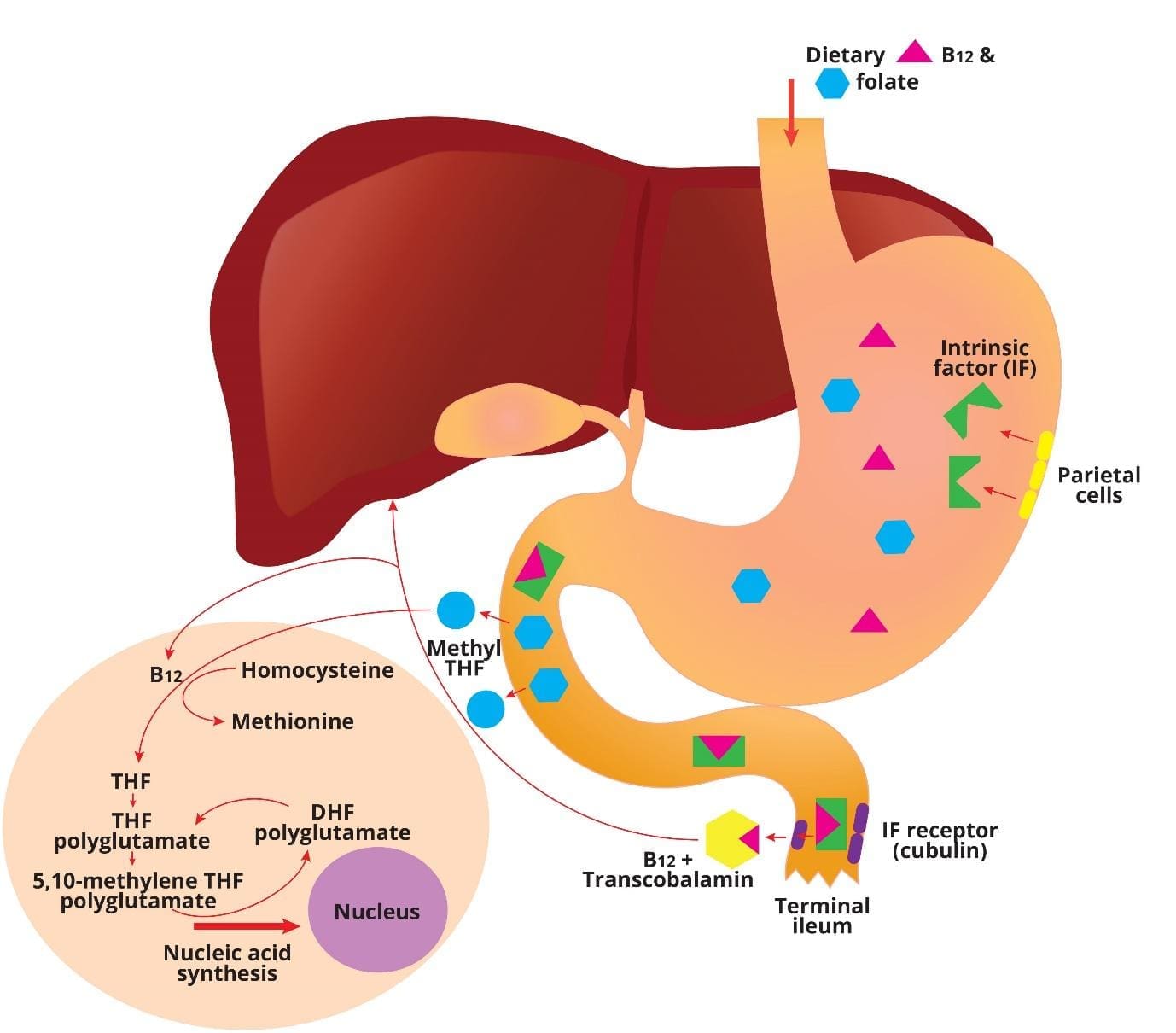
How are vitamin B12 and folate absorbed in the body?
Vitamin B12 (cobalamin) absorption
Following ingestion, some cobalamin or vitamin B12 in food is transferred to human haptocorrin in the saliva. The acidic environment of the stomach enables efficient release and transfer of the remaining food cobalamin to haptocorrin in gastric juice.
After transit to the duodenum, increase in pH enables the transfer of cobalamin from haptocorrin to intrinsic factor, a transport protein secreted by gastric parietal cells. The cobalamin-intrinsic factor complex resists digestion and travels down the gut until it encounters epithelial cells in the distal ileum that express cubilin, a specific receptor for this bimolecular complex.
The cobalamin that is absorbed in the ileum exits the basolateral side of the mucosal epithelial cell and enters the blood and binds to transcobalamin, in which it travels via the portal circulation to the liver. Circulating transcobalamin-cobalamin complexes then bind and are taken up into cells by transcobalamin receptors.
Folate absorption
Dietary folates are converted to methyl-tetrahydrofolate (THF) during absorption through the upper small intestine. Once inside the cell they are converted to folate polyglutamates. Folate binding proteins are present on cell surfaces including the enterocyte and facilitate entry of reduced folates into cells. All body cells, including those of the bone marrow, receive folate from plasma as methyl-THF.
How does cobalamin and folate work together?
