TL;DR
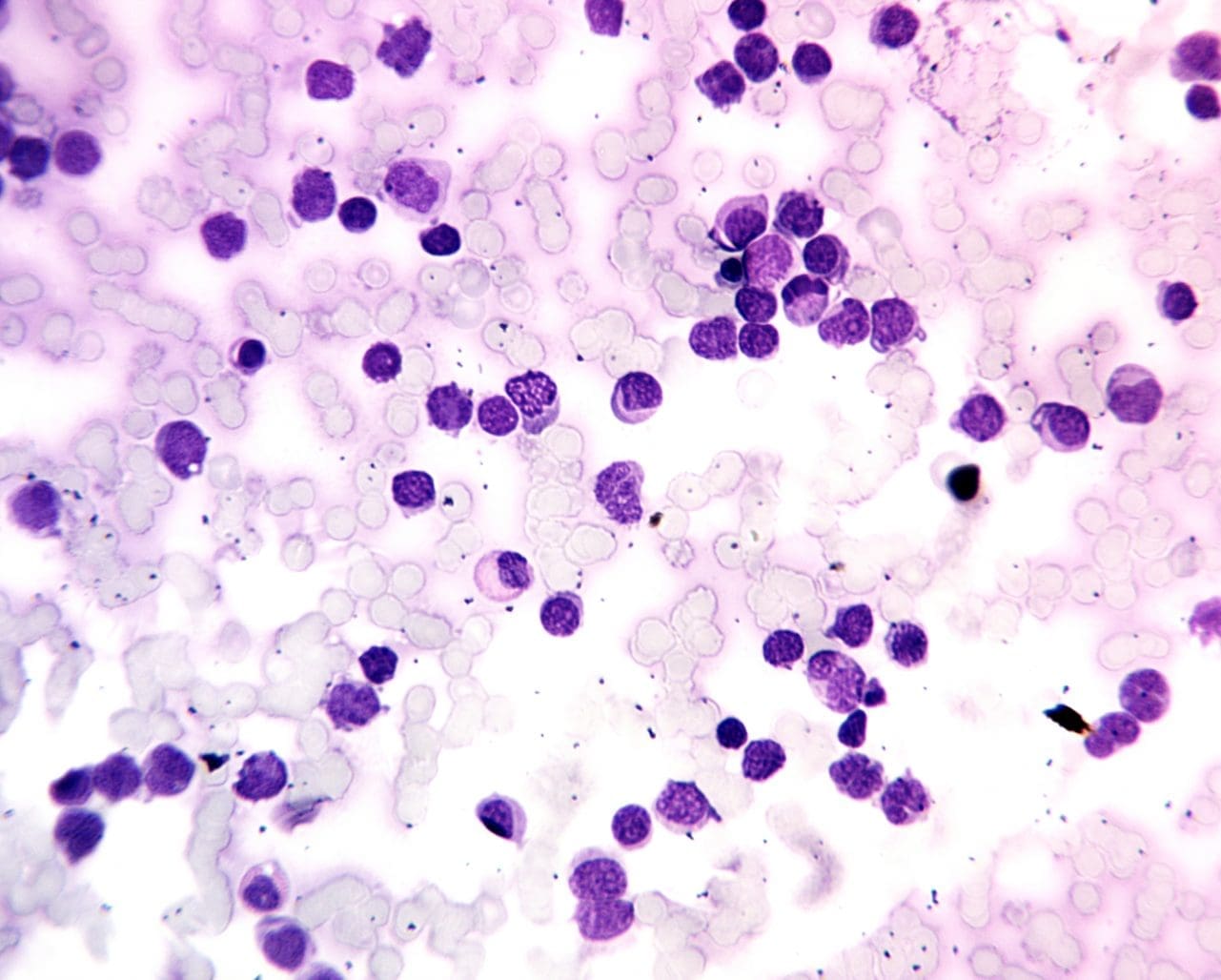
Acute leukemia is an aggressive hematologic malignancy characterized by the rapid, uncontrolled proliferation and accumulation of immature hematopoietic progenitor cells (blasts) in the bone marrow and peripheral blood, leading to the suppression of normal hematopoiesis and subsequent bone marrow failure.
- Pathophysiology ▾: Acute leukemia is a malignancy characterized by the uncontrolled, clonal proliferation of immature hematopoietic progenitor cells (blasts) in the bone marrow due to genetic mutations, leading to the suppression of normal blood cell production and resultant bone marrow failure.
- Risk Factors ▾: Major risk factors of acute leukemia include a history of Myelodysplastic Syndromes (MDS), certain genetic syndromes (like Down syndrome), prior exposure to intensive chemotherapy or radiation, and heavy tobacco use.
