Introduction
The discovery of the JAK2 V617F mutation revolutionized our understanding of myeloproliferative neoplasms (MPNs). This single nucleotide change, where a guanine (G) is replaced by a thymine (T) at position 1849 in exon 14 of the JAK2 gene, leads to a valine (V) to phenylalanine (F) substitution at amino acid position 617 of the JAK2 protein. JAK2 itself plays a critical role in a signaling pathway essential for blood cell production. Let’s delve deeper into the function of JAK2, the consequences of the V617F mutation, its prevalence in different MPNs, and the significance of its detection.
The JAK2 Pathway
The JAK-STAT pathway, with JAK2 as a key component, regulates the proliferation, differentiation, and survival of various blood cell types. JAK2 acts like a molecular switch, activated by cytokines such as erythropoietin (EPO) and thrombopoietin (TPO). Upon activation, JAK2 phosphorylates specific proteins called STATs (Signal Transducer and Activator of Transcription). Activated STATs then translocate to the nucleus, where they bind to DNA and promote the expression of genes critical for blood cell production. This finely tuned pathway ensures the controlled generation of mature red blood cells, platelets, and white blood cells.
How does the V617F Mutation Fuel MPNs?
The V617F mutation acts as a gain-of-function mutation, permanently activating JAK2 even in the absence of cytokine stimulation. This leads to constitutive (always-on) signaling through the JAK-STAT pathway, causing uncontrolled growth and proliferation of blood cell precursors in the bone marrow. The consequence? An overproduction of specific blood cell types, characteristic of the various MPNs.
Prevalence of JAK2 V617F Mutation in Different MPNs
The prevalence of the JAK2 V617F mutation varies across MPN subtypes. It is found in a staggering 95% of patients with Polycythemia Vera (PV), a condition characterized by an excess of red blood cells. In Essential Thrombocythemia (ET), marked by an elevated platelet count, the mutation is present in 50-60% of cases. For Primary Myelofibrosis (PMF), a more aggressive form of MPN with bone marrow scarring, the prevalence falls to around 50%. These variations suggest the involvement of additional genetic alterations alongside JAK2 V617F in some MPNs.
Detection: A Window into Diagnosis, Prognosis, and Treatment
Identifying the JAK2 V617F mutation plays a pivotal role in MPN diagnosis. Techniques like ARMS PCR offer a reliable and sensitive method for mutation detection. A positive test result strengthens the diagnosis of MPN, particularly for PV, where its presence is nearly ubiquitous.
Detection of the JAK2 V617F mutation is crucial for several reasons. First, it serves as a valuable diagnostic tool, aiding in the differentiation of MPNs from other blood disorders with similar symptoms. Second, the mutation status helps in risk stratification. Patients with JAK2 V617F positive MPNs generally have a higher risk of developing complications like thrombosis (blood clots) and transformation to acute myeloid leukemia (AML). Finally, the presence of the mutation informs treatment decisions. The development of JAK2 inhibitors, drugs that specifically target the mutated JAK2 protein, has revolutionized MPN therapy. These drugs can effectively control disease symptoms and improve patient outcomes.
Beyond diagnosis, JAK2 mutation status influences prognosis. Patients with MPNs harboring the V617F mutation tend to have a slightly worse prognosis compared to those without the mutation. This is likely due to the more aggressive disease course associated with the uncontrolled cell proliferation driven by the mutation.
Principle
llele-specific PCR (AS-PCR) is a technique that leverages the sensitivity of PCR to detect single nucleotide polymorphisms (SNPs) or other small genetic variations. The crucial aspect of AS-PCR is the design of primers. Specifically, the 3′ end of the primer is designed to match the specific allele being targeted. Even a single base mismatch at the 3′ end of a PCR primer can significantly reduce or completely prevent primer extension by DNA polymerase. Therefore, if the 3′ end of the primer perfectly matches the target allele, amplification occurs. If there’s a mismatch, amplification is greatly diminished. Often, additional mismatches are introduced near the 3′ end of the primer to increase the difference in melting temperature between perfect matches and mismatches, thus increasing specificity.
