TL;DR
A transfusion reaction is defined as any adverse event or unintended response in a patient that is associated with the administration of whole blood or one of its components.
| Reaction Type | Primary Cause | Pathophysiology/Mechanism | Classic Symptoms | Initial Management / Treatment |
| Acute Reactions | ||||
| Acute Hemolytic Transfusion Reaction (AHTR) ▾ | Clerical error leading to ABO incompatibility. | Recipient IgM antibodies rapidly activate complement, leading to massive intravascular hemolysis. | Fever, chills, severe back/flank pain, hypotension, red/brown urine (hemoglobinuria). | STOP transfusion. Aggressive IV fluids and diuretics (furosemide) for renal protection. Treat shock. |
| Febrile Non-Hemolytic Transfusion Reaction (FNHTR) ▾ | Recipient antibodies reacting to donor WBC antigens (HLAs), or accumulated cytokines in stored blood. | Release of pyrogenic cytokines (IL−1, IL−6) in the recipient’s bloodstream. | Temperature rise of ≥1°C from baseline, chills, rigors (must exclude AHTR and TAS). | STOP transfusion. Give acetaminophen. Restart slowly if mild and symptoms resolve. Future units should be leukoreduced. |
| Mild Allergic Reaction ▾ | Recipient IgE antibodies reacting to donor plasma proteins. | Localized mast cell degranulation and histamine release. | Urticaria (hives), pruritus (itching) only. No respiratory symptoms. | STOP transfusion. Give antihistamines (diphenhydramine). May restart if symptoms resolve. |
| Anaphylactic Reaction | Usually due to recipient anti-IgA antibodies (in IgA-deficient patients). | Systemic mast cell degranulation and massive histamine release. | Dyspnea, bronchospasm, laryngeal edema, acute hypotension/shock. | STOP transfusion. Epinephrine (first-line), IV fluids, oxygen, H1/H2 blockers. |
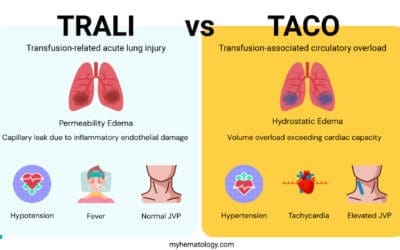
| Transfusion-Related Acute Lung Injury (TRALI) ▾ | Donor anti-HLA or anti-HNA antibodies. | Antibody-mediated activation and sequestration of recipient neutrophils in pulmonary capillaries, causing non-cardiogenic pulmonary edema. | Acute onset dyspnea and hypoxemia, bilateral pulmonary infiltrates on CXR. No evidence of left heart failure (low CVP, normal BNP). | STOP transfusion. Aggressive respiratory support (oxygen, ventilation). AVOID diuretics. |
| Transfusion-Associated Circulatory Overload (TACO) ▾ | Rapid infusion rate or excessive volume, especially in susceptible patients. | Hypervolemia leading to acute hydrostatic pulmonary edema (cardiogenic). | Acute dyspnea, orthopnea, elevated central venous pressure (CVP), signs of heart failure, elevated BNP. | STOP transfusion. Place patient upright. Oxygen. Diuretics (furosemide) to reduce plasma volume. |
| Transfusion-Associated Sepsis (TAS) ▾ | Contamination of the blood product by bacteria (e.g., Yersinia in RBCs, Staph in Platelets). | Release of bacterial endotoxins or exotoxins. | High fever, profound rigors, abrupt and severe hypotension/shock. | STOP transfusion. Send unit and patient blood for cultures. Initiate broad-spectrum IV antibiotics immediately. |
| Delayed Reactions | ||||
| Delayed Hemolytic Transfusion Reaction (DHTR) ▾ | Anamnestic response to non-ABO RBC antigens (e.g., Rh, Kidd, Duffy). | Recipient has low-titer antibody from previous sensitization. Antibody titer rises rapidly 3−10 days post-transfusion, leading to extravascular hemolysis. | Unexpected drop in Hb/Hct, mild fever, jaundice. Often mild/asymptomatic. DAT is positive. | No immediate action needed if mild. Confirm diagnosis with Blood Bank. Use phenotypically matched blood for all future transfusions. |
| Post-Transfusion Purpura (PTP) ▾ | Antibody against human platelet antigens (often HPA−1a) in previously sensitized (usually female) recipients. | Anti-platelet antibody destroys transfused HPA−1a positive platelets, and then somehow causes destruction of autologous HPA−1a negative platelets. | Abrupt onset of severe thrombocytopenia, generalized bleeding, purpura. | STOP transfusions. First-line treatment is high-dose IVIG. Use HPA-1a negative platelets only, if platelets are needed. |
| Transfusion-Associated Graft-versus-Host Disease (TA-GVHD) ▾ | Transfused immunocompetent donor T lymphocytes. | Donor T cells engraft and attack recipient tissues (skin, liver, GI tract). | High fever, erythroderma/maculopapular rash, diarrhea, hepatitis, pancytopenia. Often fatal. | Supportive care; high mortality. Prevention is key by using irradiated blood products for at-risk patients. |
Iron Overload (Transfusion Hemochromatosis) ▾ | Chronic, frequent transfusions over time (e.g., in thalassemia, myelodysplastic syndrome). | Total body iron store exceeds 20 g. Excess iron deposits in heart, liver, and endocrine organs causing organ damage. | Symptoms are related to organ failure: Congestive heart failure, liver cirrhosis, endocrinopathies (e.g., diabetes, hypogonadism). | Prevention is key. Treatment involves long-term iron chelation therapy (deferoxamine, deferasirox). |
*Click ▾ for more information
Introduction
Blood transfusion is a cornerstone of modern medicine, life-saving in trauma, surgery, and chronic hematologic conditions. However, the introduction of non-autologous blood products carries an inherent risk of adverse events, collectively known as transfusion reactions (TRs).