Vitamin B12 is needed in the conversion of methyl-THF to THF, a reaction in which homocysteine is methylated to methionine. During another reaction involving the conversion of the amino acid serine to glycine, a methyl group is transferred to THF to form N5,10-methylene THF, which is required for both de novo purine biosynthesis and for the conversion of deoxyuridylate to deoxythymidylate. Thus, vitamin B12 and folate both play critical roles in the production of the building blocks of DNA.
Vitamin B12 (cobalamin) and Folate cycle
Causes of Megaloblastic Anemia
Megaloblastic anemia is primarily caused by a defect in DNA synthesis, which results in the production of abnormally large, immature red blood cells. The main culprits behind this defect are deficiencies of vitamin B12 or folate.
Causes of B12 Deficiency
Vitamin B12 deficiency can arise from a number of factors, primarily stemming from either insufficient intake of the vitamin or problems with its absorption in the body.
Malabsorption Issues (The Most Common Cause)
The body’s ability to absorb vitamin B12 is a complex process that can be disrupted at several stages.
- Pernicious Anemia: This is a key cause, and it is an autoimmune condition where the body’s immune system attacks the cells in the stomach that produce intrinsic factor. Intrinsic factor is a protein essential for vitamin B12 absorption in the small intestine. Without it, the vitamin cannot be absorbed from food. It is believed that the autoimmune attack is initiated by gastric dendritic cells, which help to clear apoptotic parietal cells produced during the normal turnover of gastric mucosa. These dendritic cells migrate to paragastric lymph nodes, where they present peptides derived from the proton/potassium ATPase on the parietal cells to autoreactive CD4+ T lymphocytes. Over 90% of patients also have serum antibodies against parietal cells, and about 50% have antibodies against intrinsic factors.
- Stomach and Intestinal Conditions: Diseases or surgeries that affect the gastrointestinal tract can impair absorption. Examples include:
- Gastric Surgery: Procedures like a gastrectomy or gastric bypass can reduce or eliminate the stomach’s ability to produce intrinsic factor.
- Crohn’s Disease and Celiac Disease: These conditions cause inflammation and damage to the lining of the intestines, particularly the ileum, which is where B12 is absorbed.
- Atrophic Gastritis: This condition, common in older adults, leads to inflammation and thinning of the stomach lining, resulting in a reduction in both stomach acid and intrinsic factor.
- Small Intestinal Bacterial Overgrowth (SIBO): An overgrowth of bacteria in the small intestine can cause the bacteria to use up the B12 from food, leaving less for the body to absorb.
Dietary Deficiency
Vitamin B12 is found almost exclusively in animal products. While the body has a large storage capacity for B12 in the liver (often lasting years), a long-term dietary deficiency will eventually lead to problems.
- Strict Vegan Diet: Individuals who follow a vegan diet and do not consume B12-fortified foods or take supplements are at high risk.
- Malnutrition and Alcoholism: A generally poor diet or chronic alcohol use can lead to both inadequate intake and damage to the digestive system, further compounding the problem.
Medications and Other Factors 💊
Some medications and health factors can interfere with B12 metabolism and absorption.
- Medications: Certain drugs, especially if taken long-term, can reduce stomach acid or otherwise interfere with B12 absorption. Examples include proton pump inhibitors (PPIs) and metformin (a common diabetes drug).
- Older Age: As people age, their stomach acid production naturally decreases, making it more difficult to release vitamin B12 from food.

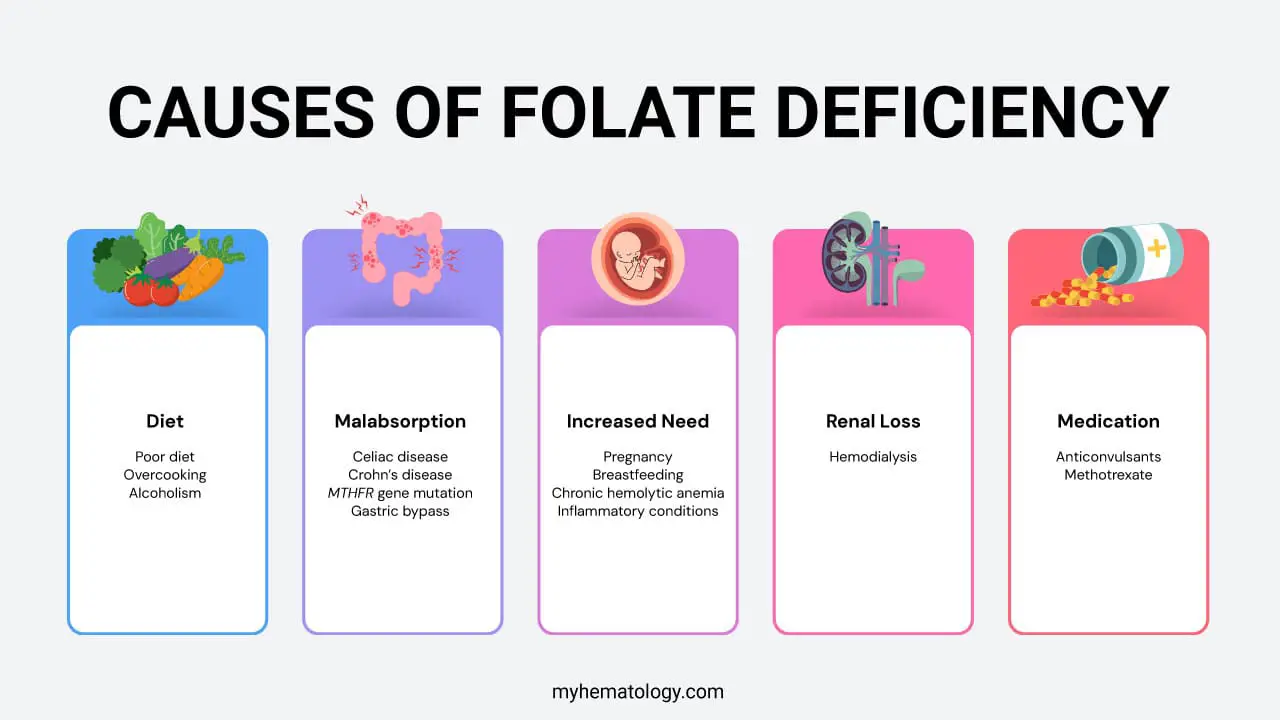
Causes of Folate Deficiency
Folate deficiency, also known as vitamin B9 deficiency, can be caused by several factors, which can be grouped into four main categories:
Inadequate Dietary Intake
This is a very common cause, as the body doesn’t store large amounts of folate. It must be consumed it regularly.
