TL;DR
Drug-induced immune hemolytic anemia are caused by drugs that trigger an immune response to attack red blood cells (RBCs), leading to hemolytic anemia.
Mechanism ▾
- Hapten Model (most common) (Drug-dependent Antibody Model)
- Immune Complex Formation (less common)
- Autoimmune Model (least common in DIIHA)
- Drug-Independent Antibody Model (less common)
Signs & Symptoms ▾
- Fatigue, pallor, shortness of breath (general anemia symptoms)
- Jaundice, dark urine (specific to hemolysis)
- Weakness, dizziness, rapid heartbeat (additional symptoms)
Complications ▾
- Increased infection risk
- Cardiac issues (tachycardia, heart failure)
- Cholelithiasis (gallstones)
- Hemosiderosis (iron overload)
- Kidney complications
- Splenic rupture (rare)
- Disseminated Intravascular Coagulation (DIC) (rare)
Diagnosis ▾
- Clinical presentation & medication history
- Laboratory investigations:
- CBC (anemia, reticulocytosis)
- Peripheral blood smear (spherocytosis, fragmented RBCs)
- Direct Antiglobulin Test (DAT) – positive (crucial)
- LDH, haptoglobin, bilirubin (may be elevated)
- Urinalysis (hemoglobinuria in severe cases)
Differential Diagnosis ▾
- Non-immune hemolytic anemias (hereditary spherocytosis, sickle cell disease)
- Immune hemolytic anemias (WAIHA, CAD, PNH)
- Microangiopathic hemolytic anemia
- Hypersplenism
Treatment & Management ▾
- Stop the offending drug (most crucial step)
- Supportive measures: blood transfusions, folic acid supplementation
- Second-line therapies (if needed)
- Corticosteroids (prednisone)
- Rituximab (B cell depletion)
- Splenectomy (last resort)
- Monitoring response: symptoms, labs (hemoglobin, DAT)
- Prognosis: Generally good with prompt diagnosis and drug removal
*Click ▾ for more information
Introduction
Drug-induced immune hemolytic anemia (DIIHA) is a rare condition where a medication triggers your body’s immune system to attack your own red blood cells (RBCs). This attack leads to the premature destruction of RBCs, a process called hemolysis.
Hemolysis refers to the breakdown of red blood cells (RBCs) and the release of their contents into the surrounding fluid, typically blood plasma. This breakdown can occur within the bloodstream (intravascular hemolysis) or outside the bloodstream in organs like the spleen (extravascular hemolysis). Hemolysis can cause various issues as RBCs are essential for carrying oxygen throughout your body.
Hemolysis is significant for several reasons:
- Reduced Oxygen Delivery: RBCs carry oxygen throughout the body bound to a molecule called hemoglobin. When RBCs rupture, less oxygen is available to be delivered to tissues, potentially leading to fatigue, weakness, and shortness of breath.
- Kidney Complications: Hemoglobin released from lysed RBCs can overload the kidneys, leading to potential kidney damage or failure if left untreated.
- Jaundice: Hemoglobin is broken down into a yellow pigment called bilirubin. When excessive hemolysis occurs, the liver can become overwhelmed by the amount of bilirubin, causing a yellowish discoloration of the skin and eyes known as jaundice.
Intravascular vs Extravascular Hemolysis
Hemolysis, the destruction of red blood cells (RBCs), can occur in two main locations:
- Extravascular Hemolysis: This happens outside the bloodstream, primarily in organs like the spleen and liver. These organs contain specialized cells called macrophages that are responsible for clearing away old or damaged RBCs. In extravascular hemolysis, these macrophages remove and break down the RBCs in a controlled manner.
- Intravascular Hemolysis: This occurs within the bloodstream itself. Here, the RBCs are directly lysed (broken open) by various factors, releasing their contents directly into the plasma. This can be a much more rapid and aggressive form of hemolysis compared to the extravascular process.