Definition: What Constitutes a Transfusion Reaction (TR)?
A transfusion reaction (TR) is defined as any unfavorable sign or symptom that occurs during, immediately after, or even days to weeks following the transfusion of blood or blood components. These transfusion reactions represent the patient’s body having an adverse response to components within the transfused unit. The components responsible can vary widely and include red blood cell antigens, white cell antigens, plasma proteins, antibodies, or even non-immunologic elements like bacteria or excess fluid volume.
Incidence and Importance: Why Vigilance is Necessary
While the vast majority of transfusions are uneventful, every transfusion carries a potential risk. Common transfusion reactions, such as mild allergic reactions or febrile non-hemolytic transfusion reactions (FNHTR), can occur in 1% to 3% of transfusions.
The true clinical importance, however, lies in the rare but potentially catastrophic severe transfusion reactions. Acute Hemolytic Transfusion Reaction (AHTR), Transfusion-Related Acute Lung Injury (TRALI), and Transfusion-Associated Circulatory Overload (TACO) are responsible for the highest rates of transfusion-related morbidity and mortality. For future clinicians, recognizing the early, often subtle signs of a severe reaction – such as a sudden drop in blood pressure or new-onset dyspnea – is paramount, as immediate cessation of the transfusion and supportive care are life-saving interventions.
Hemovigilance
To systematically minimize risks and continuously improve safety, most industrialized countries operate sophisticated hemovigilance programs . Hemovigilance is defined as a set of surveillance procedures covering the entire transfusion chain – from the donor’s vein to the recipient’s vein – to collect and analyze information on unexpected or undesirable effects resulting from blood donation or transfusion. This continuous, quality-focused feedback loop ensures that data gathered from adverse events lead directly to procedural and policy changes, thus continually maximizing the safety of the global blood supply.
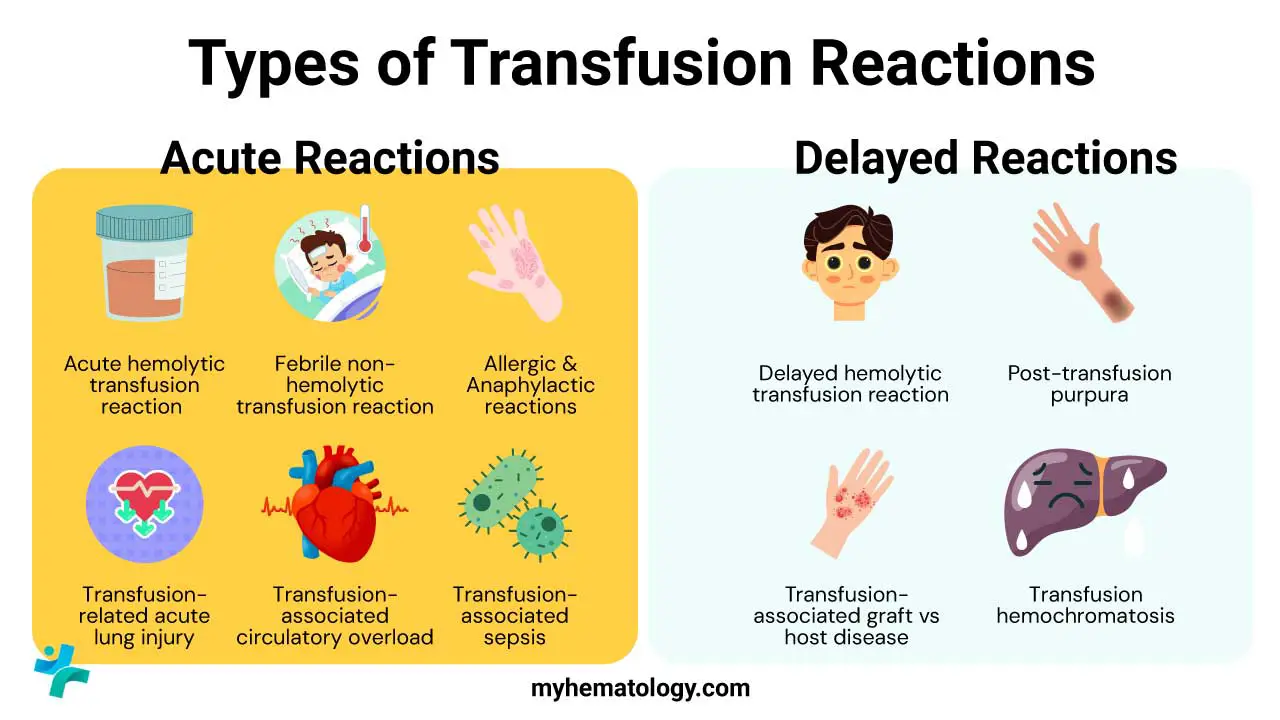
Transfusion reactions are broadly classified by their onset (Acute vs. Delayed) and their underlying mechanism (Immunologic vs. Non-immunologic). This article provides a structured, fact-based guide to the most clinically relevant transfusion reaction types, emphasizing detailed pathophysiology and adherence to clinical guidelines.