- Alcoholism: Chronic alcohol use interferes with the absorption, metabolism, and storage of folate.
- Poor Diet: Not eating enough folate-rich foods like leafy green vegetables, citrus fruits, and fortified cereals can quickly lead to a deficiency.
- Overcooking: Folate is sensitive to heat, and overcooking foods can destroy the vitamin.
Malabsorption
Conditions that affect the small intestine, where folate is absorbed, can lead to a deficiency.
- Gastrointestinal Disorders: Conditions that cause inflammation or damage to the small intestine, such as celiac disease or Crohn’s disease, can impair absorption.
- Genetic Factors: Certain genetic factors, such as mutations in the MTHFR gene, can impair the body’s ability to convert folate into its active, usable form.
- Certain surgical procedures: Surgical procedures such as gastric bypass, can also reduce the body’s ability to absorb nutrients.
Increased Need
In some cases, the body’s demand for folate is higher than normal.
- Pregnancy and breastfeeding: The rapid growth and development of the fetus and infant significantly increase the need for folate to support cell division and DNA synthesis.
- Chronic Hemolytic Anemia: In conditions where red blood cells are destroyed at a high rate (e.g., sickle cell disease), the bone marrow works overtime to produce new cells, consuming a large amount of folate in the process.
- Inflammatory Conditions: Conditions with a high rate of cell turnover, such as psoriasis or certain myeloproliferative disorders, can increase folate utilization.
Other causes
- Medications: Some drugs interfere with the body’s ability to use or absorb folate. This includes certain anti-seizure medications and methotrexate, a drug used to treat some cancers and autoimmune diseases.
- Renal Loss: Patients with chronic kidney disease, particularly those undergoing hemodialysis, lose folate during the treatment process. This necessitates supplementation to prevent deficiency.
Megaloblastic Anemia Symptoms & Signs
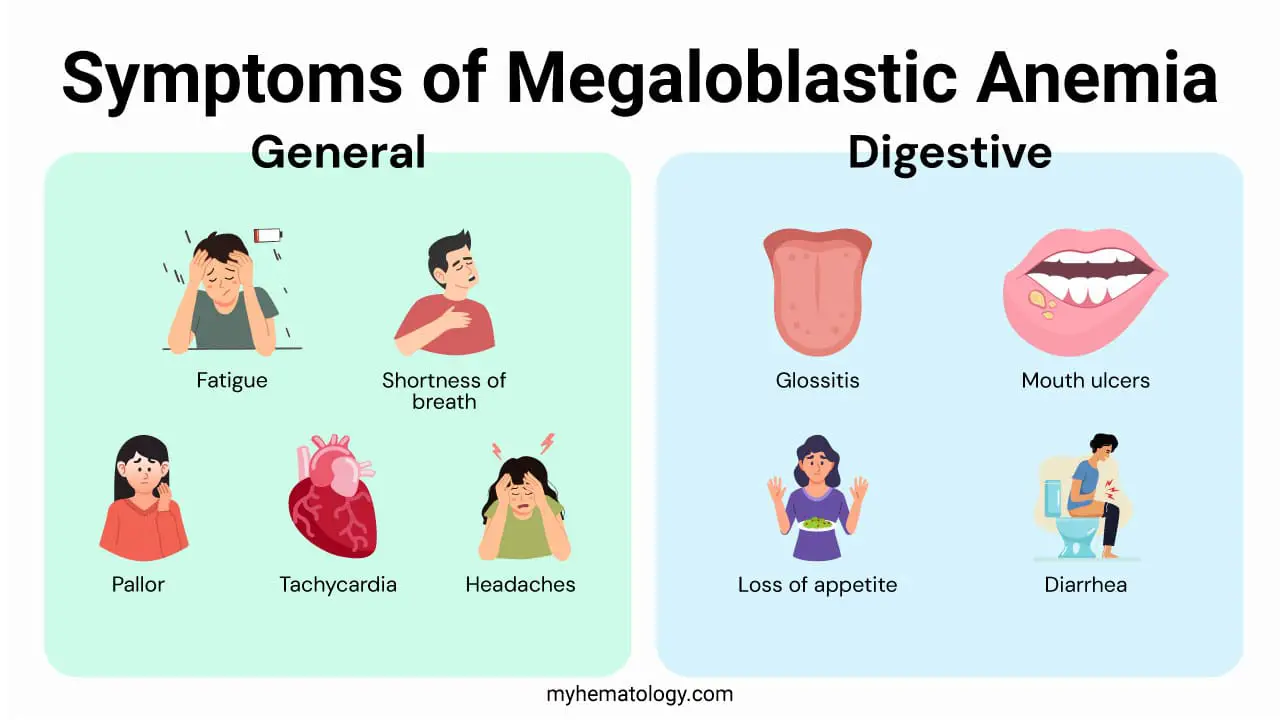
The signs and symptoms of megaloblastic anemia often develop gradually and can range from mild to severe, affecting multiple body systems.