- Symptoms ▾: Clinical presentation of acute leukemia arises from bone marrow failure, resulting in non-specific symptoms such as fatigue (anemia), recurrent infections (neutropenia), and bleeding/bruising (thrombocytopenia), along with possible organ infiltration (ALL) and bone pain.
- Laboratory Investigations ▾: Diagnosis of acute leukemia is confirmed by the presence of ≥20% blasts in the bone marrow or blood (WHO criteria), established through CBC, peripheral smear, Immunophenotyping (for lineage), and Cytogenetic/Molecular testing (for risk stratification).
- Treatment ▾: Therapy of acute leukemia is highly stratified based on patient fitness and genetics, utilizing intensive multi-agent chemotherapy (“7+3”) ± targeted agents (for fit AML), Venetoclax/HMA (for unfit AML), or a multi−phase regimen including CNS prophylaxis and prolonged maintenance (for ALL) often culminating in HSCT for high-risk disease.
*Click ▾ for more information
Introduction
Acute leukemia represents a group of aggressive hematologic malignancies characterized by the rapid, uncontrolled proliferation and accumulation of immature hematopoietic progenitor cells (blasts) in the bone marrow and peripheral blood. This malignant expansion suppresses normal hematopoiesis, leading to bone marrow failure and its resultant clinical sequelae: anemia, infection, and hemorrhage.
Acute leukemia is classified into two main types: acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL). AML, accounting for about 80% of acute leukemia cases, affects primarily adults, while ALL is more prevalent among children and adolescents. The distinction between AML and ALL lies in the type of immature blood cell involved. AML originates from myeloid cells, the precursors of red blood cells, platelets, and some white blood cells, while ALL stems from lymphoid cells, the precursors of lymphocytes, a type of white blood cell.
The rapid progression of acute leukemia sets it apart from chronic leukemias, which typically progress slowly over years. In acute leukemia, the abnormal blasts accumulate rapidly in the bone marrow, the blood cell factory, and spill over into the bloodstream. This rapid growth and spread lead to the acute presentation of symptoms and the urgent need for treatment.
Pathophysiology of Acute Leukemia
Both AML and ALL arise from acquired somatic genetic mutations that disrupt the delicate balance of proliferation, differentiation, and apoptosis within the hematopoietic stem and progenitor cell compartment. These mutations typically affect two key classes of genes.
- Class I Mutations (Proliferation): Confer a growth and/or survival advantage (e.g., FLT3 internal tandem duplications (ITD), KIT, RAS).
- Class II Mutations (Differentiation): Impair the normal differentiation of hematopoietic progenitors (e.g., RUNX1, CEBPA, PML-RARA fusion).
The combination of mutations typically leads to a “two-hit” model, resulting in a clonal population of blasts arrested at an early stage of development.
Acute Myeloid Leukemia (AML) Pathogenesis
AML originates from myeloid progenitor cells. Its pathogenesis is highly heterogeneous, driven by an array of chromosomal aberrations and molecular mutations that affect transcription factors or signaling pathways.
- Key Translocations: Recurrent genetic abnormalities often define specific AML subsets with distinct prognoses. Examples include t(8;21), inv(16), and t(15;17) (Acute Promyelocytic Leukemia, APL).
- Molecular Mutations: Mutations in NPM1 (most common), FLT3, and CEBPA are critical for risk stratification and therapeutic selection. FLT3-ITD, in particular, is associated with a high relapse risk.
Acute Lymphoblastic Leukemia (ALL) Pathogenesis
ALL arises from lymphoid progenitor cells—either B-cell (B-ALL, ≈80%) or T-cell (T-ALL, ≈20%). It is the most common pediatric malignancy but also occurs in adults, where outcomes are generally less favorable.
- Key Translocations: The t(9;22) translocation, resulting in the BCR-ABL1 fusion gene (Philadelphia chromosome-positive, Ph+), is a major driver in adult B-ALL and confers a poor prognosis without targeted therapy (Tyrosine Kinase Inhibitors, TKIs). Other significant abnormalities include t(12;21) (favorable) and KMT2A (formerly MLL) rearrangements (variable prognosis).
- Genetic Risk Subgroups: Recent classifications have identified high-risk subgroups like Ph-like ALL, which shares a gene expression profile with Ph+ ALL but is driven by alternative kinase-activating lesions (e.g., CRLF2 overexpression).
Risk Factors of Acute Leukemia
Acute leukemia is caused by genetic mutations in bone marrow cells. While the exact cause is often unknown, a number of risk factors can increase an individual’s likelihood of developing the disease. These factors are typically grouped into four main categories.
