TL;DR
Anemia of chronic disease (ACD) is a type of anemia that develops as a consequence of chronic inflammatory conditions in the body.
Causes ▾
- Chronic infections
- Autoimmune diseases
- Other chronic inflammatory conditions e.g. chronic kidney disease
- Sarcoidosis
Pathophysiology ▾
Chronic inflammation disrupts red blood cell production through multiple mechanisms:
- Suppressed EPO Production: Inflammation suppresses the production of erythropoietin (EPO), a hormone crucial for stimulating red blood cell production.
- Iron Dysregulation: Despite normal or elevated iron stores, hepcidin (an inflammatory protein) limits iron availability for red blood cell production.
- Shortened Red Blood Cell Lifespan: Chronic inflammation can slightly shorten the lifespan of circulating red blood cells.
Signs and Symptoms ▾
- Often has non-specific symptoms like fatigue, weakness, and shortness of breath. Symptoms can be easily overlooked.
Laboratory Diagnosis ▾
- CBC and PBF: Can be microcytic (small red blood cells) or normocytic (normal-sized red blood cells). Low reticulocyte count (impaired red blood cell production).
- Iron studies: Low serum iron with normal or elevated ferritin (iron entrapment, not deficiency). Low transferrin saturation (reflects limited iron availability).
- ESR and CRP: Elevated
Differential Diagnosis ▾
- Important to distinguish ACD from other types of anemia like iron deficiency anemia, vitamin B12 or folate deficiency anemia, and aplastic anemia through clinical features, laboratory tests, and sometimes bone marrow examination.
Treatment ▾
- Focuses on addressing the underlying chronic inflammatory condition.
- Erythropoietin-Stimulating Agents (ESAs) might be considered in specific cases, but their effectiveness is limited in ACD.
*Click ▾ for more information
Introduction
Anemia of chronic disease (ACD) is a type of anemia that develops as a consequence of chronic inflammatory conditions in the body. These chronic inflammatory conditions can be diverse, ranging from infections and autoimmune diseases to chronic kidney disease and cancer.
There are several key factors contributing to anemia of chronic disease (ACD) being one of the most common types of anemia:
- High Prevalence of Chronic Diseases: Chronic inflammatory conditions like infections, autoimmune diseases, and chronic kidney disease are widespread globally. As the prevalence of these underlying conditions rises, so does the likelihood of developing anemia of chronic disease (ACD) as a secondary consequence.
- Multiple Pathways to Anemia of Chronic Disease (ACD): Chronic inflammation disrupts red blood cell production in several ways. Suppressed EPO production, iron dysregulation due to hepcidin, and shortened red blood cell lifespan all contribute to anemia of chronic disease (ACD) development. This multifaceted approach to disrupting red blood cell production makes anemia of chronic disease (ACD) more likely to occur.
- Subtle Symptoms in Early Stages: Anemia of chronic disease (ACD) often presents with mild, non-specific symptoms like fatigue and weakness, which can be easily overlooked or attributed to the underlying chronic illness itself. This can lead to delayed diagnosis and potentially higher prevalence estimates as some cases might go undetected.
- Aging Population: As the global population ages, the prevalence of chronic diseases associated with anemia of chronic disease (ACD) , such as rheumatoid arthritis and heart failure, is also expected to rise. This demographic shift might further contribute to the increasing prevalence of anemia of chronic disease (ACD) .
- Improved Diagnostics, But Still Challenging: While diagnostic techniques for anemia have improved, definitively diagnosing anemia of chronic disease (ACD) can still be challenging, especially in its early stages. The presence of other types of anemia alongside anemia of chronic disease (ACD) can further complicate diagnosis. This might lead to underdiagnosis and potentially contribute to a higher perceived prevalence.
Definition of Anemia
Anemia is a condition in which the blood has a reduced ability to carry oxygen. Anemia is defined as when the hemoglobin level in the blood falls below the reference or normal range for the particular age and gender of the person. This can happen due to three main reasons:
- Low number of red blood cells: Red blood cells are the carriers of hemoglobin, a protein that binds to oxygen in the lungs and delivers it throughout the body. If we don’t have enough red blood cells, our blood will have less capacity to transport oxygen.