Mechanism Models of Drug-Induced Immune Hemolytic Anemia (DIIHA)
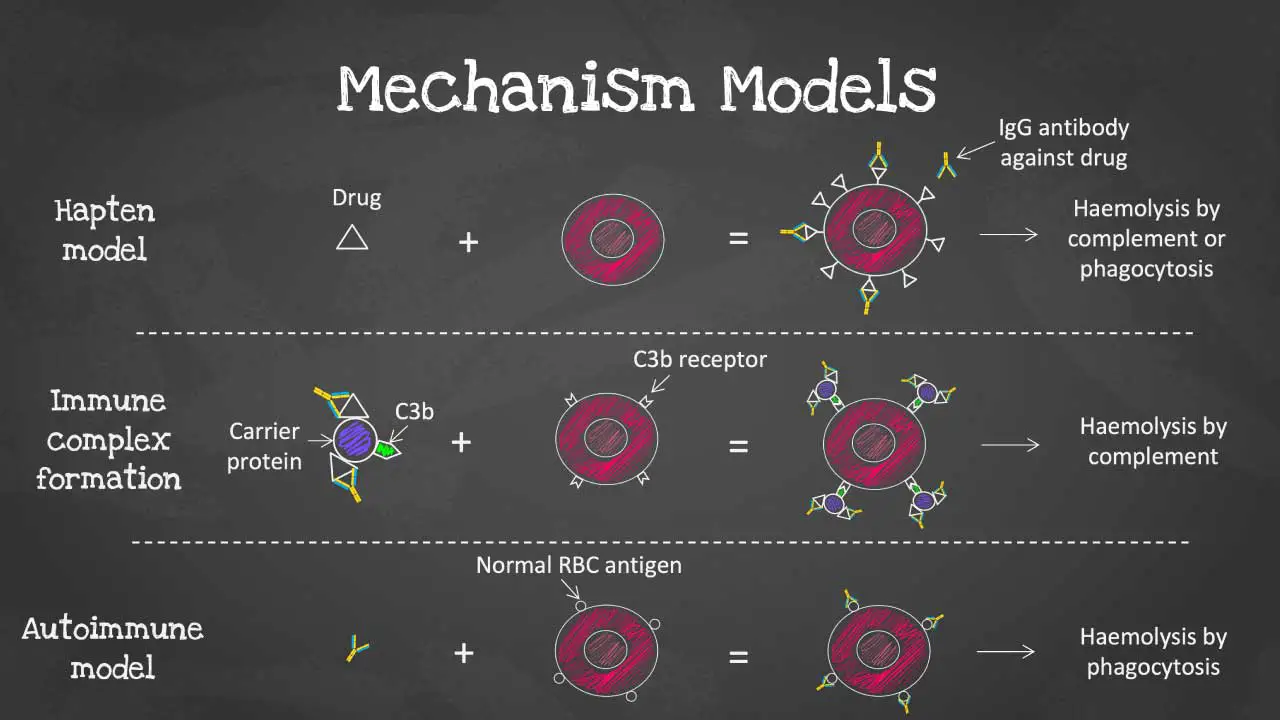
Drug-induced immune hemolytic anemia (DIIHA) arises when a medication interacts with red blood cells (RBCs) in a way that triggers the immune system to attack them. There are several proposed mechanisms for this interaction, each with its own characteristics.
Traditional Models
Hapten Model
- This is the most well-understood mechanism, particularly for drugs like penicillin.
- In this model, the drug molecule (hapten) binds covalently to proteins on the RBC membrane.
- The immune system doesn’t recognize the drug itself, but the drug-protein complex on the RBC surface appears foreign.
- The body then produces antibodies (usually IgG) specifically against this drug-protein complex.
- When these antibodies bind to the drug-coated RBCs, they are targeted for destruction by macrophages in the spleen (extravascular hemolysis).
- Because the drug remains attached to the RBC, the antibody-coated cells are continuously removed from circulation.
Immune Complex Formation
- This model suggests that some drugs can non-covalently bind to RBC membrane proteins or adsorb to the surface.
- The drug-RBC complex itself acts as an antigen, triggering the immune system to produce antibodies (IgM or IgG) against this complex.
- These antibodies can activate the complement system, a group of proteins in the blood that further attacks the drug-coated RBCs, leading to their destruction, often within the bloodstream (intravascular hemolysis).
Autoimmune Model
- This model is still under investigation.
- The theory suggests that certain drugs might directly stimulate B-lymphocytes (antibody-producing cells) to produce autoantibodies that target healthy RBCs without any drug involvement.