Prior Medical Treatments and Conditions
The risk of acute leukemia is notably increased in people who have previously been treated for other cancers or who have certain pre-existing blood disorders.
- Previous Chemotherapy or Radiation Therapy: Treatment for other cancers with certain types of chemotherapy drugs (like alkylating agents or topoisomerase II inhibitors) or high-dose radiation can damage bone marrow cells, leading to a condition called therapy-related AML or ALL years later.
- Pre-leukemic Blood Disorders: Individuals with certain chronic blood disorders have an increased risk of AML. These include:
- Myelodysplastic Syndromes (MDS): A disorder where the bone marrow produces abnormal blood cells. MDS can often transform into AML.
- Myeloproliferative Neoplasms (MPNs): Disorders like polycythemia vera or essential thrombocythemia.
- Immunosuppression: Recipients of organ transplants who are on long-term immune-suppressing drugs have a slightly increased risk of certain cancers, including ALL.
Genetic Syndromes and Inherited Factors
Certain congenital or inherited genetic conditions significantly increase acute leukemia risk.
- Genetic Syndromes:
- Down Syndrome (Trisomy 21): The most common genetic syndrome associated with an elevated risk of both ALL and AML, particularly in childhood.
- Fanconi Anemia, Bloom Syndrome, Ataxia-telangiectasia, Li-Fraumeni Syndrome, and Shwachman-Diamond Syndrome are among other rare inherited disorders that increase the risk.
- Family History: While most cases are not hereditary, having a first-degree relative (parent or sibling) with acute leukemia, especially an identical twin who develops ALL early in life, slightly increases risk.
Environmental and Lifestyle Exposures
Exposure to certain carcinogens in the environment or through lifestyle choices is a key risk factor for acute leukemia, particularly for AML.
- Smoking: This is the only proven lifestyle-related risk factor for AML. Cancer-causing substances in tobacco smoke (like benzene) enter the bloodstream and can damage the DNA in bone marrow cells.
- Chemical Exposure: Long-term, high-level exposure to certain industrial chemicals, particularly benzene (found in gasoline, motor vehicle exhaust, and some industrial solvents), is a known risk factor for AML.
- High-Dose Radiation Exposure: Exposure to very high levels of radiation, such as from nuclear accidents or atomic bomb blasts, significantly increases the risk of both AML and ALL. The risk from low-level radiation (like diagnostic X-rays or CT scans) is not clearly established but is generally considered very low.
Demographic Factors
- Age: The incidence of acute leukemia is bimodal:
- ALL is most common in young children (under age 5) and then again in adults over 50.
- AML is primarily a disease of older adults, with the average age of diagnosis being around 65.
- Gender: Most types of acute leukemia are slightly more common in males than in females.