By using primers that are specific to different alleles, AS-PCR allows for the selective amplification of only the desired allele. AS-PCR is commonly used for genotyping, which is the process of determining an individual’s genotype at a specific locus.
Advantages
- AS-PCR is exceptionally good at distinguishing between alleles that differ by a single nucleotide. This makes it ideal for detecting SNPs and other small genetic variations.
- It provides clear results with visualization of bands on a gel.
Limitations
- The success of AS-PCR heavily relies on careful primer design. Even minor errors in primer design can lead to false positives or false negatives.
- AS-PCR often requires a lot of optimization to get the best results. This can be time consuming.
- Although highly specific, AS-PCR can still produce false positives, especially if reaction conditions are not optimized or if there is contamination.
Method
This protocol is adapted from Baxter et al. (2005).
Table 1. Primer sequences for JAK2 V617F mutation.
| Primer ID | Primer sequences (5’ to 3’) |
| Reverse primer | CTG AAT AGT CCT ACA GTG TTT TCA GTT TCA |
| Forward specific | AGC ATT TGG TTT TAA ATT ATG GAG TAT ATT |
| Forward internal control | GAT CTA TAG TCA TGC TGA AAG TAG GAG AAA G |
- Wipe down the PCR work bench and pipettes with alcohol spray and turn on UV light for 10 minutes before starting any work.
- Thaw samples and reagents by hand, agitate and spin down. All components should be mixed and spun down prior to pipetting.
- Next, prepare a master mix for the PCR reaction in a 1.5 mL microcentrifuge tube on ice as shown in Table 2.
Table 2. Preparation of Master Mix for PCR reaction.
| Component | Each reaction | Final concentration |
| 5X KAPA2G Buffer | 5.0 µL | 1× |
| MgCl2 (25 mM) | 1.5 µL | 1.5 mM |
| dNTPs (10 mM) | 0.5 µL | 200 µM |
| Primer Reverse (10 µM) | 2.5 µL | 1.0 µM |
| Primer Forward Specific (10 µM) | 2.0 µL | 0.8 µM |
| Primer Forward IC (10 µM) | 1.25 µL | 0.5 µM |
| Hotstart Taq polymerase (5U/µL) | 0.1 µL | 0.5 U |
| Nuclease free water | 7.15 µL | – |
| Template DNA (can be added later e.g. Step 4) | 5.0 µL | 100 ng |
| Total | 25 µL |
- Mix master mix gently and spin down using a microcentrifuge.
- Add 20 µL of master mix to each respective PCR tube with pre-loaded 5 µL of template DNA on ice.
- Set the cycling conditions in the PCR Thermal Cycler machine as shown in Table 3 and start the run.
Table 3: Thermocycling conditions
| Step | Temperature (°C) | Time (minute(s)) |
| Initial Denaturation | 95°C | 10 minutes |
| Cycling 28 cycles | 95°C | 30 seconds |
| 60 °C | 45 seconds | |
| 72°C | 45 seconds | |
| Final Extension | 72°C | 10 minutes |
- Proceed to gel electrophoresis.