- Reduced amount of hemoglobin: Hemoglobin is the iron-rich protein inside red blood cells that gives blood its red color. If there is a deficiency in hemoglobin, even with a normal number of red blood cells, the blood will be less efficient at carrying oxygen.
- Abnormal hemoglobin function: In some cases, the hemoglobin itself might be abnormal and not function properly, even if the red blood cell count and hemoglobin quantity are within normal range. This can impair oxygen delivery.
Nearly 2 billion people worldwide were affected by anemia in 2021, with women disproportionately impacted compared to men. A recent study analyzing global anemia data from 1990 to 2021 reveals a complex interplay of factors contributing to the varying experiences of men, women, and children with this condition. Data from 2021 paints a clear picture of a gender disparity in anemia prevalence. Globally, over 31% of women were affected compared to only 17.5% of men. This difference becomes even more striking during reproductive years (ages 15-49), where women experience a prevalence of nearly 34% compared to just 11.3% in men.
Types of Anemia
Anemia can be classified in several ways, but one of the most approaches is based on the size of the red blood cells morphologically.
Classification Based on Red Blood Cell Size
- Microcytic Anemia: This type is characterized by smaller than normal red blood cells. Iron deficiency anemia is the most common cause, where the body lacks sufficient iron to produce healthy red blood cells. Other causes can include chronic blood loss or conditions affecting iron absorption.
- Normocytic Anemia: In this type, the red blood cells are normal-sized. Normocytic anemia can arise from various causes, including chronic diseases (anemia of chronic disease – ACD), bone marrow problems (aplastic anemia), or hemolytic anemia (where red blood cells are destroyed prematurely).
- Macrocytic Anemia: This type features red blood cells larger than normal. It’s often linked to deficiencies in vitamin B12 or folate, both essential for red blood cell production. Other causes can include liver disease or alcohol abuse.
Additional Classification Systems
While red blood cell size is a helpful initial classification, anemia can also be categorized based on:
- Hemoglobin level: This considers the severity of anemia by classifying anemia based on hemoglobin concentration, such as normal, mild, moderate or severe.
- Cause of the Anemia: This delves deeper into the underlying factors causing the anemia, such as deficiencies, chronic conditions, or blood loss.
Causes of Anemia of Chronic Disease (ACD)
Chronic Infections
- Bacterial infections like tuberculosis, osteomyelitis (bone infection), and endocarditis (infection of the heart valves)