Classification of Transfusion Reactions
By Timing
Classification based on the interval between the start of the transfusion and the onset of symptoms.
- Acute: Transfusion reactions occurring within hours of starting the transfusion. Examples: Acute Hemolytic Transfusion Reaction (AHTR), Febrile Non-Hemolytic Transfusion Reaction (FNHTR), Allergic Reactions (mild to anaphylactic), Transfusion-Related Acute Lung Injury (TRALI), Transfusion-Associated Circulatory Overload (TACO), and Transfusion-Associated Sepsis (TAS).
- Delayed: Transfusion reactions occurring more than 24 hours after the transfusion (sometimes weeks or months later). Examples: Delayed Hemolytic Transfusion Reaction (DHTR), Transfusion-Associated Graft-versus-Host Disease (TA-GVHD), Post-Transfusion Purpura (PTP), and Iron Overload.
By Mechanism (Immunologic vs. Non-Immunologic)
Categorizing reactions based on whether the immune system is primarily involved.
- Immunologic Reactions: Adverse events caused by interactions between immune components in the donor product (antibodies, leukocytes) and antigens in the recipient, or vice versa. Examples: AHTR (antibody-mediated RBC destruction), DHTR, TRALI (antibody-mediated lung injury), Allergic Reactions (IgE/IgG mediated), and FNHTR (cytokine or HLA antibody mediated).
- Non-Immunologic Reactions: Adverse events caused by the physical or chemical properties of the blood product, or by contamination. Examples: TACO (volume overload), TAS (bacterial contamination/endotoxin release), Non-Immune Hemolysis (due to product overheating or improper storage).
Classification and Pathophysiology of Acute Transfusion Reactions (ATRs)
Acute transfusion reactions occur during the transfusion or within 24 hours of its completion. Their severity ranges from mild, self-limiting symptoms to life-threatening emergencies.
Acute Hemolytic Transfusion Reaction (AHTR)
AHTR is the most critical and potentially fatal ATR. It is almost always caused by clerical error resulting in the transfusion of ABO-incompatible red blood cells (RBCs).
Pathophysiology
The recipient possesses pre-formed, naturally occurring IgM antibodies (e.g., anti-A or anti-B) against the transfused donor RBC antigens. These IgM antibodies bind rapidly to the transfused cells, activating the classical complement cascade (C1q, C2, C4, C3). This leads to massive, rapid intravascular hemolysis via the Membrane Attack Complex (MAC, C5b-9).
The lysis releases:
- Hemoglobin: Exceeding haptoglobin binding capacity, leading to hemoglobinemia and subsequent hemoglobinuria.
- Cytokines and Procoagulant Factors: Activation of the clotting cascade results in Disseminated Intravascular Coagulation (DIC) and widespread systemic inflammation.
- Vasoactive Mediators: Including bradykinin and IL-1, leading to shock and acute renal failure (ARF) due to renal tubular damage from hypotension and hemoglobin precipitation.
Febrile Non-Hemolytic Transfusion Reaction (FNHTR)
FNHTR is one of the most common reactions, typically non-life-threatening.
Pathophysiology
Primarily caused by the recipient’s antibodies reacting with Human Leukocyte Antigens (HLA) on residual donor white blood cells (WBCs). The main mechanism is the release of pyrogenic cytokines (IL-1β, IL-6, TNF-α) from residual WBCs (primarily monocytes and lymphocytes) that accumulate in the blood component during storage.These cytokines cross the blood-brain barrier and elevate the hypothalamic set-point, causing fever and rigors.
Prevention: Pre-storage leukoreduction is highly effective and has significantly decreased the incidence of FNHTR.
Allergic and Anaphylactic Reactions
These result from hypersensitivity to plasma proteins in the donor blood product.
Pathophysiology
- Mild Allergic Reaction: Thought to be due to recipient IgE antibodies reacting with soluble donor plasma proteins. Leads to mast cell degranulation and localized release of histamine, causing pruritus and urticaria (hives).
- Anaphylactic Reaction: A severe, generalized type I hypersensitivity reaction, often seen in patients with congenital IgA deficiency who have formed anti-IgA antibodies. Rapid, massive mast cell degranulation leads to widespread vasodilation, bronchospasm, and circulatory collapse.
