TL;DR
Aplastic anemia (AA) causes pancytopenia (reduction of all blood cell types: RBCs, WBCs and platelets) due to aplasia (failure) of the bone marrow to function (to produce blood cells).
- Causes▾:
- Congenital e.g. Fanconi anemia, dyskeratosis congenita
- Acquired e.g. idiopathic, drugs, chemical agents, infections
- Signs and symptoms ▾: Lethargy, pallor (anemia), infections especially mouth and throat (neutropenia), bruising, epistaxis, gum bleeding (thrombocytopenia)
- Pathophysiology of idiopathic aplastic anemia (IAA) ▾: Hematopoietic stem cells targeted by gamma-interferon and tumor necrosis factor secreted by cytotoxic T-cells.
- Laboratory investigations and diagnosis ▾:
- Complete blood count: Anemia, leukopenia, thrombocytopenia, ↓ reticulocytes.
- Bone marrow smear: Hypoplasia with mainly lymphocytes and plasma cells present
- Cytogenetic analysis for differential diagnosis from hypoplastic myelodysplasia (abnormalities expected)
- Treatment and management ▾: Treat the underlying cause, transfusion of packed red cells and platelet concentrates as supportive treatment, prevention of infection and specific treatment e.g. antilymphocyte or antithymocyte globulin
*Click ▾ for more information
What is aplastic anemia (AA)?
Aplastic anemia (AA), a rare but serious blood disorder, occurs when the bone marrow, the spongy tissue inside bones that produces blood cells, fails to produce enough new blood cells of all lineages including red blood cells, white blood cells and platelets. This deficiency can lead to life-threatening complications such as infections, bleeding, and heart problems.
In aplastic anemia (AA), the underlying defect is a substantial reduction in the number of hematopoietic pluripotent stem cells and a fault in the remaining stem cells or an immune reaction against them, which makes them unable to divide and differentiate sufficiently to populate the bone marrow. It will cause a reduction in marrow stem cells thus inadvertently increasing the fat spaces to more than 75% of the marrow with no abnormal cells seen.
What causes bone marrow failure?
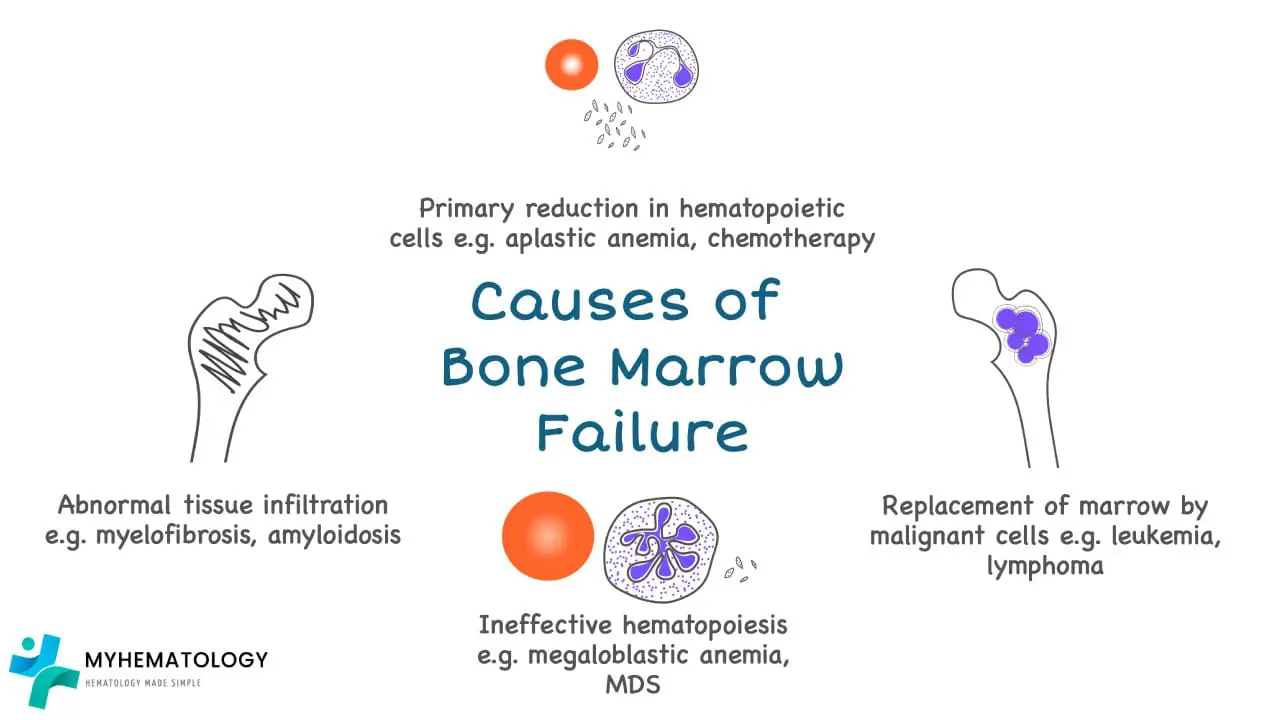
Bone marrow failure can be caused by aplastic anemia, or marrow infiltration by malignant cells or abnormal cells, ineffective erythropoiesis and abnormal tissue infiltration for example in myelofibrosis.