General Symptoms (Common to both B12 and Folate Deficiency)
These symptoms are a direct result of the anemia caused by the reduced number of healthy red blood cells.
- Fatigue and Weakness: The most common and often first symptom. The body’s tissues aren’t receiving enough oxygen.
- Shortness of Breath: Even with light physical activity, patients may feel out of breath.
- Pale Skin: The lack of red blood cells reduces the normal rosy color of the skin.
- Rapid Heartbeat: The heart works harder to compensate for the reduced oxygen-carrying capacity of the blood.
- Headaches and Dizziness: These can be related to insufficient oxygen supply to the brain.
Digestive Symptoms
These symptoms are also common to both B12 and folate deficiencies.
- Glossitis: A sore, red, and swollen tongue that may appear smooth. This is a classic symptom.
- Mouth Ulcers: Sores can develop inside the mouth.
- Changes in Appetite: Many people experience a loss of appetite, which can lead to weight loss.
- Gastrointestinal Distress: Symptoms like diarrhea, nausea, and bloating can occur.
Neurological Symptoms (Unique to B12 Deficiency)
This is a critical distinguishing feature. Folate deficiency generally does not cause these symptoms, and treating B12 deficiency with only folate can mask the anemia while allowing the neurological damage to progress.
- Paresthesias: A “pins and needles” or tingling sensation in the hands and feet.
- Balance and Coordination Issues: Difficulty with balance, an unsteady gait, and general clumsiness.
- Cognitive Changes: Confusion, memory loss, and difficulty concentrating.
- Mood Changes: Irritability, depression, and, in severe cases, psychosis or dementia-like symptoms.
- Vision Problems: Disturbed or blurred vision.
How do I test for megaloblastic anemia?
Lab investigations for megaloblastic anemia are a multi-step process that starts with standard blood tests and proceeds to more specific tests to determine the exact cause of the deficiency.
Initial Blood Tests
Complete Blood Count (CBC)
This test is the cornerstone of diagnosis. It will typically show:
- Anemia: Low red blood cell count and low hemoglobin.
- Macrocytosis: An elevated Mean Corpuscular Volume (MCV), usually greater than 100 fL. This indicates that the red blood cells are abnormally large.
- Pancytopenia: In severe cases, there may be a decrease in all three blood cell lines: red cells, white cells, and platelets.
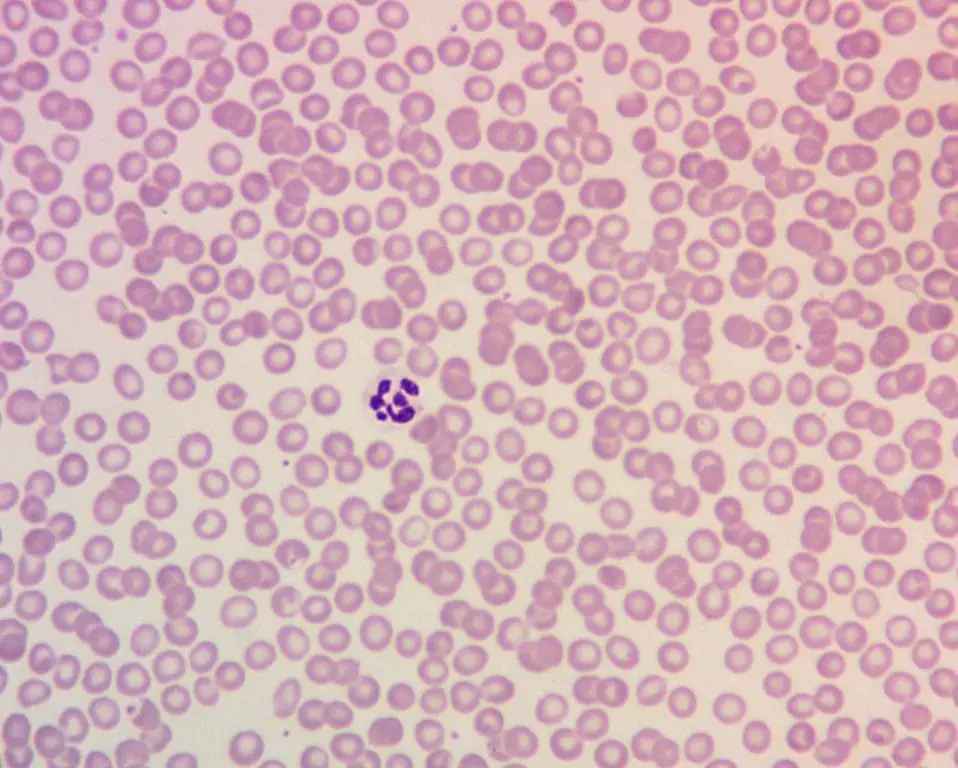
Peripheral Blood Smear
- Macro-ovalocytes: Large, oval-shaped red blood cells.