Transfusion-Related Acute Lung Injury (TRALI)
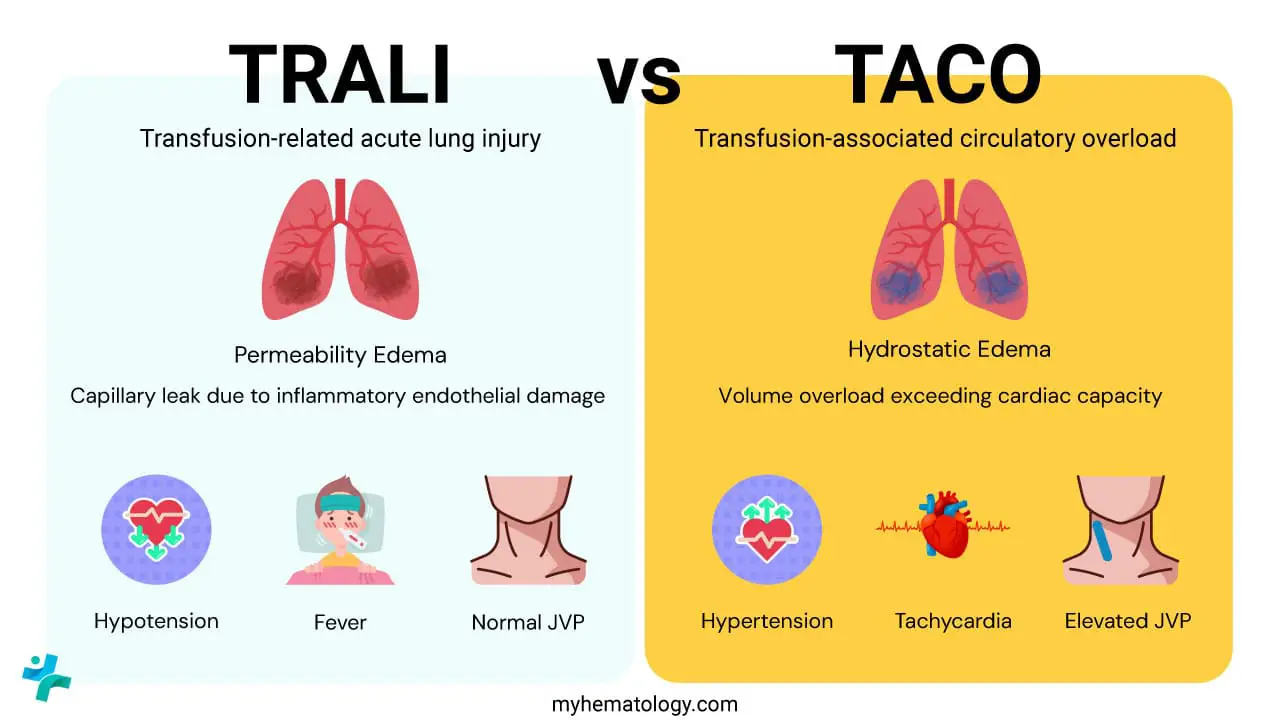
TRALI is a leading cause of transfusion-related morbidity and mortality, characterized by non-cardiogenic pulmonary edema.
Pathophysiology (Two-Hit Model)
- First Hit: The patient’s underlying clinical condition (e.g., surgery, sepsis, trauma) causes endothelial activation and neutrophil priming in the pulmonary microvasculature.
- Second Hit: Anti-HLA or anti-HNA (Human Neutrophil Antigen) antibodies from the donor plasma (often multiparous female donors) react with recipient neutrophils, or, less commonly, biologically active lipids/cytokines accumulate in the stored blood product.
This interaction causes neutrophil aggregation and activation within the pulmonary capillaries.bActivated neutrophils release toxic mediators (proteases, oxygen radicals) that damage the pulmonary capillary endothelium, leading to plasma leakage into the alveolar space and pulmonary edema.
Transfusion-Associated Circulatory Overload (TACO)
TACO is a non-immunologic reaction caused by the rapid rate or excessive volume of transfusion, leading to hydrostatic pulmonary edema.
Pathophysiology
The circulatory system cannot tolerate the volume load, especially in patients with pre-existing cardiac or renal dysfunction. Increased hydrostatic pressure in the pulmonary capillaries forces fluid out of the vessels and into the interstitial and alveolar spaces.
Key Differentiating Feature: Elevated Brain Natriuretic Peptide (BNP) levels and evidence of positive fluid balance are characteristic, distinguishing it from TRALI (which is volume-independent).
Transfusion-Associated Sepsis (Bacterial Contamination)
Transfusion-Associated Sepsis (TAS) is a severe, acute, non-immunologic transfusion reaction that results from the infusion of blood or a blood component contaminated with viable bacteria or, less commonly, other microorganisms.
Pathophysiology
The core issue is the contamination of the blood unit, most often during phlebotomy, with organisms that can survive or thrive during storage. Upon infusion, the high load of viable bacteria and their potent toxins (lipopolysaccharide/endotoxins) enter the recipient’s circulation. This leads to a rapid, overwhelming SIRS (Systemic Inflammatory Response Syndrome) response, resulting in widespread and often irreversible vascular damage, massive vasodilation, and profound septic shock, which can be fatal within hours.