- This is similar to autoimmune hemolytic anemia (AIHA) where the body attacks its own RBCs without an external trigger.
- More research is needed to fully understand the role of this mechanism in DIIHA.
Current Models
Drug-dependent antibodies
The drug-dependent antibody model in DIIHA is the most common and well-understood mechanism compared to the drug-independent antibody and autoimmune models.
Drug-Dependent Antibody Model
- Direct Interaction: In this model, the drug molecule itself directly binds to red blood cell (RBC) membrane proteins. This binding is crucial for the immune response to occur.
- Antigen Formation: The drug-RBC complex creates a new antigen, essentially a foreign structure not normally present on healthy RBCs.
- Immune Response: The immune system recognizes this altered RBC as a threat and produces antibodies (primarily IgG) specifically against the drug-RBC complex.
- Antibody Action: These antibodies bind only to RBCs that have the drug molecule attached, marking them for destruction by macrophages in the spleen (extravascular hemolysis).
Key Differences from Other Models
- Drug’s Role: Unlike the drug-independent model, the drug directly interacts with the RBC in this case. In the autoimmune model, there’s no direct drug-RBC interaction.
- Specificity: The antibodies generated in the drug-dependent model are highly specific to the drug-RBC complex, unlike the potentially broader targeting in the drug-independent model.
Example
- A classic example of the drug-dependent model is DIIHA caused by penicillin. Penicillin covalently binds to RBC membrane proteins, forming an antigen that triggers an immune response.
Drug-independent antibodies
There’s another less frequent mechanism where the drug itself doesn’t directly bind to RBCs, but rather triggers the production of antibodies that can still attack them. This is known as the drug-independent antibody mechanism, and one possible explanation for it is molecular mimicry.
- Molecular Mimicry: In this scenario, the drug structurally resembles a protein or molecule naturally present on healthy tissues, including RBC membranes.
- Immune Response: The body’s immune system mistakenly identifies the drug as a threat and mounts an immune response against it. This can involve B cells producing antibodies that target the drug’s structure.
- Cross-Reactivity: The problem arises because the antibodies generated against the drug can also cross-react with the similar protein on healthy RBCs. These antibodies bind to the RBCs, marking them for destruction by macrophages (extravascular hemolysis) or activating the complement system (potentially leading to intravascular hemolysis).
Key Points about Drug-Independent Antibodies
- This mechanism is less well-understood compared to the hapten or immune complex models.
- It’s thought to be involved in some cases of DIIHA associated with certain medications like methyldopa (used for high blood pressure).
- Molecular mimicry isn’t the only possible explanation for drug-independent antibodies. Other mechanisms like the drug altering the RBC surface in a way that’s recognized as foreign by the immune system are also being explored.
Difficulties in Studying Drug-Independent Antibodies
- It’s challenging to directly identify the specific antigen on RBCs that the antibodies are targeting.
- The drugs involved might not have a clear structural similarity to any known RBC proteins, making it difficult to pinpoint the cause of mimicry.
Key differences between the models of DIIHA
| Model | Drug Interaction with RBC | Antigen Formation | Antibody Specificity | Prevalence |
| Hapten (Drug-Dependent) | Direct, covalent (or potentially non-covalent) binding | Drug-RBC complex | Highly specific to drug-RBC complex | Most common |
| Immune Complex Formation | Non-covalent drug-protein binding | Drug-protein complex | Can be broader, targeting both drug-protein complex and potentially unmodified RBC antigens | Less common |
| Autoimmune | None (drug doesn’t directly bind RBC) | Altered RBC surface (exact mechanism unknown) | Specific to RBC antigens | Least common in DIIHA |
| Drug-Independent Antibody | None (drug doesn’t directly bind RBC) | Molecular mimicry (drug resembles RBC antigen) | Broader, targeting both drug and RBC antigens | Less common than hapten model |
Other Relevant Models
- Non-immunologic Protein Adsorption: Some drugs, particularly beta-lactamase inhibitors, might non-specifically bind to proteins on the RBC membrane. This can lead to a positive Direct Antiglobulin Test (DAT) but might not involve true antibody-mediated destruction.
- Genetic Predisposition: Certain individuals might have a genetic susceptibility that makes them more prone to developing DIIHA upon exposure to specific drugs.