- Race/Ethnicity: ALL is slightly more common in white people than in black people.
Sign and Symptoms of Acute Leukemia
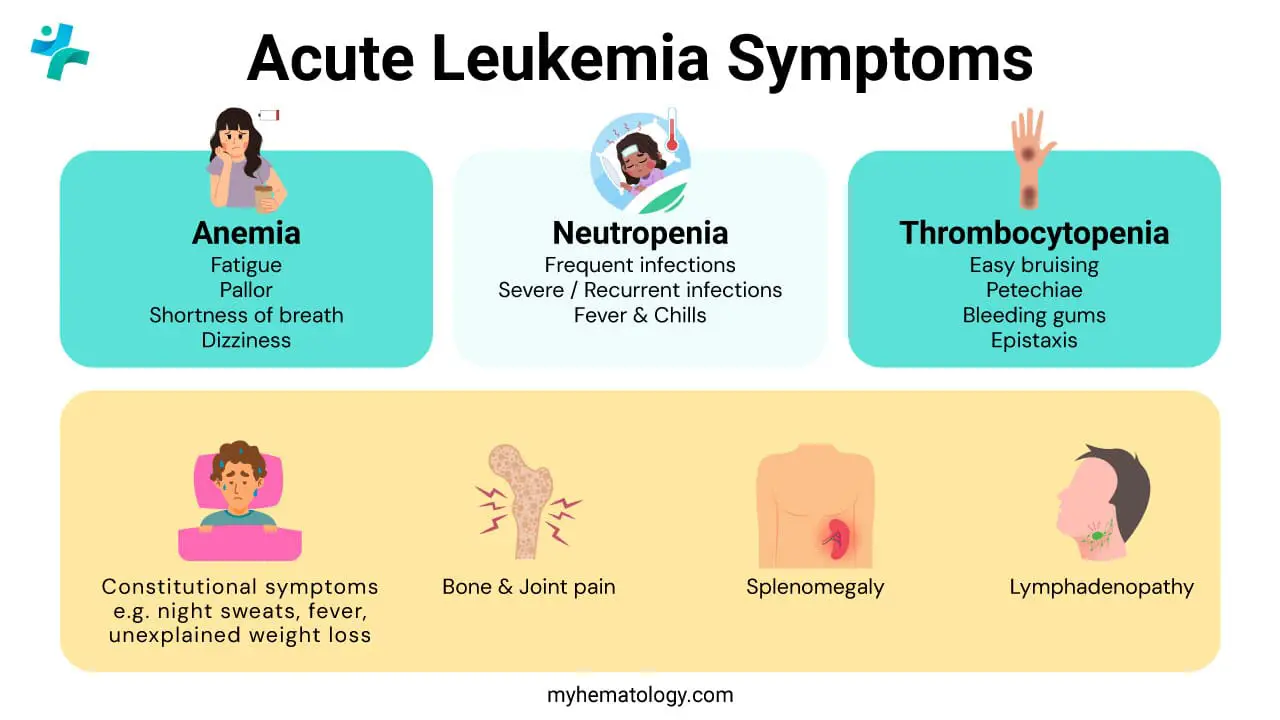
Acute leukemia develops very rapidly, and its symptoms tend to appear and worsen quickly, usually over days to a few weeks. The signs and symptoms of acute leukemia are primarily caused by the rapid, uncontrolled growth of abnormal, immature white blood cells (blasts) in the bone marrow, which crowds out the production of healthy blood cells (red blood cells, normal white blood cells, and platelets).
Symptoms due to Anemia (Low Red Blood Cells)
A low number of red blood cells (anemia) results in a reduced ability to carry oxygen throughout the body.
- Fatigue and Weakness: Profound tiredness that doesn’t improve with rest.
- Pallor (Pale Skin): A noticeable paleness or “washed-out” appearance due to the lack of oxygen-carrying cells.
- Shortness of Breath (Dyspnea): Difficulty breathing, especially with physical activity.
- Dizziness or Lightheadedness.
- Headaches (less common but possible).
Symptoms due to Thrombocytopenia (Low Platelets)
A low number of platelets (thrombocytopenia) impairs the blood’s ability to clot effectively, leading to easy or excessive bleeding.
- Easy Bruising: Bruising more easily than usual, or bruises appearing without a clear injury.
- Petechiae: Tiny, flat, red or purple spots on the skin, often clustered on the legs, caused by bleeding from small capillaries under the skin.
- Abnormal Bleeding:
- Frequent or severe nosebleeds.
- Bleeding gums.
- Heavy or prolonged menstrual bleeding (menorrhagia) in women.
Symptoms due to Neutropenia (Low Functional White Blood Cells)
While the overall white blood cell count may be high (leukocytosis), the number of functional infection-fighting cells (neutrophils) is low, leading to a weakened immune system.
- Frequent Infections: Getting infections more often than usual.
- Severe or Recurrent Infections: Infections that are more severe, difficult to treat, or keep coming back.
- Fever and Chills: Often associated with an infection, but the fever can also be directly caused by the leukemia itself.
Other General or Specific Symptoms
- Bone and Joint Pain: Caused by the buildup of leukemia cells (blasts) within the bone marrow, most common in children with ALL. This can sometimes cause limping or reluctance to walk.
- Unexplained Weight Loss and Loss of Appetite.
- Night Sweats and Fever (known as B symptoms).
- Swollen Lymph Nodes (Lymphadenopathy): Painless lumps in the neck, armpit, or groin (more common in ALL).
- Abdominal Fullness or Pain: Caused by the enlargement of the liver and/or spleen (hepatosplenomegaly) as leukemia cells collect in these organs. This can lead to feeling full after only eating a small amount.
- Neurological Symptoms (Rare but Serious): If the leukemia spreads to the central nervous system (brain and spinal cord), it can cause:
- Severe headaches.
- Blurred vision.
- Nausea and vomiting.
- Seizures or trouble with balance.