| Transfusion Reaction | Causes | Pathophysiology | Classic Symptoms |
| Acute Hemolytic Transfusion Reaction (AHTR) | ABO incompatibility (clerical error is the primary cause) | Intravascular hemolysis, complement activation, massive cytokine/bradykinin release, acute kidney injury. | Fever, chills, back/flank pain, red or brown urine (hemoglobinuria), hypotension, shock. |
| Febrile Non-Hemolytic Transfusion Reaction (FNHTR) | Cytokines accumulated during blood product storage (especially platelets/WBC-containing products). | Recipient reacting to donor leukocyte antigens (HLAs) or release of pyrogenic cytokines (IL-1, IL-6, TNF-a). | Temperature rise of ≥1°C from baseline, chills, rigors (in the absence of hemolysis). |
| Allergic/Anaphylactic Reaction | Recipient IgE or IgG antibodies directed against donor plasma proteins. | Mast cell/basophil degranulation, histamine release, leading to smooth muscle contraction and vasodilation. | Mild: Urticaria, pruritus. Severe/Anaphylactic: Dyspnea, bronchospasm, laryngeal edema, hypotension, shock. |
| Transfusion-Related Acute Lung Injury (TRALI) | Donor anti-HLA or anti-HNA antibodies reacting with recipient neutrophils. | Activation and sequestration of neutrophils in the pulmonary microvasculature, causing acute non-cardiogenic pulmonary edema. | Acute dyspnea, hypoxemia, bilateral pulmonary infiltrates (within 6 hours of transfusion). |
| Transfusion-Associated Circulatory Overload (TACO) | Too rapid or too large a volume of transfusion. | Hypervolemia leads to increased hydrostatic pressure and left heart failure (cardiogenic pulmonary edema). | Acute onset of respiratory distress, orthopnea, elevated central venous pressure (CVP), elevated BNP. |
| Transfusion-Associated Sepsis (Bacterial Contamination) | Contamination of the blood product during collection or processing (often Yersinia enterocolitica in RBCs; S. epidermidis in Platelets). | Release of bacterial endotoxins or exotoxins. | High fever, profound rigors, severe hypotension/shock, vomiting, DIC. |
Classification and Pathophysiology of Delayed Transfusion Reactions (DTRs)
Delayed reactions occur more than 24 hours after the transfusion, often days to weeks later.
Delayed Hemolytic Transfusion Reaction (DHTR)
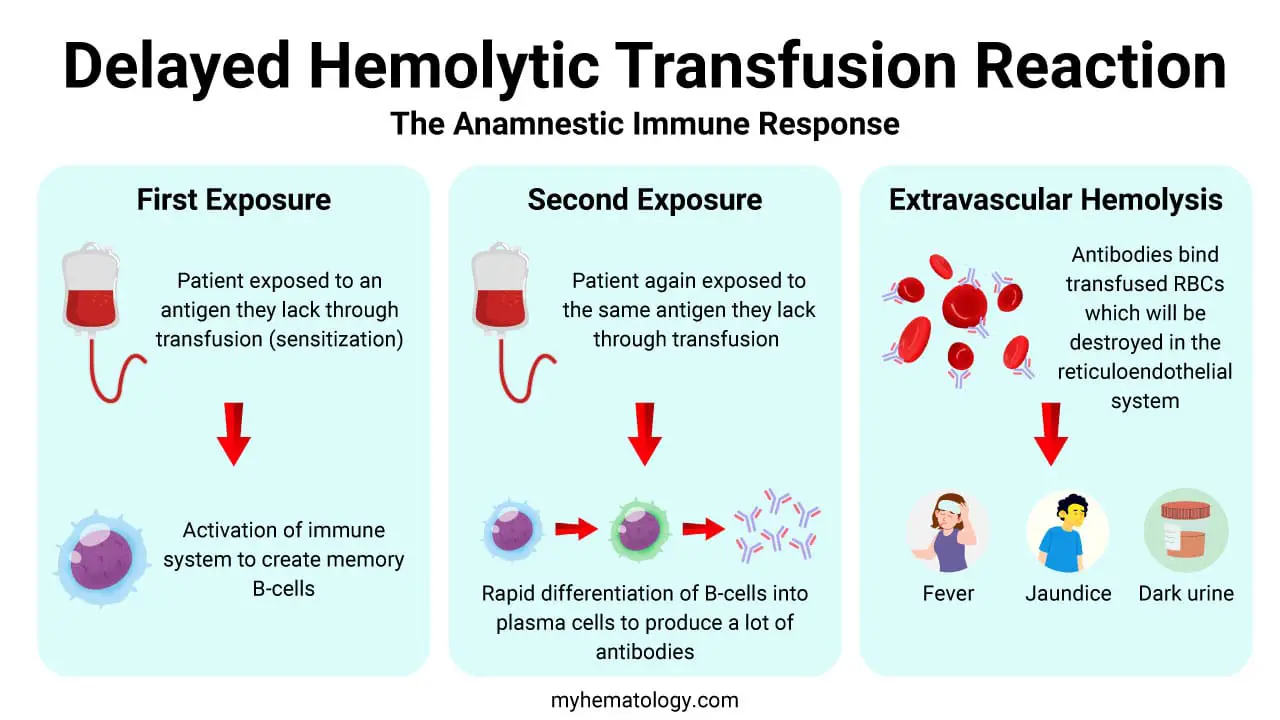
Delayed Hemolytic Transfusion Reaction (DHTR) is caused by anamnestic antibody response to previously encountered RBC antigens (e.g., Rh, Kidd), typically occuring 3 – 10 days after a blood transfusion.
Pathophysiology
Typically results from a secondary anamnestic immune response in a patient previously sensitized by transfusion or pregnancy (often to non-ABO antigens like Rhesus, Kidd, or Duffy). The antibody titer was too low to be detected during pre-transfusion testing.