- Viral infections like HIV/AIDS and hepatitis B or C
- Parasitic infections like malaria
Autoimmune Diseases
- Rheumatoid arthritis
- Systemic lupus erythematosus (SLE)
- Inflammatory bowel disease (IBD) including Crohn’s disease and ulcerative colitis
Other Chronic Inflammatory Conditions
- Chronic kidney disease (CKD)
- Certain cancers (lymphoma, carcinoma, sarcoma)
Additional Less Common Causes
- Certain autoimmune vasculitides (inflammation of blood vessels)
- Sarcoidosis (inflammatory disease affecting multiple organs)
Pathophysiology of ACD
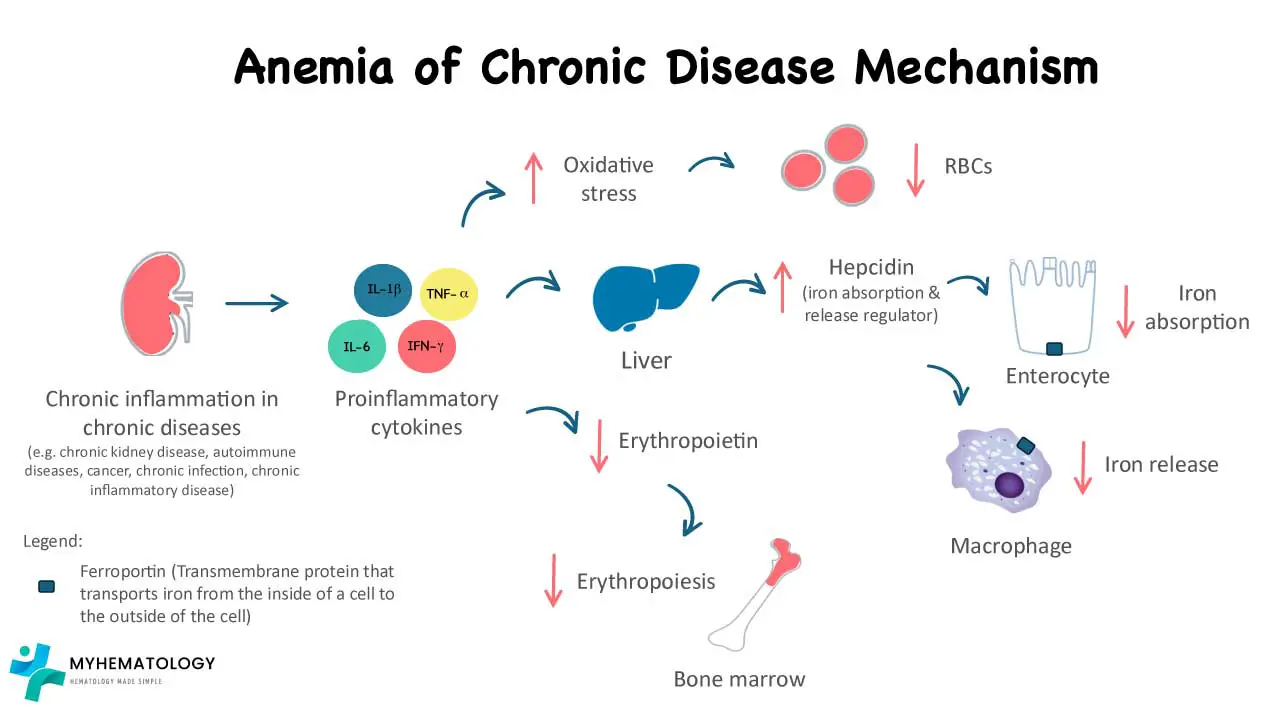
ACD arises from a complex interplay between the chronic inflammatory response and several factors affecting red blood cell production.
Chronic Inflammation and Cytokines
- Chronic inflammatory conditions trigger the release of various inflammatory cytokines, including interleukin-1 (IL-1) and interleukin-6 (IL-6).
- IL-1: This cytokine directly suppresses the production of erythropoietin (EPO) by the kidneys. EPO is a hormone crucial for stimulating red blood cell production in the bone marrow. Reduced EPO levels lead to a decrease in red blood cell production.
- IL-6: While its exact role is still being explored, IL-6 is believed to contribute to iron dysregulation and potentially shorten the lifespan of red blood cells.
Iron Dysregulation and Hepcidin
- Chronic inflammation leads to the production of a protein called hepcidin by the liver.
- Hepcidin binds to ferroportin, a protein on the surface of macrophages and intestinal enterocytes, which are responsible for iron release and absorption, respectively, regulating iron release from storage sites in the body (macrophages and hepatocytes).
- In anemia of chronic disease (ACD), even though total body iron stores might be normal or even elevated, hepcidin restricts iron release, making it less available for hemoglobin synthesis in red blood cells. This creates a state of “functional iron deficiency” within the bone marrow, despite having sufficient iron stores.
Other Contributing Factors
- Shortened Red Blood Cell Lifespan: Chronic inflammation might have a mild suppressive effect on the lifespan of circulating red blood cells, further contributing to lower red blood cell counts.
- Nutritional Deficiencies: Chronic illness can sometimes lead to poor dietary intake or malabsorption, potentially contributing to deficiencies in vitamins and minerals like folate and vitamin B12, which are essential for red blood cell production.
Key Points to Remember
- The combined effect of suppressed EPO production, iron dysregulation due to hepcidin, and potentially shortened red blood cell lifespan leads to the development of anemia in anemia of chronic disease (ACD) .