Interpretation
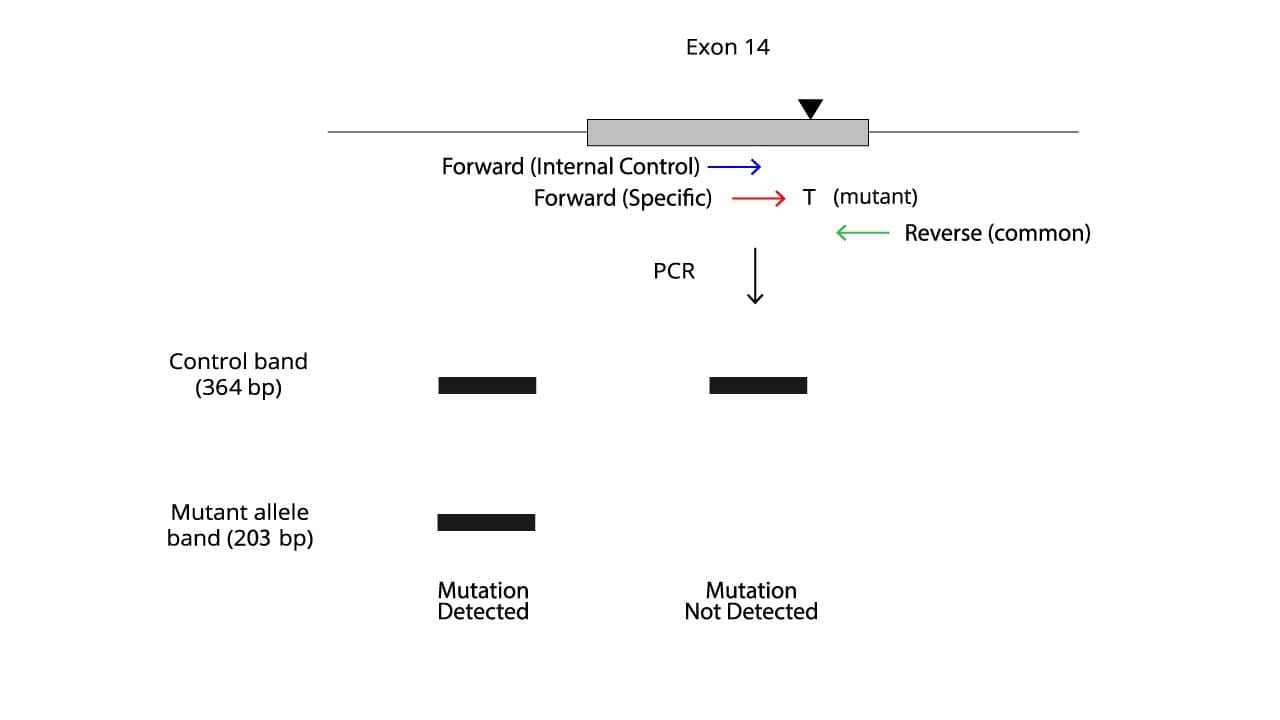
The presence or absence of specific bands on the gel determines the mutation status.
- A band corresponding to the control band only indicates a wild-type sequence and also that the PCR is working.
- A band corresponding to the mutant allele band indicates the presence of the JAK2 V617F mutation.
- Ideally, no amplification of the control band suggests issues with the PCR reaction, a failed reaction.
Troubleshooting
Allele-specific PCR, while a valuable tool, can be susceptible to several errors. Here are some common troubleshooting issues:
- Non-specific amplification: This occurs when primers bind to unintended sequences, leading to additional bands besides the expected ones.
- Primer design issues: Mismatches or primer degeneracy (lack of specificity) can lead to non-specific binding.
- PCR conditions: High primer or template concentration, inappropriate annealing temperature, or presence of PCR inhibitors can also contribute.
- Lack of amplification: This indicates the PCR reaction failed to amplify any product.
- Insufficient DNA: Low DNA concentration in the sample can lead to weak or absent amplification.
- Primer problems: Issues like primer degradation, incorrect primer sequences, or presence of inhibitors can prevent amplification.
- PCR conditions: Suboptimal reaction conditions like incorrect MgCl2 concentration or annealing temperature can hinder amplification.
- Faint bands:
- Low DNA quality: Degraded DNA can lead to inefficient amplification.
- Suboptimal PCR conditions: Reviewing factors like primer concentration, annealing temperature, or extension time might be necessary.
- Single band for both wild-type and mutant:
- Specific primer issues: Mismatches in the inner primers might not be stringent enough, allowing amplification of both wild-type and mutant sequences with the same primer.
- Contamination: Improper handling of samples or reagents can introduce contaminating DNA, leading to extraneous bands.