Upon re-exposure, B cells rapidly produce a high titer of IgG alloantibodies, which bind to the transfused RBCs. The IgG-coated cells are primarily cleared by the reticuloendothelial system (macrophages in the spleen and liver) via Fc receptor binding (extravascular hemolysis). This process is slower and less severe than the intravascular hemolysis of AHTR.
Clinical Presentation: Jaundice, mild fever, and a drop in hemoglobin concentration that is greater than expected.
Transfusion-Associated Graft-Versus-Host Disease (TA-GVHD)
A rare but nearly universally fatal complication, typically occurring 1 – 4 weeks after a transfusion of blood or blood products.
Pathophysiology
Infusion of viable donor T-lymphocytes into an immunocompromised recipient or a recipient who shares HLA haplotypes with the donor (directed or family donations). The donor T cells recognize the recipient’s HLA as foreign and proliferate, attacking recipient tissues (graft-versus-host).
Prevention: Gamma irradiation of cellular blood components is required to prevent T-cell proliferation.
Post-Transfusion Purpura (PTP)
PTP is a rare but severe immunologic complication characterized by the abrupt onset of profound thrombocytopenia, typically occurring 5 to 10 days after a transfusion of blood or blood components containing platelets. It most often affects women who have been sensitized during previous pregnancies or transfusions.
Pathophysiology
The primary mechanism involves alloimmunization against Human Platelet Antigens (HPA), usually HPA−1a. The recipient (HPA−1a negative, e.g., a multiparous woman) is exposed to HPA−1a positive platelets (from a donor or during pregnancy), leading to the creation of potent antibodies (anti-HPA−1a). Upon a subsequent transfusion of HPA−1a positive blood, the circulating anti-HPA−1a antibodies rapidly destroy the transfused platelets. Crucially, a mysterious “bystander” effect occurs. The same antibodies, or perhaps immune complexes formed with donor HPA−1a antigen, also trigger the destruction of the recipient’s own HPA−1a negative platelets. This destruction of autologous platelets is what causes the profound thrombocytopenia, leading to bleeding and purpura.
Iron Overload
Iron Overload, or Transfusion Hemochromatosis, is a non-immunologic, chronic consequence of receiving multiple blood transfusions over an extended period. It leads to the toxic accumulation of iron in body tissues, which is a major cause of morbidity and mortality in chronically transfused patients (e.g., those with thalassemia, sickle cell disease, or myelodysplastic syndrome).
Pathophysiology
Each unit of packed contains approximately 200 to 250 mg of elemental iron. This is an amount the body cannot excrete. Under normal conditions, the body maintains iron balance primarily by regulating absorption, but there is no active physiological mechanism for excreting excess iron. In patients receiving chronic transfusions, iron is deposited in the parenchyma of vital organs once the total body iron exceeds the binding capacity of transferrin. The iron-laden macrophages and hepatocytes release toxic forms of iron (non-transferrin-bound iron or NTBI). This excess iron causes cellular damage via the production of reactive oxygen species (free radicals). Organ toxicity typically manifests when the body’s iron stores exceed, leading to:
- Cardiac failure (the leading cause of death in these patients).
- Liver cirrhosis (due to iron deposition in hepatocytes).
- Endocrinopathies (e.g., diabetes mellitus, hypogonadism) due to damage to the pancreas and pituitary gland.
Investigation and Management of a Suspected Transfusion Reaction
The investigation of a transfusion reaction is a critical, systematic process that starts at the bedside and moves quickly to the laboratory. The primary goal is twofold: to stabilize the patient and to rapidly rule out Acute Hemolytic Transfusion Reaction (AHTR) and Transfusion-Associated Sepsis (TAS), as these are the most immediately life-threatening.
The investigation proceeds through three critical phases: immediate clinical triage, the initial laboratory work-up (the “Big Four”), and secondary investigations based on preliminary findings.
Immediate Clinical Triage (The Bedside Stop)
The moment a transfusion reaction is suspected, the following immediate steps are mandatory:
- STOP THE TRANSFUSION IMMEDIATELY: Clamp the transfusion line. This is the single most important intervention.
- MAINTAIN I.V. ACCESS: Keep the existing intravenous line open by replacing the blood product with a slow infusion of 0.9% normal saline. Do not flush the blood back into the patient.
- CLERICAL CHECK (Bedside): A critical step to rule out the most common cause of fatal reactions (AHTR). The nurse and a colleague must recheck all documentation:
- Match the patient’s identity (name, hospital number) on the wristband against the blood component tag and the blood request form.
- Check the unit number, ABO and Rh types on the blood unit tag against the compatibility form.
- Any clerical error triggers a full AHTR work-up immediately.
- Clinical Assessment: Monitor and record vital signs (temperature, pulse, BP, RR, SpO2) every 5 minutes. Treat life-threatening symptoms (e.g., hypotension with fluids/pressors, anaphylaxis with epinephrine).
- Notify the Blood Bank/Transfusion Service: Inform the lab immediately that a transfusion reaction has occurred and send the required samples/materials STAT (see below).
Initial Laboratory Work-up (The “Big Four” and Core Samples)
The laboratory’s initial investigation is focused on detecting evidence of acute, immune-mediated hemolysis.
Materials Sent to the Transfusion Service
The following are sent to the lab for the initial work-up:
- The implicated blood bag (and the administration set/tubing attached).
- A freshly drawn post-transfusion blood sample (usually an EDTA tube and a clotted tube, drawn carefully to avoid in vitro hemolysis).