Important Points
- These models are not mutually exclusive, and some degree of overlap might occur in certain cases.
- The specific mechanism involved depends on the particular drug and individual patient factors.
Common medications associated with DIIHA
Several medications can trigger Drug-induced Immune Hemolytic Anemia (DIIHA).
Antibiotics
These are the medications most frequently associated with drug-induced immune hemolytic anemia (DIIHA). Some examples include:
- Cephalosporins: Cefotetan, ceftriaxone, cephalexin
- Penicillins: Ampicillin, amoxicillin-clavulanate (Augmentin)
- Fluoroquinolones: Ciprofloxacin, levofloxacin
Immunosuppressants
Medications used to suppress the immune system can also cause drug-induced immune hemolytic anemia (DIIHA) in some cases. Examples include:
- Azathioprine (Imuran)
- Cyclophosphamide (Cytoxan)
Other Medications
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): Ibuprofen, naproxen (less common)
- Methyldopa (Aldomet): Used for high blood pressure
- Quinidine: Used for heart rhythm problems (less common)
- Dapsone: Used for treating leprosy and dermatitis herpetiformis
Important Points
- This list is not exhaustive, and drug-induced immune hemolytic anemia (DIIHA) can be caused by various other medications.
- The risk of drug-induced immune hemolytic anemia (DIIHA) associated with a particular medication can vary depending on factors like the individual’s susceptibility and the dose used.
- A thorough medication history is crucial for diagnosing drug-induced immune hemolytic anemia (DIIHA).
Additional Considerations
- Some herbal remedies and dietary supplements can also potentially trigger drug-induced immune hemolytic anemia (DIIHA), although this is less common.
- In some cases, the specific medication causing drug-induced immune hemolytic anemia (DIIHA) might not be readily identifiable.
Clinical Presentation
The signs and symptoms of drug-induced immune hemolytic anemia (DIIHA) can vary depending on the severity of the condition and the rate of red blood cell destruction.
General Symptoms
- Fatigue: This is a frequent complaint due to reduced oxygen delivery to tissues caused by anemia. People with DIIHA might experience tiredness and a lack of energy that interferes with daily activities.
- Pallor: Pale skin, mucous membranes, and nail beds can be a sign of anemia as there are fewer red blood cells circulating to deliver oxygen, leading to a lack of the usual pinkish color.
- Shortness of Breath: When the body doesn’t receive enough oxygen, shortness of breath or difficulty breathing can occur, especially during exertion.
Symptoms Specific to Hemolysis
- Jaundice: The breakdown of red blood cells releases bilirubin, a yellow pigment. When the liver can’t keep up with processing the excess bilirubin, it builds up in the bloodstream, causing a yellowish discoloration of the skin and eyes.
- Dark urine: Hemoglobin released from lysed red blood cells can be filtered by the kidneys and appear in the urine, making it appear darker than usual.
- Splenomegaly: The spleen plays a role in removing damaged red blood cells. In DIIHA, the spleen might become enlarged (splenomegaly) due to increased workload of clearing hemolyzed cells.
Additional Symptoms (may not be present in all cases)
- Weakness
- Dizziness
- Rapid heartbeat (tachycardia)
- Abdominal pain
- Fever (less common)
Progression of Symptoms
The severity of symptoms can vary depending on the rate of hemolysis. In some cases, the onset might be gradual with mild fatigue and paleness. In severe cases, hemolysis can be rapid and life-threatening, leading to significant shortness of breath, jaundice, and weakness.
Complications
Drug-induced immune hemolytic anemia (DIIHA) can lead to various complications if left untreated.
- Increased Risk of Infections: In drug-induced immune hemolytic anemia (DIIHA), the destruction of RBCs leads to anemia, which can impair the immune system’s ability to fight infections. This can make individuals with DIIHA more susceptible to infections and potentially lead to slower healing.
- Cardiac Issues: Anemia can put a strain on the heart as it tries to compensate for the reduced oxygen delivery to tissues. This can manifest as:
- Tachycardia (rapid heartbeat): The heart beats faster to pump more blood and try to deliver enough oxygen.
- Heart failure: In severe cases, the heart muscle may become weak and struggle to pump blood effectively.