Troubleshooting Tips
- Review primer design: Ensure primers are specific and have appropriate melting temperatures.
- Optimize PCR conditions: Experiment with primer concentrations, annealing temperature, and extension times.
- Verify DNA quality and quantity: Use techniques like gel electrophoresis to assess DNA integrity and perform quantification assays.
- Maintain proper technique: Minimize contamination risks by following sterile techniques.
- Include positive and negative controls: Ensure the assay is functioning correctly.
By understanding these potential errors and implementing appropriate troubleshooting steps, you can optimize your allele-specific PCR for reliable JAK2 V617F mutation detection.
Frequently Asked Questions (FAQs)
What does it mean when JAK2 V617F mutation is detected?
Detecting a JAK2 V617F mutation is a strong indication that a person is likely to have a Myeloproliferative Neoplasm (MPN). MPNs are a group of blood cancers characterized by uncontrolled growth of bone marrow cells, leading to an overproduction of red blood cells, white blood cells, or platelets.
However, it’s important to understand some key points:
- Not definitive diagnosis: While a positive JAK2 V617F mutation test is highly suggestive of MPN, it’s not a definitive diagnosis on its own. Other tests like a bone marrow biopsy and analysis of blood cell counts are needed for a confirmed diagnosis and to determine the specific type of MPN (e.g., Polycythemia Vera, Essential Thrombocythemia, Primary Myelofibrosis).
- Not everyone with MPN has the mutation: While the JAK2 V617F mutation is prevalent in MPNs, it’s not present in all cases. There can be other mutations in the JAK2 gene or other genes that contribute to MPN development. A negative JAK2 V617F test doesn’t necessarily rule out MPN.
- However, the presence of the mutation doesn’t guarantee you’ll develop MPN. Many people with the mutation never experience any related issues.
Here’s a breakdown of what a positive JAK2 V617F mutation suggests:
- Increased risk: Individuals with the mutation generally have a higher risk of developing complications associated with MPNs, such as blood clots (thrombosis) and transformation to acute myeloid leukemia (AML).
- Targeted therapy options: The presence of the mutation opens doors for specific treatment strategies. JAK2 inhibitors, drugs that target the mutated JAK2 protein, have revolutionized MPN therapy, offering effective control of symptoms and improved patient outcomes.
Next steps
If a JAK2 V617F mutation is detected, a hematologist will interpret the results in conjunction with other clinical findings and tests. This will guide the diagnosis, determine the best course of treatment, and monitor the disease progression.
Can JAK2 mutation disappear?
In most cases, the JAK2 V617F mutation itself won’t disappear. It’s a permanent change in the genetic code of a cell. However, there are some situations where the detectability of the mutation might be reduced or even become undetectable.
- Limited Disappearance: Complete disappearance of the JAK2 V617F mutation from all bone marrow cells is extremely rare.
- Reduced Allele Burden: In some cases, with specific treatments like hydroxyurea (HU), the proportion of cells carrying the mutated JAK2 gene (allele burden) can decrease significantly. This might even lead to undetectable levels of the mutation in some tests. However, the mutation itself is still present in a smaller number of cells.
- Treatment Considerations
- Hydroxyurea (HU): This chemotherapy drug can sometimes reduce the overall number of bone marrow cells, including those with the JAK2 mutation. This can lead to a lower detectable level of the mutation in some patients.
- Bone Marrow Transplant (BMT): In rare cases, a successful BMT can replace diseased bone marrow with healthy cells, effectively eliminating the JAK2 mutation from the blood system.
What happens if JAK2 is negative?
A negative JAK2 test result, specifically for the JAK2 V617F mutation, doesn’t necessarily mean absence of Myeloproliferative Neoplasms (MPNs). Here’s a breakdown of what a negative result might indicate:
- No MPN: It’s possible there’s no MPN at all. The JAK2 V617F mutation is prevalent in MPNs, but it’s not the only cause.