- The first post-transfusion urine sample (to check for hemoglobinuria).
The Core Investigation
The lab performs a series of preliminary tests on the pre- and post-transfusion samples.
| Investigation | Purpose | Interpretation |
| Visual Inspection of Plasma/Serum | Detects free hemoglobin in the blood. | Pink/red plasma in the post-transfusion sample (but not the pre-transfusion sample) is strong evidence of intravascular hemolysis (e.g., AHTR). |
| Direct Antiglobulin Test (DAT) / Direct Coombs | Tests for RBCs coated with antibody (IgG) or complement (C3d) in vivo. | A positive DAT on the post-transfusion sample (if previously negative) indicates an immune reaction (e.g., AHTR, DHTR). |
| Repeat ABO and Rh Typing | Confirms the patient’s and donor unit’s blood types. | Discrepancies confirm a clerical error resulting in ABO incompatibility. |
| Repeat Antibody Screen and Crossmatch | Checks for newly detectable alloantibodies in the patient’s serum that might have caused the reaction. | Incompatibility confirms immune sensitization. |
If the visual inspection, DAT, and ABO/Rh typing are all negative, AHTR is effectively ruled out. The investigation then shifts to non-immune or non-hemolytic causes.
Secondary Investigations (Differential Diagnosis)
If the initial work-up is negative for AHTR, or if clinical symptoms persist, tailored tests are ordered to differentiate between the other major transfusion reactions.
| Suspected Transfusion Reaction | Key Differential Symptoms | Secondary Laboratory Tests |
| Transfusion-Associated Sepsis (TAS) | High fever (≥2°C rise), rigors, profound shock. | Blood cultures (from patient and remaining unit); Gram stain of the unit content (if suspicious). CBC (for neutropenia/leukocytosis), DIC screen (PT, aPTT, fibrinogen, D-dimer). |
| Transfusion-Related Acute Lung Injury (TRALI) vs. Transfusion-Associated Circulatory Overload (TACO) | Acute hypoxemia and bilateral pulmonary edema on Chest X−ray. | BNP (B-type Natriuretic Peptide) / NT−proBNP: Elevated in TACO (cardiogenic); Normal/Low in TRALI (non-cardiogenic). Fluid balance assessment; HLA/HNA antibody testing (for donor/recipient) may be pursued later for TRALI. |
| Delayed Hemolytic Transfusion Reaction (DHTR) | Unexplained drop in hemoglobin 3 to 30 days post-transfusion, new jaundice. | LDH, Bilirubin (Indirect): Elevated. Haptoglobin: Decreased. New alloantibody identification (via IAT and panel). |
| Anaphylaxis | Rapid hypotension, bronchospasm, urticaria. | Serum Tryptase: Measured immediately and then 3 hours post-reaction (marker of mast cell degranulation). IgA level and Anti−IgA antibody testing: If severe anaphylaxis is suspected (especially in a patient with a known history of reactions). |
| Febrile Non-Hemolytic Transfusion Reaction (FNHTR) | Isolated temperature rise (≥1°C), chills, rigors, headache (must be a diagnosis of exclusion). | None needed if AHTR and Sepsis are ruled out. Often considered “minor” and is confirmed if all other investigations are negative. |
By following this investigative pathway, clinicians can narrow down the potential cause from a broad clinical picture to a specific laboratory diagnosis, guiding appropriate treatment and ensuring the future safety of the patient’s blood transfusions.
Specific Treatment Guidelines for Transfusion Reactions
| Reaction Type | Key Treatment/Management | Prophylaxis for Future Transfusions |
| AHTR | Aggressive IV fluids, Furosemide (to maintain urine output > 100 ml/hr), treat DIC and shock. Do not restart. | Meticulous ID checks; pre-transfusion protocols. |
| FNHTR | Antipyretics (Acetaminophen/Paracetamol); consider Meperidine for severe rigors. Transfusion may be restarted after exclusion of AHTR and physician consultation. | Use Leukoreduced blood components. |
| Mild Allergic | Antihistamines (Diphenhydramine). Transfusion may be restarted if symptoms resolve. | Pre-medication with Antihistamines. Use Washed RBCs or platelets. |
| Anaphylactic | Epinephrine (IM, followed by IV if needed), IV fluids, Antihistamines, Corticosteroids. Do not restart. | Use Washed RBCs or platelets; IgA-deficient products for anti-IgA patients. |
| TRALI | Supportive care: O2, mechanical ventilation (often needed). Diuretics are typically contraindicated. Do not restart. | Use male-only or nulliparous female plasma products. |
| TACO | Supportive care: Diuretics (Furosemide), O2. Slow rate of transfusion (over > 4 hours). | Pre-transfusion diuretic use; slower infusion rate, split units. |
| TA-GVHD | Immunosuppression (often ineffective). | Gamma-Irradiated blood components (required for all immunocompromised patients or directed donations). |
Prevention and Safe Transfusion Practices
Error Prevention: The Bedside Check and Documentation
- Patient Identification: This is the single most important step to prevent-incompatible AHTRs. MUST be done at the bedside by TWO licensed personnel (or one person using an electronic barcode system).
- Mandatory Verification: Must verify FIVE key items: Recipient name, date of birth, MRN, blood product compatibility/type, and blood unit number.