- Cholelithiasis (Gallstones): The breakdown of RBCs releases bilirubin, which can form gallstones in the gallbladder. These stones can cause significant pain and inflammation.
- Hemosiderosis (Iron Overload): Hemoglobin, the protein inside RBCs, contains iron. When excessive hemolysis occurs, the body can’t keep up with processing the released iron. This iron overload can damage various organs, including the liver, heart, and pancreas.
- Kidney Complications: In severe hemolysis, large amounts of hemoglobin can be filtered by the kidneys. This can overwhelm the kidneys and potentially lead to kidney damage or failure.
Additional Complications
- Splenic Rupture (rare): The enlarged spleen in DIIHA can be susceptible to rupture, especially with trauma. This is a serious medical emergency requiring immediate surgery.
- Disseminated Intravascular Coagulation (DIC): In rare cases, severe DIIHA can trigger a life-threatening condition called DIC. This involves widespread blood clotting and bleeding throughout the body.
Laboratory Investigations
In diagnosing Drug-induced Immune Hemolytic Anemia (DIIHA), laboratory investigations play a crucial role. Here’s a breakdown of some key tests and their expected findings:
Complete Blood Count (CBC)
- Anemia: This is the hallmark finding in drug-induced immune hemolytic anemia (DIIHA). The CBC will typically show a decrease in red blood cell (RBC) count, hemoglobin concentration, and hematocrit (percentage of RBCs in whole blood).
- Reticulocytosis: The body tries to compensate for RBC destruction by increasing reticulocyte production (immature RBCs). A mild to moderate increase in reticulocytes might be seen.
Peripheral Blood Smear
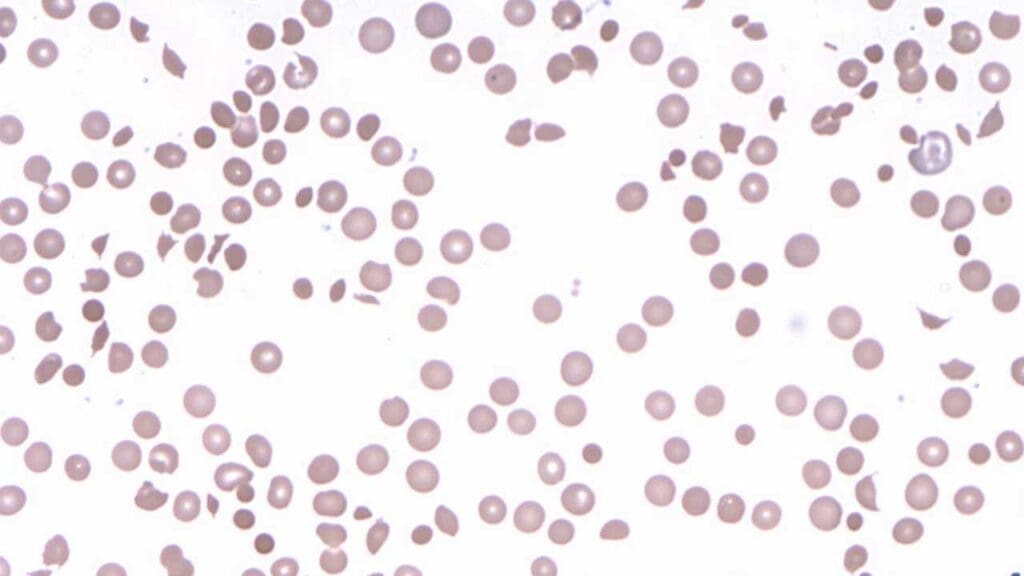
In DIIHA, the smear might reveal:
- Spherocytosis: RBCs may appear more spherical than usual due to damage from the immune attack.
- Fragmented RBCs: Fragments of RBCs might be present, indicating ongoing destruction.
- Polychromasia: Immature RBCs (reticulocytes) with a bluish hue might be visible.
Direct Antiglobulin Test (DAT)
This test detects antibodies or complement proteins bound to RBCs, a strong indicator of immune involvement in hemolysis.
- Positive DAT: A positive DAT is a crucial finding in drug-induced immune hemolytic anemia (DIIHA).