Most frequent cause of bone marrow aplasia is due to drugs or radiation therapy especially in malignancy treatment where the drugs may interfere with DNA replication or other critical aspects of cell growth and are therefore toxic to normal cells in the body that are continuously proliferating.
Myelophthisic anemia occurs when bone marrow space is secondarily invaded by one of several different types of disease processes most frequently metastatic cancer for example breast and lung carcinomas. Metastatic cancers replace the marrow and often activate marrow fibroblasts leading to extensive deposition of collagen. This reactive fibrosis distorts the marrow microenvironment thus disrupts the production of hematopoietic cells and leads to release of immature marrow elements.
Aplastic or hypoplastic anemia (AA) is defined as pancytopenia resulting from aplasia of the bone marrow.
What is pancytopenia?
Pancytopenia is a condition characterized by a low count of all three major types of blood cells: red blood cells, white blood cells, and platelets. This can lead to a variety of symptoms, including fatigue, weakness, shortness of breath, pale skin, easy bruising or bleeding, and frequent infections.
The causes of pancytopenia can be divided into two main categories: primary and secondary. Primary pancytopenia is caused by a problem with the bone marrow, the spongy tissue inside bones that produces blood cells. This can be due to a number of factors, including:
- Aplastic anemia
- Leukemia
- Lymphoma
- Myelodysplastic syndromes (MDS)
- Vitamin B12 deficiency
Secondary pancytopenia is caused by a problem outside of the bone marrow that is affecting the production or destruction of blood cells. This can be due to a number of factors, including:
- Infections: Some infections, such as HIV and hepatitis C, can affect the production of blood cells.
- Medications: Some medications, such as chemotherapy drugs and antibiotics, can cause a reduction in blood cell counts.
- Autoimmune disorders: In some cases, the immune system mistakenly attacks and destroys blood cells.
- Radiation exposure: Exposure to radiation can damage the bone marrow and lead to pancytopenia.
- Splenomegaly: This is an enlargement of the spleen, which can trap blood cells and reduce their numbers in the bloodstream.
What are the causes of aplastic anemia (AA)?
The causes of aplastic anemia can be broadly categorized into two main groups: acquired and inherited. In a significant number of cases, the exact cause remains unknown, and this is referred to as idiopathic aplastic anemia.