- Electronic Crossmatch: Reliance on automated laboratory systems (LIMS) and electronic patient identification systems (EPIS) to eliminate manual data entry errors.
Modification of Blood Products
- Leukoreduction (Filtering): The removal of white blood cells (WBCs) from blood components to prevent FNHTR (by removing WBCs that cause cytokine release), reduce risk of CMV (Cytomegalovirus) transmission, and reduce HLA alloimmunization. Now standard practice for almost all components (RBCs and platelets).
- Irradiation: Exposure of blood components to gamma radiation (or X-ray) to inactivate donor T lymphocytes to prevent TA-GVHD (Transfusion-Associated Graft-versus-Host Disease) by ensuring donor lymphocytes cannot proliferate and attack recipient tissues. This is mandatory for high-risk patients (e.g., immunocompromised, fetal/neonatal transfusions, directed donations from relatives).
Managing Infection Risk (TAS)
- Aseptic Technique: Strict aseptic collection and processing of donor units to prevent initial contamination.
- Platelet Monitoring: Since platelets are stored at room temperature (20−24°C), they are the most common source of TAS. Mitigation includes rapid processing and, increasingly, bacterial detection/culture testing prior to release.
Pre-Medication (A Measured Approach)
- When to Use: Only appropriate for patients with a documented history of non-severe allergic reactions or FNHTR.
- Standard Regimen: Acetaminophen (for fever/FNHTR) and Diphenhydramine (for mild allergic symptoms) 30 minutes prior to the start of transfusion.
- Caution: Pre-medication should NOT be used routinely, as it can mask the early signs of a severe reaction (AHTR or TAS), delaying life-saving intervention.
Managing Volume/Rate (TACO Prevention)
- Patient-Specific Rate: Transfusion rate must be adjusted for patients at risk of TACO (e.g., elderly, small children, history of heart failure, renal failure).
- Volume Reduction: Consider giving packed RBCs in smaller aliquots or administering them in conjunction with a diuretic (furosemide).
Frequently Asked Questions (FAQs)
What is the most common cause of a fatal transfusion reaction?
The most common cause of a fatal transfusion reaction is clerical error resulting in the transfusion of ABO-incompatible blood, which leads to Acute Hemolytic Transfusion Reaction (AHTR).
How does pre-storage leukoreduction prevent Febrile Non-Hemolytic Transfusion Reactions (FNHTR)?
Leukoreduction filters out the majority of donor white blood cells (WBCs) from the blood component before storage. This prevents the WBCs from breaking down and releasing accumulated pyrogenic cytokines (like IL-1 and IL-6) during storage, which are the primary cause of the fever and chills in FNHTR.
What single test is most crucial to confirm acute hemolysis?
The Direct Antiglobulin Test (DAT or Direct Coombs Test) on the post-transfusion sample is critical. A positive DAT indicates that the patient’s red blood cells are coated with antibodies (IgG) or complement components (C3d), providing strong evidence of an immune-mediated reaction. Additionally, looking for free plasma hemoglobin and hemoglobinuria confirms the lysis.
Can a transfusion reaction occur weeks later?
Yes. Delayed Transfusion Reactions (DTRs) can occur days to weeks after transfusion, with the most common being Delayed Hemolytic Transfusion Reaction (DHTR). This results from a secondary immune response to blood group antigens (other than ABO), where IgG antibodies are rapidly produced and cause extravascular hemolysis.
Glossary of Medical Terms
- Alloimmunization: The production of antibodies by a recipient against alloantigens (antigens) present on the transfused red blood cells, platelets, or white blood cells of a donor.
- Extravascular Hemolysis: Destruction of red blood cells primarily by macrophages in the spleen and liver after the cells have been coated with IgG antibodies (positive DAT). (Seen in DHTR)
- Intravascular Hemolysis: Rapid destruction of red blood cells occurring within the blood vessels, typically mediated by the complete activation of the complement cascade leading to the formation of the Membrane Attack Complex (MAC) and cellular lysis. (Seen in AHTR)
- Leukoreduction: A process used to filter and remove the majority of white blood cells (WBCs) from blood components to reduce the risk of FNHTR, alloimmunization to HLA antigens, and transmission of WBC-associated viruses (CMV).
- Complement Cascade: A system of plasma proteins that, when activated (e.g., by IgM antibodies in AHTR), results in a cascade of reactions leading to the formation of the Membrane Attack Complex (MAC) and cellular lysis.
- Hemovigilance: A set of surveillance procedures covering the entire transfusion chain, from the collection of blood to the follow-up of recipients, designed to detect, analyze, and prevent adverse effects of blood transfusion.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Suddock JT, Crookston KP. Transfusion Reactions. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482202/
- Raval, J. S., Griggs, J. R., & Fleg, A. (2020). Blood Product Transfusion in Adults: Indications, Adverse Reactions, and Modifications. American family physician, 102(1), 30–38.
- Semple, J. W., Rebetz, J., & Kapur, R. (2019). Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood, 133(17), 1840–1853. https://doi.org/10.1182/blood-2018-10-860809
- Wang, Y., Rao, Q., & Li, X. (2022). Adverse transfusion reactions and what we can do. Expert review of hematology, 15(8), 711–726. https://doi.org/10.1080/17474086.2022.2112564