- Specificity: The type of antibody identified on the RBCs (IgG, IgM, or both) can provide further clues about the specific mechanism involved (e.g., IgG typically seen in the hapten model).
Other Tests
- LDH (Lactate Dehydrogenase): This enzyme is released from damaged RBCs. Elevated LDH levels suggest ongoing hemolysis.
- Haptoglobin: This protein binds to free hemoglobin released from lysed RBCs. Low haptoglobin levels indicate recent hemolysis.
- Blood bilirubin: Increased bilirubin levels can suggest hemolysis and potential liver involvement in processing the excess bilirubin.
- Urinalysis: Presence of hemoglobin in the urine (hemoglobinuria) might be seen in severe cases.
Importance of Considering Medication History
A thorough review of the patient’s medication history is crucial. Identifying a newly started medication or a change in dosage shortly before the onset of symptoms can be highly suggestive of drug-induced immune hemolytic anemia (DIIHA).
Differential Diagnosis of DIIHA
Drug-induced immune hemolytic anemia (DIIHA) can present with symptoms similar to other types of hemolytic anemia.
Non-Immune Hemolytic Anemias
- Hereditary Spherocytosis: A genetic disorder causing abnormally shaped RBCs that are more susceptible to destruction by the spleen.
- Sickle Cell Disease: Another genetic condition where RBCs have an abnormal sickle shape, leading to hemolysis and potential complications.
- Enzyme Deficiencies: Deficiencies in certain enzymes like glucose-6-phosphate dehydrogenase (G6PD) can make RBCs more vulnerable to damage by oxidative stress, leading to hemolysis.
Immune Hemolytic Anemias (Non-Drug Induced)
- Warm Autoimmune Hemolytic Anemia (WAIHA): The immune system attacks healthy RBCs in the absence of any medication trigger.
- Cold Agglutinin Disease (CAD): Antibodies in this condition target RBCs at cooler temperatures, causing hemolysis.
- Paroxysmal Nocturnal Hemoglobinuria (PNH): A rare blood disorder where a specific genetic mutation leads to RBC destruction.
Other Conditions
- Microangiopathic Hemolytic Anemia: This can occur in various conditions like thrombotic thrombocytopenic purpura (TTP) or disseminated intravascular coagulation (DIC) where mechanical damage to RBCs happens within small blood vessels.
- Hypersplenism: Enlarged spleen can lead to the destruction of healthy RBCs along with other blood cells.
Distinguishing DIIHA from Others
A comprehensive evaluation is necessary to differentiate drug-induced immune hemolytic anemia (DIIHA) from these conditions. Here are some factors to consider:
- Medication history: A recent initiation or change in medication is a crucial clue for drug-induced immune hemolytic anemia (DIIHA).
- Direct Antiglobulin Test (DAT): A positive DAT is suggestive of immune-mediated hemolysis, but the specific antibody type identified can help narrow down the cause (e.g., IgG in drug-induced immune hemolytic anemia (DIIHA) vs. IgM in cold agglutinin disease (CAD)).
- Other tests: Additional investigations like blood smears, reticulocyte count, and haptoglobin levels can provide further clues about the type of hemolytic anemia present.
Importance of Accurate Diagnosis
The specific cause of hemolytic anemia has significant implications for treatment. Therefore, accurately differentiating drug-induced immune hemolytic anemia (DIIHA) from other conditions is crucial for ensuring the most effective management strategy.
Treatment and Management
Key Principle: The cornerstone of drug-induced immune hemolytic anemia (DIIHA) management is the prompt identification and removal of the offending drug.
First-Line Therapy: Stop the Culprit Drug
- Stopping the offending medication is the most crucial step. This allows the immune system to “calm down” and the remaining healthy RBCs to survive.
- A thorough review of the medication history is essential to identify the culprit.
- If the medication is essential for another condition, alternative medications with a lower risk of DIIHA might be considered, in consultation with specialists in the relevant field.
Supportive Measures
- Blood Transfusions: In severe cases with significant anemia and compromised oxygen delivery, red blood cell transfusions might be necessary to improve oxygen-carrying capacity and alleviate symptoms.
- Folic Acid Supplementation: Folic acid is essential for RBC production. Supplementation might be necessary to support the body’s efforts to replenish RBCs after hemolysis.