- Hypersegmented Neutrophils: A specific and sensitive finding where neutrophils (a type of white blood cell) have an increased number of segments in their nucleus (typically five or more).
- Howell-Jolly Bodies: Small, dark, round inclusions in red blood cells that are remnants of the nucleus.
Vitamin Level Testing
Once megaloblastic anemia is suspected, the next step is to pinpoint whether it is due to a B12 or folate deficiency.
Serum Vitamin B12 and Folate Levels
- A low serum B12 level (<200 pg/mL) is a strong indicator of deficiency. However, levels in the “borderline” range (200-400 pg/mL) may require further investigation.
- A low serum folate level (<4 ng/mL) suggests a folate deficiency.
Confirmatory and Differentiating Tests
If the initial vitamin levels are borderline or inconclusive, these tests provide a more definitive diagnosis.
Methylmalonic Acid (MMA) and Homocysteine (HCY) Levels
These are metabolites that accumulate when the B12 and folate pathways are disrupted.
- In B12 deficiency, both MMA and HCY levels will be elevated. This is because B12 is a required co-factor for the enzymes that metabolize both of these substances.
- In folate deficiency, only HCY levels will be elevated, while MMA levels remain normal. This key difference helps differentiate between the two deficiencies.
- Caution: Elevated MMA can also be a sign of kidney failure, so a doctor must consider this possibility.
Investigations for the Underlying Cause
If a B12 deficiency is confirmed, especially if pernicious anemia is suspected, further tests are done to identify the root cause.
- Parietal Cell Antibody Test: While less specific than the IF antibody test, a positive result can also support a diagnosis of pernicious anemia.
- Intrinsic Factor (IF) Blocking Antibody Test: This is the most specific test for pernicious anemia. A positive result indicates that the body’s immune system is producing antibodies that block intrinsic factor, which is necessary for B12 absorption.
How is megaloblastic anemia treated?
The treatment and management of megaloblastic anemia depend entirely on the underlying cause, which is most commonly a deficiency in either vitamin B12 or folate. The primary goal is to correct the deficiency, replenish the body’s stores of the vitamin, and address any underlying condition that is causing the deficiency.
Treatment for Vitamin B12 Deficiency
The method of treatment for B12 deficiency depends on the severity of symptoms and the cause of the deficiency.
Initial Treatment (for severe deficiency or neurological symptoms)
- Intramuscular Injections: The standard initial treatment for severe B12 deficiency, especially in cases with neurological symptoms or malabsorption issues like pernicious anemia, is a series of injections. This ensures the vitamin bypasses the digestive system and is absorbed directly into the bloodstream. These injections are typically given daily or every other day for a period of one to two weeks to rapidly replenish B12 stores.
Maintenance Treatment
- Intramuscular Injections: For conditions that impair B12 absorption permanently (such as pernicious anemia, atrophic gastritis, or after gastric surgery), lifelong injections are necessary. The frequency is typically reduced to once every 1-3 months, depending on the patient’s needs and the country’s guidelines.
- High-Dose Oral Supplements: For some individuals, particularly those with a milder deficiency or those who have corrected the underlying cause (e.g., a short-term dietary issue), high-dose oral supplements (1000-2000 mcg daily) can be effective. A small percentage of oral B12 can be absorbed without intrinsic factor, making this a viable option for some patients, even those with pernicious anemia. However, it requires strict adherence and is not suitable for those with severe symptoms.
- Dietary Changes: If the deficiency is purely due to dietary choices (e.g., a vegan diet), increasing intake of B12-fortified foods (like cereals, plant milks, and nutritional yeast) or taking regular oral supplements can be sufficient.
Treatment for Folate Deficiency
Oral Folic Acid Supplements
The primary treatment for folate deficiency is the use of oral folic acid tablets. These are generally very effective, as the body can absorb this synthetic form of the vitamin easily. The typical dosage is 1-5 mg daily, for several months, until blood folate levels return to normal and the anemia has resolved.
Addressing the Underlying Cause
Just as with B12 deficiency, it is crucial to address the cause of the folate deficiency. This may involve:
- Improving dietary habits.
- Managing underlying gastrointestinal diseases like celiac disease or Crohn’s disease.
- Changing medications that interfere with folate metabolism, in consultation with a healthcare provider.
Important Precaution: A critical aspect of treatment is to never give folate supplements alone to a patient with megaloblastic anemia until a vitamin B12 deficiency has been ruled out. As discussed previously, folate can temporarily correct the anemia but will not stop the progressive, and potentially irreversible, neurological damage caused by an underlying B12 deficiency.