- Leukostasis (Medical Emergency): A very high white blood cell count can cause blood vessels to clog, potentially leading to breathing problems and neurological symptoms.
| Feature | Acute Lymphoblastic Leukemia (ALL) | Acute Myeloid Leukemia (AML) |
| Origin Cell | Immature lymphoid cells (lymphoblasts) | Immature myeloid cells (myeloblasts) |
| Organ Involvement | More commonly spreads to the lymphoid system. | More commonly associated with gum and skin tissue infiltration. |
| More Common Signs | Swollen lymph nodes (neck, groin, armpits) and a large spleen/liver (hepatosplenomegaly) are often more prominent. Testicular swelling (in males) and signs of Central Nervous System (CNS) involvement (e.g., headaches, vomiting) are also more likely. | Gum swelling/hypertrophy (gums look overgrown and bleed easily) and skin lesions (small bumps, sometimes called leukemia cutis) are more characteristic. |
| Patient Age | Most common acute leukemia in children (peak age 2–5). | Most common acute leukemia in adults (median age is late 60s). |
Laboratory Investigations for Acute Leukemia
In the realm of acute leukemia, where aberrant blood cells wreak havoc, laboratory investigations serve as invaluable tools for diagnosis, monitoring, and guiding treatment decisions. These investigations provide a comprehensive view into the intricacies of the disease, shedding light on the type, extent, and progression of acute leukemia.
Complete Blood Count (CBC)
Common findings in acute leukemia include anemia (low RBCs/hemoglobin) and thrombocytopenia (low platelets). WBC count can be low, normal, or very high, but the proportion of functional mature cells (like neutrophils) is usually reduced (neutropenia).
Peripheral Blood Smear (PBS)
The most critical finding of acute leukemia is the presence of blasts (immature white blood cells) in the circulating blood, which are normally confined to the bone marrow.
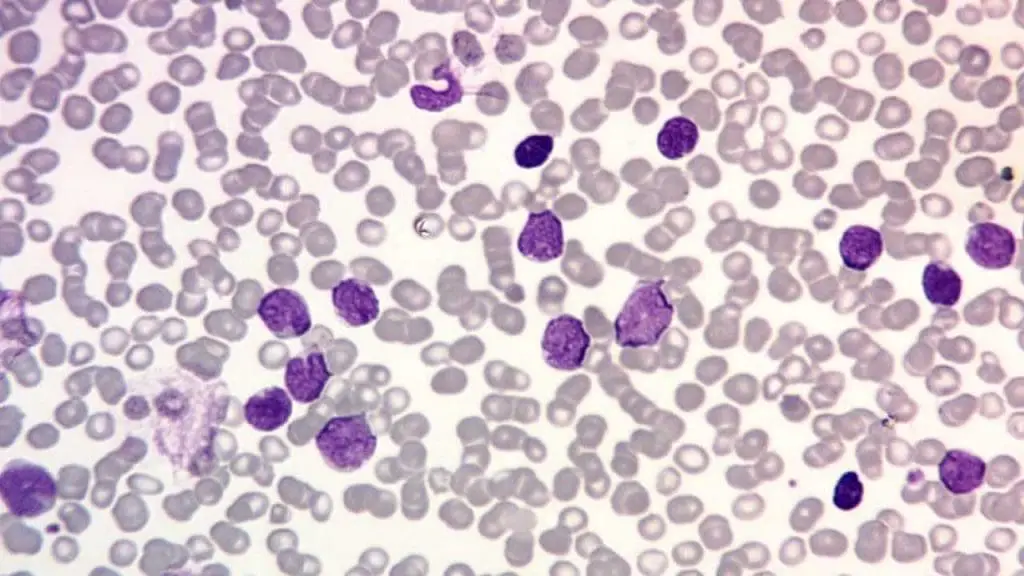
Bone Marrow Aspiration and Biopsy
Diagnosis of acute leukemia is confirmed if ≥20% of the cells in the bone marrow are blasts (according to WHO criteria, though certain genetic abnormalities can confirm a diagnosis even with a lower blast count).
Immunophenotyping (Flow Cytometry)
This is the gold standard for determining the cell lineage (myeloid vs. lymphoid) and subtype.
- ALL Diagnosis: Blasts express lymphoid markers (e.g., CD19, CD22, CD10 for B-ALL; CD3, CD7 for T-ALL).
- AML Diagnosis: Blasts express myeloid markers (e.g., CD13, CD33, CD117, and intracellular Myeloperoxidase (MPO)).
Immunophenotyping is also crucial for detecting minimal residual disease (MRD) during and after treatment.
Cytogenetic Analysis
Acute leukemia often arises from genetic abnormalities that disrupt the normal processes of cell growth and differentiation. Cytogenetic analysis, utilizing specialized techniques like karyotyping and fluorescence in situ hybridization (FISH), allows for the detection of these chromosomal aberrations.
Identifying these genetic abnormalities has significant implications for diagnosis, risk stratification, and treatment selection. For instance, certain genetic alterations, such as the Philadelphia chromosome (Ph+) in acute myeloid leukemia, are associated with specific treatment approaches and prognostic factors.