- While iron stores might appear normal, the body struggles to utilize it effectively for red blood cell production due to hepcidin’s action.
Signs and Symptoms of Anemia of Chronic Disease (ACD)

Anemia of chronic disease (ACD) is often a subtle condition, and its symptoms can be easily overlooked or attributed to the underlying chronic illness itself.
General Symptoms
- Fatigue and weakness
- Shortness of breath, especially during exertion
- Pale skin
- Dizziness or lightheadedness
- Difficulty concentrating or thinking clearly (cognitive difficulties)
- Decreased exercise tolerance
Additional Symptoms
- Chest pain
- Headaches
- Restlessness or difficulty sleeping
- Cold hands and feet
- Loss of appetite
Complications of Anemia of Chronic Disease (ACD)
While anemia of chronic disease (ACD) itself is rarely life-threatening, it can lead to various complications that can significantly impact a patient’s quality of life.
Worsened Heart Failure Symptoms
In patients with existing heart failure, anemia of chronic disease (ACD) can worsen symptoms like shortness of breath, fatigue, and exercise intolerance. This is because anemia reduces the oxygen-carrying capacity of the blood, making it even harder for the heart to pump blood effectively.
Increased Risk of Infections
A well-functioning immune system relies on adequate oxygen delivery. Anemia of chronic disease (ACD) can impair the immune system’s ability to fight infections, potentially increasing the risk of developing infections or experiencing slower healing after an infection.
Impaired Quality of Life
The fatigue, weakness, and shortness of breath associated with anemia of chronic disease (ACD) can significantly impact a person’s daily activities, work performance, and overall well-being. This can lead to feelings of frustration, social isolation, and decreased quality of life.
Increased Risk of Hospitalization
Patients with anemia of chronic disease (ACD) and its complications might require more frequent hospital admissions due to issues like worsening heart failure symptoms or infections.
Cognitive Impairment (in some cases)
Chronic anemia can affect oxygen delivery to the brain, potentially leading to problems with memory, concentration, and overall cognitive function.
Laboratory Investigations
Diagnosing anemia of chronic disease (ACD) can be challenging due to its overlapping symptoms with other types of anemia and the potential absence of prominent symptoms in the early stages.
Complete Blood Count (CBC)
This is the initial test for evaluating anemia.
- Microcytic or Normocytic Anemia: Anemia of chronic disease (ACD) can present with either microcytic (small red blood cells) or normocytic (normal-sized red blood cells).
- Low Reticulocyte Count: Reticulocytes are young red blood cells. A low reticulocyte count indicates decreased bone marrow production of red blood cells.
Iron Studies
While iron deficiency is not the primary cause in anemia of chronic disease (ACD), these tests provide valuable information.
- Low Serum Iron: Serum iron levels might be low, but this doesn’t necessarily indicate iron deficiency in anemia of chronic disease (ACD).
- Normal or Increased Ferritin: Ferritin is a protein that stores iron. In anemia of chronic disease (ACD), ferritin levels can be normal or even elevated despite low serum iron, reflecting iron entrapment due to hepcidin.
- Low Transferrin Saturation: Transferrin is a protein that transports iron in the blood. Transferrin saturation reflects the percentage of transferrin occupied by iron. In anemia of chronic disease (ACD), transferrin saturation is typically low.
Additional Tests
- Inflammatory Markers: C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) might be elevated due to the underlying chronic inflammatory condition.
- Bone Marrow Aspiration/Biopsy (rarely): In some complex cases, a bone marrow examination might be necessary to rule out other causes of anemia. The bone marrow storage (reticuloendothelial) iron is normal, but erythroblast iron is reduced.
Differential diagnosis of ACD from other types of anemia
Distinguishing anemia of chronic disease (ACD) from other types of anemia can be crucial for guiding appropriate treatment.
Iron Deficiency Anemia (IDA)
- Iron Studies: In IDA, serum iron, ferritin, and transferrin saturation are typically low, reflecting true iron deficiency. In anemia of chronic disease (ACD), ferritin might be normal or elevated despite low serum iron due to hepcidin-mediated entrapment.