Management and Monitoring
- Patient Education: Educating patients on the importance of long-term adherence to their treatment plan and dietary recommendations is a cornerstone of effective management. This is especially true for those with chronic conditions like pernicious anemia who will require lifelong therapy.
- Regular Follow-up: Regardless of the treatment, regular follow-up with a healthcare provider is essential. This includes repeat blood tests (CBC, B12, and folate levels) to ensure the treatment is effective and that the deficiency does not recur.
- Prognosis: The prognosis for megaloblastic anemia is generally excellent once the deficiency is diagnosed and treated correctly. However, if neurological damage has occurred due to a long-standing B12 deficiency, some symptoms may not be fully reversible.
Frequently Asked Questions (FAQs)
What is the difference between megaloblastic anemia and non-megaloblastic anemia?
Both megaloblastic and non-megaloblastic anemia are forms of macrocytic anemia, meaning they are characterized by abnormally large red blood cells (macrocytes). This is identified by a Mean Corpuscular Volume (MCV) greater than 100 fL. The key difference lies in the underlying cause and the specific appearance of the cells on a blood smear.
Megaloblastic vs. Non-Megaloblastic Anemia
| Feature | Megaloblastic Anemia | Non-Megaloblastic Anemia |
| Primary Cause | Impaired DNA synthesis in bone marrow. | Diverse causes, not directly related to a DNA synthesis defect. |
| Underlying Etiology | Deficiency of Vitamin B12 or Folate. | Chronic Alcoholism, liver disease, hypothyroidism, myelodysplastic syndromes (MDS), or increased red blood cell destruction (hemolysis). |
| Red Blood Cell Appearance | Large, oval-shaped red blood cells (macro-ovalocytes). | Large, but round-shaped red blood cells. |
| Key Blood Smear Finding | Hypersegmented neutrophils (a type of white blood cell with more than 5 nuclear segments). This is a hallmark feature. | Absence of hypersegmented neutrophils. |
| Metabolite Levels | Elevated homocysteine (HCY). Elevated methylmalonic acid (MMA) with B12 deficiency. Normal MMA with folate deficiency. | Normal HCY and MMA levels, unless there is a co-existing condition. |
| Neurological Symptoms | Common, especially with B12 deficiency (e.g., paresthesias, balance issues, cognitive decline). | Rare, unless related to the underlying cause (e.g., alcoholism). |
Is megaloblastic anemia a serious illness?
While megaloblastic anemia itself isn’t inherently a life-threatening condition, its seriousness depends on the severity and the underlying cause.
Left untreated, it can lead to serious complications like heart problems, neurological damage (especially with B12 deficiency), and pregnancy complications. However, the good news is that early diagnosis and treatment are highly effective. With proper management, most people experiencing megaloblastic anemia recover fully and don’t experience long-term effects.
How common is megaloblastic anemia?
Overall, it’s not considered rare. Estimates suggest less than 1,000 people in the US have the disease, but this likely underestimates the true number due to undiagnosed cases.
- Macrocytosis (large red blood cells), a common sign, affects 2-4% of the population, with 60% having anemia, often due to folate or B12 deficiency.
- Vitamin B12 deficiency becomes more prevalent in older adults, with around 1 in 7,500 people developing pernicious anemia each year.
- Folate deficiency is also common, particularly among pregnant women, individuals with dietary limitations, and those with absorption issues.
How can I reduce my risk of developing megaloblastic anemia?
Here are some steps you can take to reduce your risk of developing megaloblastic anemia:
Diet
- Eat a balanced diet rich in vitamin B12 and folate: Include foods like:
- Vitamin B12: Animal products like meat, poultry, fish, eggs, and dairy. Fortified cereals and nutritional yeast can also be good sources.
- Folate: Leafy green vegetables, legumes, fruits (especially citrus fruits), fortified cereals and grains, and nuts and seeds.
- Consider supplementation: If you’re concerned about your intake or have risk factors like strict vegetarian/vegan diets, malabsorption issues, or pregnancy, talk to your doctor about vitamin B12 and/or folate supplements.
Lifestyle
- Moderate alcohol intake: Excessive alcohol consumption can interfere with vitamin B12 absorption.
- Avoid smoking: Smoking can increase your vitamin B12 requirements.
- Manage underlying conditions: If you have any conditions that affect nutrient absorption, like celiac disease or inflammatory bowel disease, work with your doctor to manage them effectively.
What is the difference between the terms megaloblastic and macrocytic?
The terms “megaloblastic” and “macrocytic” are related but distinct when describing red blood cells and anemia. Megaloblastic refers to the abnormal size and development of red blood cell precursors in the bone marrow called megaloblasts. These cells are larger than normal but functionally impaired, leading to ineffective red blood cell production. Not all macrocytic anemias are megaloblastic. Other causes can lead to large red blood cells without involving megaloblasts, such as liver disease or certain medications. Megaloblasts are associated with various causes, including vitamin B12 and folate deficiencies, genetic disorders, and certain medications.
Macrocytic simply describes red blood cells that are larger than normal. This is measured by the Mean Corpuscular Volume (MCV) in a blood test. It can be a sign of megaloblastic anemia, but not always. Other conditions can also cause macrocytosis without involving megaloblasts.