- Other Mutations: There might be other mutations in the JAK2 gene besides V617F that contribute to MPN development. These mutations are less common and require more specialized tests for detection.
- Mutations in Other Genes: MPNs can also arise from mutations in other genes besides JAK2. These mutations can affect different signaling pathways involved in blood cell production.
What to do next with a negative JAK2 test:
- Further Investigation: If there are symptoms suggestive of MPN despite a negative JAK2 test, a hematologist might recommend further investigations. This could include:
- Blood tests: Complete blood count (CBC) with differential to assess cell counts.
- Bone marrow biopsy: Examining bone marrow samples under a microscope can reveal signs of abnormal cell growth characteristic of MPNs.
- Genetic testing: Looking for mutations in other genes associated with MPNs beyond JAK2.
- Diagnosis: Based on all the findings, including clinical symptoms, blood tests, and bone marrow analysis.
Are you born with JAK2?
No, a person is not born with the JAK2 V617F mutation. The JAK2 gene itself is a normal gene present in everyone, and it plays an important role in signaling pathways for blood cell production.
The JAK2 V617F mutation is an acquired mutation, meaning it develops at some point in life. It is not an inherited disease. Here’s a breakdown of how it can occur:
- Somatic Mutation: The JAK2 V617F mutation happens in somatic cells, which are all body cells except for sperm and egg cells. This means the mutation occurs after conception and isn’t present in the germline (reproductive cells).
- Sporadic Event: The exact cause of the JAK2 V617F mutation is not entirely clear, but it’s believed to be a random event during cell division. Errors in copying DNA during cell replication can lead to mutations like this.
- No Hereditary Risk: Since the mutation occurs in somatic cells, it can’t be passed down to their children. There’s no increased risk for offsprings to develop the mutation or MPNs simply because a person has it.
Can JAK2 test be wrong?
Yes, JAK2 testing, specifically for the JAK2 V617F mutation, can have limitations and produce inaccurate results.
Types of Errors
- False-positive results
- Test limitations: Techniques like allele-specific PCR may have limited sensitivity, potentially missing low-level mutations or detecting background noise.
- Non-specific primer binding: Mismatches in primer design can lead to amplification of unintended sequences, mimicking the presence of the mutation.
- Sample contamination: Contamination with DNA from other sources (e.g., normal cells) can lead to false positives.
- False-negative results
- Low mutant allele burden: If the proportion of cells carrying the mutation is very low, the test might not be sensitive enough to detect it.
- Inhibitor presence: Substances in the blood sample can interfere with the PCR reaction, hindering detection of the mutation.
- Mosaicism: Unequal distribution of the mutation among blood cells can lead to a negative result if the sample doesn’t contain enough mutated cells.
Minimizing Errors
- Test selection: Utilizing validated assays with high sensitivity and specificity for JAK2 V617F mutation detection is crucial. Techniques like quantitative PCR or next-generation sequencing can offer greater accuracy.
- Optimizing procedures: Following proper protocols for sample collection, DNA extraction, and PCR setup minimizes contamination and technical errors.
- Repeat testing: In some cases, especially if clinical suspicion for MPN is high despite a negative JAK2 test, repeating the test with a different assay or sending the sample to a reference laboratory can be considered.
Clinical Context
- A negative JAK2 test result should be interpreted within the context of the patient’s clinical presentation. Symptoms suggestive of MPN, complete blood count findings, and other laboratory investigations all play a role in diagnosis.
- Consider alternative diagnoses: If a high suspicion for MPN persists despite a negative JAK2 test, explore mutations in other genes associated with MPN (e.g., MPL, CALR) or other causes of bone marrow dysfunction.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR; Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19-25;365(9464):1054-61. doi: 10.1016/S0140-6736(05)71142-9. Erratum in: Lancet. 2005 Jul 9-15;366(9480):122. PMID: 15781101.
- Thapa B, Fazal S, Parsi M, et al. Myeloproliferative Neoplasms. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531464/
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.