Major Cytogenetic Subtypes in Acute Leukemia
| Leukemia Type | Cytogenetic Abnormality (Fusion Gene) | Characteristics & Prognosis |
| Acute Myeloid Leukemia (AML) | t(8;21)(q22;q22.1)/RUNX1-RUNX1T1 | Favorable Prognosis. Often associated with AML M2 (FAB classification) and typically responds well to standard chemotherapy. |
| inv(16)(p13.1q22)/t(16;16)/CBFB-MYH11 | Favorable Prognosis. Often associated with AML M4 with eosinophilia (M4Eo). Responds well to standard therapy. | |
| t(15;17)(q24.1;q24.1)/PML-RARA | Favorable Prognosis. Defines Acute Promyelocytic Leukemia (APL). Highly curable with All-Trans Retinoic Acid (ATRA) and arsenic trioxide (targeted therapy). | |
| Monosomy 5 or del(5q) | Adverse/Poor Prognosis. Associated with a complex karyotype and resistance to chemotherapy. | |
| Monosomy 7 or del(7q) | Adverse/Poor Prognosis. Often seen in therapy-related AML (t-AML) and carries a high risk of relapse. | |
| Complex Karyotype (3 or more abnormalities) | Adverse/Poor Prognosis. Very high risk of resistance and poor survival. | |
| Acute Lymphoblastic Leukemia (ALL) | t(9;22)(q34.1;q11.2)/BCR-ABL1 (Philadelphia Chromosome) | Adverse/Poor Prognosis (historically). Now treatable with Tyrosine Kinase Inhibitors (TKIs), significantly improving outcomes. Common in adult ALL. |
| t(12;21)(p13.2;q22.1)/ETV6-RUNX1 | Favorable Prognosis. The most common translocation in childhood ALL, associated with a good response to therapy. Often cryptic (not visible on standard karyotype). | |
| KMT2A (formerly MLL) rearrangements | Adverse/Poor Prognosis. Most common in infants (<1 year old) and often associated with a very high WBC count. | |
| High Hyperdiploidy (51-65 chromosomes) | Favorable Prognosis (especially in children). | |
| Hypodiploidy (44 or fewer chromosomes) | Adverse/Poor Prognosis |
Monitoring Disease Progression
Laboratory investigations play a crucial role in monitoring the progression of acute leukemia and the response to treatment. Serial CBCs and bone marrow examinations are regularly performed to assess changes in blast count, peripheral blood cell counts, and the eradication of abnormal blasts.
These serial investigations provide valuable information for evaluating the effectiveness of treatment, identifying potential complications, and guiding treatment adjustments. Monitoring the presence of minimal residual disease (MRD), low levels of undetectable blasts, is crucial for assessing the risk of relapse and guiding long-term follow-up strategies.
Treatment and Management Approach for Acute Leukemia
The clinical management of acute leukemia is complex, typically involving intensive combination chemotherapy followed by consolidation and, potentially, hematopoietic stem cell transplantation (HSCT). Treatment goals include achieving complete remission (CR) and, increasingly, Minimal Residual Disease (MRD) negativity, a strong prognostic factor for relapse-free survival.
Acute Myeloid Leukemia (AML) Treatment
1. Induction Chemotherapy: The standard induction regimen for fit patients remains the “7+3” protocol (Cytarabine for 7 days, Daunorubicin or Idarubicin for 3 days).
- APL Exception: APL is treated as a distinct medical emergency with All-Trans Retinoic Acid (ATRA) combined with Arsenic Trioxide (ATO), resulting in a high cure rate.
2. Consolidation: Consolidation aims to eliminate residual disease. Options include high-dose cytarabine (HiDAC), or allogeneic HSCT, which offers the greatest anti-leukemic effect.
3. Targeted and Novel Therapies:
- FLT3 Inhibitors: Midostaurin and Gilteritinib are used in FLT3-mutated AML.
- IDH1/IDH2 Inhibitors: Ivosidenib and Enasidenib for respective mutations.
- Low-Intensity Regimens: Venetoclax (a BCL2 inhibitor) combined with Azacitidine is now the standard of care for many older or unfit patients, showing improved outcomes over hypomethylating agents (HMAs) alone.
Acute Lymphoblastic Leukemia (ALL) Treatment
ALL treatment typically involves a multi-phase, lengthy protocol lasting 2 to 3 years.
1. Induction, Consolidation, and Maintenance: Intensive, multi-agent chemotherapy forms the backbone of treatment (e.g., Vincristine, Corticosteroids, L-Asparaginase).
2. Central Nervous System (CNS) Prophylaxis: Intrathecal chemotherapy is mandatory due to the high risk of CNS involvement.