In summary, megaloblastic refers to the type of cell development, while macrocytic refers to the size of the red blood cells. Megaloblastic anemia is a specific type of macrocytic anemia caused by abnormal precursor cells. Not all macrocytic anemias are megaloblastic, and having macrocytic red blood cells requires further investigation for diagnosis.
How does vitamin B12 deficiency cause macrocytic anemia?
When your body lacks vitamin B12, it struggles to properly utilize B9 (folate) for DNA synthesis in developing red blood cells. This disrupts their maturation process, leading to the formation of abnormally large but dysfunctional cells called megaloblasts. These giants can’t properly divide or leave the bone marrow, resulting in fewer, oversized red blood cells in circulation – classic features of macrocytic anemia. This deficiency impacts oxygen delivery throughout your body, causing fatigue, weakness, and other symptoms.
What are the potential complications of untreated megaloblastic anemia?
Untreated megaloblastic anemia can lead to several potential complications, some of which can be very serious.
Neurological Issues
- Vitamin B12 deficiency, a major cause of megaloblastic anemia, can significantly impact the nervous system. Prolonged deficiency can lead to nerve damage, causing symptoms like:
- Numbness and tingling in the hands and feet.
- Muscle weakness and difficulty walking.
- Vision problems, including blurred vision and double vision.
- Cognitive decline, confusion, and memory problems.
- In severe cases, dementia may develop.
Heart Problems
- When your body lacks healthy red blood cells to carry oxygen efficiently, your heart has to work harder to pump blood and deliver oxygen to your tissues. This can lead to:
- Enlarged heart (cardiomegaly).
- Heart failure, where the heart struggles to pump enough blood to meet the body’s needs.
- Increased risk of heart attack.
Other Complications
- Pregnancy complications: Untreated megaloblastic anemia during pregnancy can increase the risk of:
- Neural tube defects in the baby.
- Premature birth.
- Low birth weight.
- Bone problems: Folate deficiency, another cause of megaloblastic anemia, can weaken bones and increase the risk of fractures.
- Psychological problems: Fatigue and other symptoms of anemia can lead to depression and anxiety.
What dietary changes can help prevent or manage megaloblastic anemia?
Here are some dietary changes that can help prevent or manage megaloblastic anemia by increasing your intake of vitamin B12 and folate.
For Vitamin B12
Animal products: These are the most reliable sources of vitamin B12. Include them in your diet regularly.
- Meat: Beef, lamb, pork, chicken, and turkey.
- Poultry: Chicken, duck, and goose.
- Fish and shellfish: Salmon, tuna, mackerel, sardines, clams, and oysters.
- Eggs: A good source of both B12 and folate.
- Dairy products: Milk, cheese, and yogurt.
For Folate
- Leafy green vegetables: These are packed with folate. Aim for at least one serving per day, such as spinach, kale, collard greens, romaine lettuce, and Swiss chard.
- Other vegetables: Broccoli, asparagus, Brussels sprouts, beets, and okra are also good sources.
- Fruits: Citrus fruits like oranges, grapefruit, and tangerines are good sources of folate. Other fruits like papaya, mango, and kiwi also contain some folate.
- Legumes: Beans, lentils, and chickpeas are excellent sources of folate and other nutrients.
- Fortified foods: Many breakfast cereals, grains, and nutritional yeast are fortified with folic acid, the synthetic form of folate.
Additional tips
- Cook food gently: Overcooking can destroy folate, so steam, stir-fry, or microwave vegetables instead of boiling them.
- Eat fortified foods in moderation: While helpful, don’t rely solely on fortified foods as they may not offer the same range of nutrients as naturally folate-rich options.
- Consider supplements: If you have difficulty meeting your needs through diet alone, talk to your doctor about vitamin B12 or folate supplements.
What other health conditions can be mistaken for megaloblastic anemia?
Several health conditions can sometimes be mistaken for megaloblastic anemia due to overlapping symptoms or similar blood test findings.
- Iron deficiency anemia: This is the most common type of anemia and also presents with fatigue, weakness, and pale skin. However, unlike megaloblastic anemia, red blood cells in iron deficiency are smaller and paler (microcytic). Additional tests like iron levels and reticulocyte count can help differentiate between the two.
- Other macrocytic anemias: Not all macrocytic anemias (large red blood cells) are megaloblastic. Conditions like:
- Liver disease: Can affect red blood cell production and lead to macrocytosis without involving abnormal precursors.
- Alcoholism: May interfere with vitamin B12 absorption and cause macrocytosis.
- Certain medications: Can have a similar effect on red blood cell size.
- Other conditions with similar symptoms: Fatigue, a common symptom of megaloblastic anemia, can also occur in various conditions like:
- Thyroid disorders: Hypothyroidism can cause fatigue and other symptoms that might be mistaken for anemia.
- Chronic fatigue syndrome: Features persistent fatigue without a clear medical cause.
- Nutritional deficiencies: Deficiencies in other vitamins and minerals besides B12 and folate can also cause fatigue and weakness.
Differentiating diagnosis
- A thorough medical history, physical examination, and blood tests are crucial for accurate diagnosis.
- Red blood cell size, shape, and number, along with vitamin B12 and folate levels should be assessed.
- In some cases, bone marrow examination might be necessary to determine the exact cause of the anemia.
How does megaloblastic anemia affect different age groups?