Second-Line Therapies (if needed)
- Corticosteroids: These medications can suppress the immune response and reduce hemolysis. Prednisone is the most commonly used corticosteroid in drug-induced immune hemolytic anemia (DIIHA). Corticosteroids are typically used for a limited duration due to potential side effects with long-term use.
- Rituximab: This medication targets B cells, the immune cells responsible for antibody production. It can be effective in severe or persistent drug-induced immune hemolytic anemia (DIIHA) cases. Rituximab is an immunosuppressive therapy and requires careful monitoring for potential infections.
- Splenectomy (removal of the spleen): In rare and severe cases where other treatments fail, splenectomy might be considered. The spleen plays a role in RBC destruction, and removing it can reduce hemolysis. Splenectomy is a major surgery and increases the risk of infections. It’s typically reserved as a last resort after careful consideration of risks and benefits.
Monitoring Response
- Closely monitor clinical symptoms like fatigue, jaundice, and shortness of breath.
- Regularly assess laboratory parameters like hemoglobin, reticulocyte count, and LDH to track the response to treatment and identify any ongoing hemolysis.
- Monitor the Direct Antiglobulin Test (DAT) to assess the presence of antibodies on RBCs. A gradual decrease in the positive DAT result can indicate a successful treatment response.
Prognosis
- The prognosis for drug-induced immune hemolytic anemia (DIIHA) is generally good with prompt diagnosis and removal of the offending drug.
- Most patients recover completely with minimal long-term consequences.
- Factors like the severity of hemolysis and underlying medical conditions can influence the individual’s recovery timeline.
Education and Prevention
- Educate patients about drug-induced immune hemolytic anemia (DIIHA) and the importance of reporting any new or worsening symptoms while taking medications.
- Maintain a detailed medication history, including over-the-counter medications and supplements, to minimize the risk of future drug-induced immune hemolytic anemia (DIIHA) episodes.
- Consider consulting a specialist in hematology (blood disorders) for complex cases or patients with high-risk medications.
Additional Considerations
- The specific treatment approach for drug-induced immune hemolytic anemia (DIIHA) might be tailored based on the severity of the condition, the patient’s overall health status, and the specific medication involved.
Frequently Asked Questions (FAQs)
What is the onset of drug induced anemia?
The onset of drug-induced immune hemolytic anemia (DIIHA) can vary depending on several factors, including:
- The specific drug: Some medications are more likely to cause a rapid immune response, leading to a faster onset of DIIHA.
- Prior exposure to the drug: Individuals who have been previously exposed to the same drug or a similar medication in the same class might have a quicker onset due to pre-existing sensitization.
- Individual immune system: Variations in individual immune responses can influence the speed at which antibodies are produced against the drug-RBC complex.
Here’s a breakdown of the typical timeframe for DIIHA onset:
- Median time: The median time for the onset of DIIHA after starting the culprit drug is approximately 6 days.
- Range: However, the onset can range anywhere from hours to months after initial drug exposure.
- Rapid Onset: Cases with a very rapid onset (within hours) are more likely seen in individuals with prior sensitization.
- Delayed Onset: DIIHA might not develop until weeks or even months after starting the medication in some cases.
Can levothyroxine cause drug induced immune hemolytic anemia?
Levothyroxine, a synthetic thyroid hormone replacement medication, is generally not considered a common cause of drug-induced immune hemolytic anemia (DIIHA). Levothyroxine doesn’t have a known mechanism for direct RBC interaction.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program (2009) 2009 (1): 73–79.
- Maquet J, Lafaurie M, Michel M, Lapeyre-Mestre M, Moulis G. Drug-induced immune hemolytic anemia: detection of new signals and risk assessment in a nationwide cohort study. Blood Adv (2024) 8 (3): 817–826.
- https://medlineplus.gov/ency/article/000578.htm
- Chan Gomez J, Saleem T, Snyder S, Joseph M, Kanderi T. Drug-Induced Immune Hemolytic Anemia due to Amoxicillin-Clavulanate: A Case Report and Review. Cureus. 2020 Jun 17;12(6):e8666. doi: 10.7759/cureus.8666. PMID: 32699666; PMCID: PMC7370667.