- Transferrin Saturation: Transferrin saturation is a useful differentiator. While it’s low in both ACD and IDA, the reasons differ. In IDA, low transferrin saturation reflects a lack of iron to bind to transferrin. In ACD, it reflects adequate iron stores but limited availability due to hepcidin.
- Clinical Presentation: IDA often presents with more prominent symptoms like severe fatigue, pale skin, and pica (cravings for non-food items). ACD symptoms might be milder or non-specific.
Vitamin B12 or Folate Deficiency Anemia
- Macrocytic Anemia: These deficiencies typically cause macrocytic anemia (large red blood cells), whereas anemia of chronic disease (ACD) can present with microcytic (small red blood cells) or normocytic (normal-sized red blood cells).
- Specific Tests: Vitamin B12 and folate levels can be measured to confirm these deficiencies. These tests are not routinely used in diagnosing anemia of chronic disease (ACD).
- Clinical Features: Vitamin B12 or folate deficiency might cause neurological symptoms like tingling or numbness in the extremities, which are not typically seen in anemia of chronic disease (ACD).
Aplastic Anemia
- Bone Marrow Examination: A bone marrow biopsy might be necessary to differentiate anemia of chronic disease (ACD) from aplastic anemia, a condition where the bone marrow has a severely reduced capacity to produce blood cells.
- Severity: Aplastic anemia is a more severe condition compared to anemia of chronic disease (ACD) and requires specialized treatment.
Additional Considerations
- In some cases, a combination of anemia types might be present. For example, a patient with chronic inflammatory bowel disease (CIBD) might have anemia of chronic disease (ACD) along with iron deficiency due to blood loss.
- A thorough medical history, physical examination, and appropriate laboratory tests are essential for accurate diagnosis.
Treatment and Management
The primary goal of managing anemia of chronic disease (ACD) is to address the underlying chronic inflammatory condition that’s causing it.
Treating the Underlying Condition
Effective management of the chronic inflammatory disease (infection, autoimmune disorder, etc.) is crucial for improving red blood cell production and reducing inflammation. This might involve medications, lifestyle changes, and other specific therapies depending on the underlying condition.
Erythropoietin-Stimulating Agents (ESAs) (Considered in Specific Cases)
In some cases, Erythropoietin-Stimulating Agents (ESAs) might be used to stimulate red blood cell production in the bone marrow. However, their use in anemia of chronic disease (ACD) is more restricted compared to other types of anemia due to:
- Limited Response: The inflammatory response in anemia of chronic disease (ACD) can still hinder the effectiveness of ESAs.
- Iron Availability: If iron stores are truly low, even with ESAs, red blood cell production might be limited. Iron supplementation might be necessary in these cases.
- Risk of Complications: ESAs can be associated with an increased risk of blood clots. Careful monitoring and risk-benefit analysis are essential before using ESAs in anemia of chronic disease (ACD).
Iron Supplementation (Limited Role in ACD)
Unlike iron deficiency anemia, iron supplementation is not the primary treatment for anemia of chronic disease (ACD). This is because iron stores are often normal or elevated in anemia of chronic disease (ACD) due to hepcidin-mediated entrapment. Supplementation might even worsen inflammation in some cases. However, in specific situations where iron deficiency co-exists with anemia of chronic disease (ACD), iron supplementation might be considered to improve red blood cell production.
Blood Transfusions (Rarely Used)
Blood transfusions are generally reserved for severe cases of anemia of chronic disease (ACD) with life-threatening complications like heart failure or for patients undergoing major surgery. Transfusions should be used cautiously due to potential risks like iron overload and bloodborne infections.
Supportive Measures
- Maintaining a healthy diet rich in iron, vitamins, and minerals can support overall health and red blood cell production.
- Managing fatigue and improving sleep quality can enhance well-being.
- Addressing any psychological concerns or anxiety associated with chronic illness can be helpful.
Frequently Asked Questions (FAQs)
What are the diagnostic criteria for anemia of chronic disease?