3. Targeted Therapy in Ph+ ALL: The addition of Tyrosine Kinase Inhibitors (TKIs), such as Imatinib or Dasatinib, to chemotherapy has revolutionized the prognosis for Ph+ ALL.
4. Immunotherapies and Novel Agents:
- Chimeric Antigen Receptor (CAR) T-cell Therapy: Tisagenlecleucel (CD19 targeting) is a significant advance for pediatric and young adult relapsed/refractory B-ALL.
- Blinatumomab (BiTE): A bispecific T-cell engager antibody used for B-ALL in the MRD-positive or relapsed/refractory setting.
- Inotuzumab Ozogamicin (Antibody-Drug Conjugate): Targets CD22.
Common Treatment Strategies for ALL and AML
| ALL | AML |
| Induction (e.g. Vincristine, Asparaginase, Dexamethasone ± Anthracycline ± TKI) ↓ CNS Prophylaxis (Intrathecal Methotrexate) ↓ Consolidation/Intensification (High-dose Cytarabine, Methotrexate, Cyclophosphamide, Etoposide, ± Immunotherapy) ↓ Risk Assessment/Transplant Decision ↓ Maintenance (2-3 years of oral 6-MP and Methotrexate, pulsed Vincristine/Steroids, ± TKI) | Intensive Track Induction: Standard: “7+3” (Cytarabine for 7 days + Anthracycline for 3 days) ↓ Consolidation (High-dose Cytarabine or HSCT) Lower-Intensity Track First line (Venetoclax +Hypomethylating Agent) or Alternative / Targeted Options (Targeted Monotherapies or HMA Monotherapy) |
Supportive Care
Supportive care, a crucial component of acute leukemia management, focuses on alleviating symptoms, managing side effects, and addressing complications arising from the disease and its treatment. This encompasses a range of interventions, including blood transfusions to combat anemia, platelet transfusions to prevent bleeding, and administration of medications to manage infections and pain.
Supportive care also plays a significant role in addressing the psychological and emotional impact of acute leukemia. Providing emotional support, counseling, and psychosocial services can help patients and their families cope with the challenges and uncertainties associated with the disease.
Conclusion and Future Directions
Acute leukemia remains a therapeutic challenge, but advancements in molecular diagnostics and targeted therapy have significantly improved survival rates, particularly for children and younger adults. The future of acute leukemia management lies in refining risk-adapted therapies, integrating novel immunotherapies (like CAR-T cells and BiTEs) earlier in the treatment landscape, and developing effective strategies to overcome drug resistance and eradicate MRD. For medical and allied health professionals, staying current with ASH and EHA guidelines and understanding the molecular heterogeneity of these diseases is paramount to delivering optimal, personalized patient care.
Frequently Asked Questions (FAQs)
What is the primary difference between AML and ALL based on cell lineage?
AML originates from myeloid precursors (which give rise to red blood cells, platelets, and most white blood cells like neutrophils and monocytes), whereas ALL originates from lymphoid precursors (which give rise to B and T lymphocytes). This difference determines the immunophenotyping markers used for diagnosis and often the choice of induction chemotherapy agents.
What is Minimal Residual Disease (MRD) and why is it important?
MRD refers to the small number of residual leukemia cells that remain in the patient after therapy, despite achieving a morphologic complete remission (CR). MRD detection (using highly sensitive techniques like flow cytometry or NGS) is a powerful prognostic factor. MRD-negativity is strongly associated with better outcomes, and MRD-positivity often triggers preemptive therapy or dictates the need for HSCT.
When is Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) indicated for acute leukemia?
HSCT is typically reserved for patients deemed to be at high or intermediate risk of relapse based on their cytogenetics and molecular profile (particularly in AML) or those who have relapsed. It is generally performed during the first CR for high-risk patients to leverage the potent graft-versus-leukemia (GVL) effect, but requires a suitable donor and patient fitness.
Doesn’t BCR-ABL translocation cause Chronic Myeloid Leukemia (CML)?
The BCR−ABL1 translocation, which creates the Philadelphia chromosome (Ph+), is the hallmark genetic abnormality of Chronic Myeloid Leukemia (CML). However, this same translocation can also cause a specific, high-risk subset of Acute Lymphoblastic Leukemia (ALL).