Megaloblastic anemia can affect individuals across all age groups, but the specific impacts and risk factors can vary depending on age.
Children
- Causes: Most common causes of megaloblastic anemia in children include:
- Dietary deficiencies: Especially in strict vegetarians/vegans or children with limited dietary variety, leading to B12 or folate deficiency.
- Congenital conditions: Rare genetic disorders affecting vitamin B12 metabolism or folate absorption can cause megaloblastic anemia.
- Impacts: Growth delays, developmental problems, fatigue, paleness, and poor appetite. Early diagnosis and treatment are crucial to minimize impact on growth and development.
- Additional concerns: Children with underlying medical conditions like celiac disease or inflammatory bowel disease are at increased risk due to potential nutrient absorption issues.
Adults
- Causes: Most common causes of megaloblastic anemia include:
- Vitamin B12 deficiency: Often due to dietary limitations (vegans, older adults), malabsorption issues, or medications interfering with B12 absorption.
- Folate deficiency: Less common but can occur due to dietary limitations, malabsorption, or pregnancy/breastfeeding.
- Impacts: Fatigue, weakness, shortness of breath, pale skin, and tingling/numbness in extremities. Neurological problems can develop with prolonged B12 deficiency.
- Additional concerns: Older adults are more prone to B12 deficiency due to decreased stomach acid production and potential changes in dietary habits.
Elderly
- Causes: Similar to adults, with additional factors like:
- Increased prevalence of age-related conditions: Malabsorption issues like celiac disease or medications affecting B12 absorption become more common.
- Cognitive decline: May make individuals less aware of dietary needs or hinder medication adherence.
- Impacts: Similar to adults, with potentially more severe consequences due to underlying health conditions.
- Increased risk of falls: Due to fatigue and weakness.
- Cognitive decline: May be exacerbated by B12 deficiency.
- Additional concerns: Regular monitoring and personalized management are crucial to prevent complications and ensure overall well-being.
What are the specific risks and considerations for pregnant women with megaloblastic anemia?
Pregnant women with megaloblastic anemia face unique risks and considerations compared to the general population.
Risks to the mother
- Increased risk of complications: Untreated megaloblastic anemia can lead to
- Preeclampsia: A serious pregnancy complication with high blood pressure and potential organ damage.
- Preterm birth: Delivering the baby before 37 weeks.
- Low birth weight: The baby being smaller than expected for gestational age.
- Postpartum hemorrhage: Excessive bleeding after childbirth.
- Neurological damage: Vitamin B12 deficiency, a major cause, can cause nerve damage if left untreated. This can lead to weakness, numbness, and cognitive problems.
Risks to the baby
- Neural tube defects: Folate deficiency, another cause, significantly increases the risk of these birth defects affecting the brain and spinal cord.
- Growth problems: The baby may not grow and develop as expected due to nutrient deficiencies.
- Increased risk of miscarriage: Studies suggest a potential link between untreated anemia and miscarriage, although more research is needed.
Considerations for management
- Early diagnosis and treatment are crucial: Prompt intervention minimizes risks to both mother and baby.
- Identifying the underlying cause: Addressing the root cause of the anemia, like B12 deficiency or dietary limitations, is essential.
- Prenatal vitamins: Folic acid supplementation is standard for all pregnant women, but those with anemia may need additional vitamin B12 supplements.
- Regular monitoring: Close monitoring of blood counts, vitamin levels, and fetal development is critical.
- Multidisciplinary care: Collaboration between obstetricians, hematologists, and nutritionists is often recommended for optimal management.
Are there any genetic factors that contribute to the development of megaloblastic anemia?
Inborn errors of metabolism: These are genetic mutations affecting enzymes involved in vitamin B12 or folate metabolism. This can lead to impaired absorption, utilization, or transport of these vitamins, ultimately causing megaloblastic anemia. Examples include:
- Megaloblastic anemia type I (cblC/cblD deficiency): Affects the conversion of vitamin B12 to its active form.
- Megaloblastic anemia type II (cblB deficiency): Affects the transport of vitamin B12 inside cells.
- Congenital folate malabsorption: Impairs the absorption of folate from the gut.
Fanconi anemia: This genetic disorder affects DNA repair mechanisms and can lead to bone marrow problems, including megaloblastic anemia initially before presenting with aplastic anemia.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Brosh RM Jr, Bellani M, Liu Y, Seidman MM. Fanconi Anemia: A DNA repair disorder characterized by accelerated decline of the hematopoietic stem cell compartment and other features of aging. Ageing Res Rev. 2017 Jan;33:67-75. doi: 10.1016/j.arr.2016.05.005. Epub 2016 May 17. PMID: 27223997; PMCID: PMC5114166.
- Hariz A, Bhattacharya PT. Megaloblastic Anemia. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537254/
- Aslinia, F., Mazza, J. J., & Yale, S. H. (2006). Megaloblastic anemia and other causes of macrocytosis. Clinical medicine & research, 4(3), 236–241. https://doi.org/10.3121/cmr.4.3.236
- Moulin, V., Grandoni, F., Castioni, J., & Lu, H. (2018). Pancytopenia in an adult patient with thiamine-responsive megaloblastic anaemia. BMJ case reports, 2018, bcr2018225035. https://doi.org/10.1136/bcr-2018-225035