There aren’t strictly defined diagnostic criteria for Anemia of Chronic Disorders (ACD) because there’s no single test that definitively diagnoses it. Diagnosis relies on a combination of factors:
Clinical Features
- Presence of a chronic inflammatory condition (infection, autoimmune disease, etc.) lasting for at least 3 months.
Laboratory Findings
- Anemia: Microcytic (small red blood cells) or normocytic (normal-sized red blood cells) anemia based on a complete blood count (CBC).
- Iron Paradox:
- Low serum iron levels.
- Normal or elevated ferritin levels (indicating iron stores but limited availability due to hepcidin).
- Low Transferrin Saturation: Reflects low percentage of transferrin carrying iron.
- Low Reticulocyte Count: Suggests decreased red blood cell production in the bone marrow.
Additional Considerations
- While not routinely used for diagnosis, elevated inflammatory markers (C-reactive protein or erythrocyte sedimentation rate) might be present due to the underlying chronic condition.
- In some complex cases, a bone marrow examination might be necessary to rule out other causes of anemia.
How long does it take to recover from anemia of chronic disease?
Unfortunately, there’s no one-size-fits-all answer to how long it takes to recover from Anemia of Chronic Disorders (ACD) because the recovery timeline depends on several factors:
- Severity of the Underlying Condition: Effectively managing the chronic inflammatory disease that’s causing ACD is essential for improvement. The speed of recovery hinges on controlling the underlying condition and reducing inflammation.
- Degree of Anemia: The severity of the anemia itself also influences recovery time. More severe anemia might take longer to improve compared to mild cases.
- Treatment Approach: The specific treatment approach used, such as addressing nutritional deficiencies or using medications like ESAs (in specific cases), can impact the recovery rate.
- Individual Factors: Individual factors like overall health status and response to treatment can also influence how quickly red blood cell counts normalize.
Here’s a general timeframe to consider
- With effective treatment of the underlying condition: In some cases, with successful management of the chronic inflammatory condition, noticeable improvement in anemia can occur within weeks to months.
- More complex cases: In situations with severe underlying conditions or where controlling inflammation is challenging, recovery might take several months or even longer.
Why is TIBC low in anemia of chronic disease?
In Anemia of Chronic Disease (ACD), the total iron-binding capacity (TIBC) is typically low even though there might be adequate or even elevated iron stores in the body.
Understanding Transferrin and TIBC
- Transferrin: This is a protein in the blood that plays a critical role in iron transport. It acts like a carrier, binding to iron and shuttling it throughout the body for various functions, including red blood cell production.
- TIBC: Total Iron-Binding Capacity is an indirect measurement of transferrin levels in the blood. The higher the TIBC, the more iron transferrin can potentially bind to.
The Role of Hepcidin in ACD
- Chronic inflammation triggers the production of hepcidin, a regulatory protein produced by the liver.
- Hepcidin binds to ferroportin, a protein on the surface of macrophages and intestinal enterocytes. These cells are responsible for iron release from stores and absorption from the diet, respectively.
- By binding to ferroportin, hepcidin essentially locks up iron within these cells, hindering its release into circulation even though iron stores might be normal or elevated.
How Hepcidin Affects TIBC
- Since iron is trapped within cells due to hepcidin, less iron is available to bind to transferrin in the bloodstream.
- As a consequence, a significant portion of transferrin remains unbound.
- This translates to a lower TIBC because the total capacity for iron binding (transferrin) is not being fully utilized.
Key Points
- Low TIBC in ACD reflects limited iron availability due to hepcidin, not a true lack of iron stores.
- It contrasts with iron deficiency anemia, where TIBC is high because there’s insufficient iron to bind to all the available transferrin.
Analogy
Imagine transferrin as taxis and iron as passengers. In a normal situation (without ACD), most taxis (transferrin) are carrying passengers (iron). TIBC would be high, reflecting a full capacity. In ACD, due to hepcidin, iron is stuck at the “depots” (macrophages and enterocytes). Even though there are plenty of taxis, most are empty, leading to a lower TIBC.