BCR-ABL1 in CML vs. ALL
| Feature | Chronic Myeloid Leukemia (CML) | Acute Lymphoblastic Leukemia (ALL) |
| Prevalence of Ph+ | ≈95% of all CML cases. | ≈25−30% of adult ALL cases and ≈3−5% of pediatric ALL cases. |
| Disease Onset | Typically arises in a pluripotent hematopoietic stem cell, causing a slow, progressive overproduction of all myeloid lineage cells, but primarily mature or maturing granulocytes. | Typically arises in a lymphoid progenitor cell (usually B-cell lineage), causing a rapid, uncontrolled proliferation of immature lymphoblasts. |
| Pathophysiology | The BCR-ABL1 fusion protein drives constitutive activation of tyrosine kinase pathways, promoting cell survival and proliferation. In CML, this primarily affects the myeloid lineage leading to chronic accumulation of mature cells. | The same active BCR-ABL1 protein drives the proliferation and differentiation arrest, but it is believed to occur in a cell that is already committed to the lymphoid lineage or in a multipotent stem cell that preferentially develops a lymphoid phenotype. |
| Clinical Course | Starts in a chronic phase and can transform into an accelerated or blast crisis phase (which often looks like an acute leukemia). | Presents immediately as an acute disease characterized by bone marrow failure and rapid progression. |
| Prognosis/Treatment | Highly treatable with Tyrosine Kinase Inhibitors (TKIs), which have revolutionized survival. | Requires intensive multi-agent chemotherapy plus TKIs (e.g., Imatinib, Dasatinib) and is considered high-risk, though TKI use has greatly improved outcomes. |
The “Stem Cell Origin” Hypothesis
The difference in presentation (chronic vs. acute, myeloid vs. lymphoid) is thought to be related to the cellular context in which the BCR-ABL1 translocation occurs and the specific fusion protein variant formed:
- CML Variant (p210): The most common fusion is the p210 protein (encoded by the b2a2 or b3a2 transcripts). This variant is highly associated with CML and is believed to have occurred in a very early, pluripotent stem cell, giving rise to both myeloid and lymphoid abnormalities, but predominantly a CML phenotype.
- ALL Variant (p190): The p190 protein (encoded by the e1a2 transcript) is shorter and has higher tyrosine kinase activity. It is more frequently associated with Ph+ALL and is hypothesized to occur in a more lymphoid-committed progenitor, or its heightened activity is simply more conducive to rapid, acute lymphoblastic transformation.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
Glossary of Medical Terms
- Blast: An immature hematopoietic progenitor cell; in acute leukemia, these are the malignant, non-functional cells that accumulate.
- Pancytopenia: A condition in which the number of all three major blood cell lines (red blood cells, white blood cells, and platelets) is reduced.
- Immunophenotyping: The process of characterizing cells using antibodies against specific cell surface or intracellular markers, typically performed by flow cytometry, to determine cell lineage (myeloid vs. lymphoid).
- Cytogenetics: The study of chromosomes, used to detect large structural abnormalities like translocations, inversions, and deletions (e.g., t(9;22)).
- Allogeneic HSCT: Hematopoietic stem cell transplantation where the stem cells are donated by another person (the allograft), relying on the graft-versus-leukemia (GVL) effect.
References
- National Cancer Institute (2023). Acute Myeloid Leukemia Treatment (PDQ®) – Health Professional Version. National Institutes of Health.
- Pollyea DA, Altman JK, Assi R, Bixby D, Fathi AT, Foran JM, Gojo I, Hall AC, Jonas BA, Kishtagari A, Lancet J, Maness L, Mangan J, Mannis G, Marcucci G, Mims A, Moriarty K, Mustafa Ali M, Neff J, Nejati R, Olin R, Percival ME, Perl A, Przespolewski A, Rao D, Ravandi F, Shallis R, Shami PJ, Stein E, Stone RM, Sweet K, Thota S, Uy G, Vachhani P, Cassara CJ, Freedman-Cass DA, Stehman K. Acute Myeloid Leukemia, Version 3.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023 May;21(5):503-513. doi: 10.6004/jnccn.2023.0025. PMID: 37156478.
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022 Jul;36(7):1720-1748. doi: 10.1038/s41375-022-01620-2. Epub 2022 Jun 22. Erratum in: Leukemia. 2023 Sep;37(9):1944-1951. PMID: 35732829; PMCID: PMC9214472.
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023 Feb 1;108(2):306-307. doi: 10.3324/haematol.2022.280803. PMID: 36722401; PMCID: PMC9890027.
- Puckett Y, Chan O. Acute Lymphocytic Leukemia. [Updated 2023 Aug 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Vakiti A, Reynolds SB, Mewawalla P. Acute Myeloid Leukemia. [Updated 2024 Apr 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507875/
- Steinicke, T.L., Benfatto, S., Capilla-Guerra, M.R. et al. Rapid epigenomic classification of acute leukemia. Nat Genet (2025). https://doi.org/10.1038/s41588-025-02321-z