Why is ferritin high in anemia of chronic disease?
In Anemia of Chronic Disease (ACD), ferritin levels are often normal or elevated even though there’s limited iron available for red blood cell production. This might seem counterintuitive, but here’s why ferritin is high in ACD:
Understanding Ferritin
- Ferritin is a protein found in cells throughout the body, particularly in the liver, spleen, and bone marrow. It acts as a storage unit for iron, binding to excess iron and keeping it safe for future use.
- A high ferritin level generally indicates sufficient or even overloaded iron stores in the body.
The Role of Hepcidin in ACD
- Chronic inflammation triggers the production of hepcidin, a regulatory protein produced by the liver.
- Hepcidin binds to ferroportin, a protein on the surface of macrophages and intestinal enterocytes. These cells store iron from breakdown of red blood cells (macrophages) and absorb dietary iron (enterocytes).
- By binding to ferroportin, hepcidin essentially locks up iron within these cells, hindering its release into circulation for red blood cell production.
Why Ferritin is High Despite Limited Availability
- Even though iron is trapped within cells due to hepcidin, the total body iron stores remain adequate or elevated. This is reflected by the high ferritin levels.
- The iron is simply not accessible for red blood cell production because hepcidin restricts its release.
Key Points
- High ferritin in ACD indicates iron stores are present, but they are not available for erythropoiesis (red blood cell production) due to hepcidin.
- This is a key difference from iron deficiency anemia (IDA) where ferritin levels are typically low because there’s a genuine lack of iron stores in the body.
Analog
Imagine ferritin as a safe deposit box for iron. In a healthy situation, the safe deposit box (ferritin) has some iron stored. In ACD, the safe deposit box (ferritin) is full of iron, but it’s locked shut (due to hepcidin) and inaccessible for red blood cell production. This leads to high ferritin but limited usable iron.
Can osteoarthritis cause anemia of chronic disease?
Osteoarthritis is a degenerative joint disease primarily characterized by wear and tear of cartilage in the joints. While some low-grade inflammation might be present in OA, it’s different from the systemic and persistent inflammation seen in conditions that cause ACD.
Can diabetes cause anemia of chronic disease?
Diabetes itself does not directly cause Anemia of Chronic Disease (ACD), but there can be a complex interplay between the two conditions. Chronic complications of diabetes, such as nephropathy (kidney disease) or neuropathy (nerve damage), can sometimes trigger secondary inflammatory processes. This could contribute to ACD in some cases.
Can hypertension cause anemia of chronic disease?
No, hypertension (high blood pressure) is not a direct cause of Anemia of Chronic Disease (ACD). ACD arises from chronic inflammatory conditions, whereas high blood pressure is a cardiovascular condition with distinct causes. However, there can be some indirect connections and considerations.
Who treats anemia of chronic disease?
A patient with Anemia of Chronic Disease (ACD) will typically be managed by a collaborative team of healthcare professionals including the primary care physician, the hematologist and the specialist for the underlying chronic condition.
References
- Madu AJ, Ughasoro MD. Anaemia of Chronic Disease: An In-Depth Review. Med Princ Pract. 2017;26(1):1-9. doi: 10.1159/000452104. Epub 2016 Sep 28. PMID: 27756061; PMCID: PMC5588399.
- GBD 2021 Anaemia Collaborators. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990-2021: findings from the Global Burden of Disease Study 2021. Lancet Haematol. 2023 Sep;10(9):e713-e734. doi: 10.1016/S2352-3026(23)00160-6. Epub 2023 Jul 31. Erratum in: Lancet Haematol. 2023 Oct;10(10):e796. Erratum in: Lancet Haematol. 2024 Jan;11(1):e10. PMID: 37536353; PMCID: PMC10465717.
- Svenson N, Bailey J, Durairaj S, Dempsey-Hibbert N. A simplified diagnostic pathway for the differential diagnosis of iron deficiency anaemia and anaemia of chronic disease. International Journal of Laboratory Hematology. 2021;43:1644–1652.