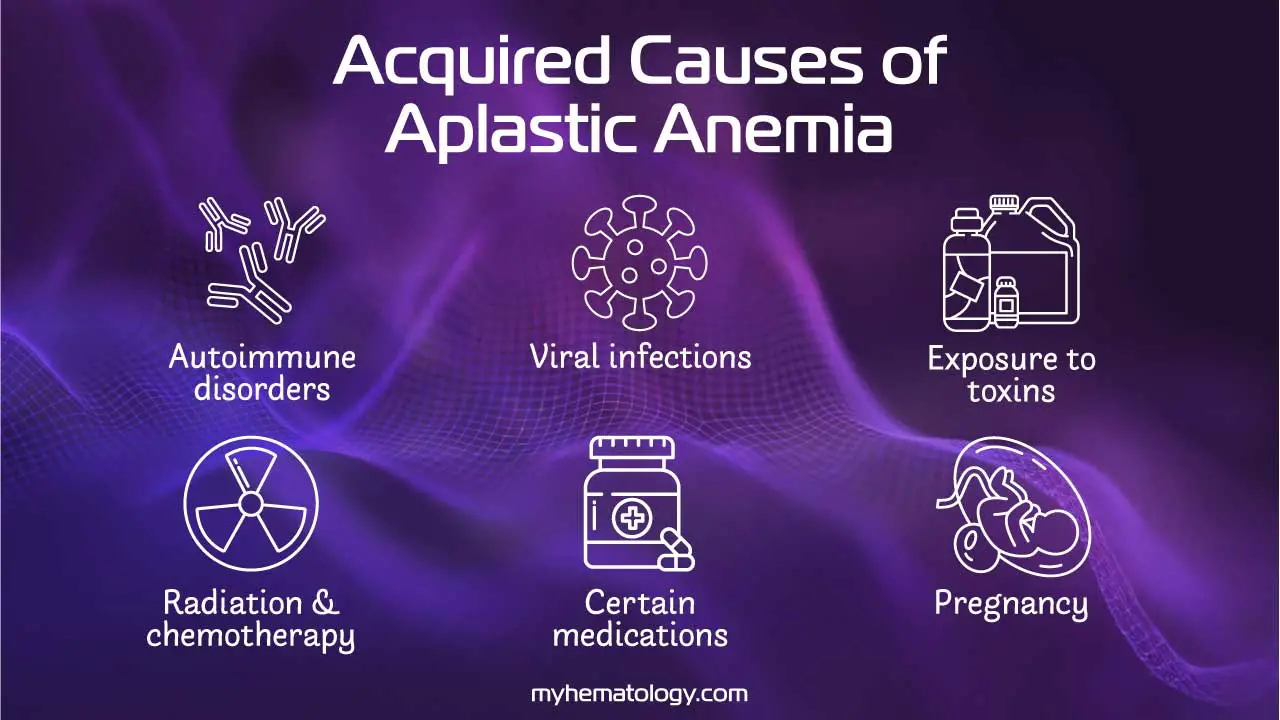
Acquired Aplastic Anemia
Acquired aplastic anemia is the most common form and is often an autoimmune disease. In most cases, the patient’s immune system, specifically T cells, mistakenly attacks and destroys the hematopoietic stem cells in the bone marrow. This immune-mediated attack can be triggered by various factors, including:
- Autoimmune disorders: In some cases, the immune system mistakenly attacks and destroys stem cells in the bone marrow. This is thought to be the most common cause of aplastic anemia.
- Viral infections: Some viruses, such as hepatitis C, parvovirus B19, and Epstein-Barr virus, have been linked to aplastic anemia.
- Exposure to toxins: Certain chemicals, such as benzene, pesticides, and heavy metals, have been linked to aplastic anemia.
- Radiation and chemotherapy: These treatments, which are often used to treat cancer, can damage the bone marrow and lead to aplastic anemia.
- Medications: Some medications, such as chloramphenicol and phenytoin, have been linked to aplastic anemia (AA).
- Pregnancy: In some cases, a woman’s immune system may attack her own bone marrow during pregnancy. This is a rare cause of aplastic anemia.
Inherited Aplastic Anemia
Inherited aplastic anemia is much less common and is caused by a genetic defect that impairs the function of hematopoietic stem cells. These are often part of a larger group of disorders called inherited bone marrow failure syndromes. The most well-known examples include:
- Fanconi Anemia: This is the most common inherited form. Patients with Fanconi anemia often have physical abnormalities in addition to bone marrow failure, such as skeletal defects, particularly in the thumbs, and skin pigmentation changes.
- Dyskeratosis Congenita: This is a disorder characterized by a classic triad of abnormal skin pigmentation, nail dystrophy, and oral leukoplakia, along with progressive bone marrow failure.
- Shwachman-Diamond Syndrome: This is a rare genetic disorder that causes pancreatic insufficiency, skeletal abnormalities, and bone marrow failure, often with neutropenia as the most prominent feature.
What is the pathophysiology of idiopathic aplastic anemia (IAA)?
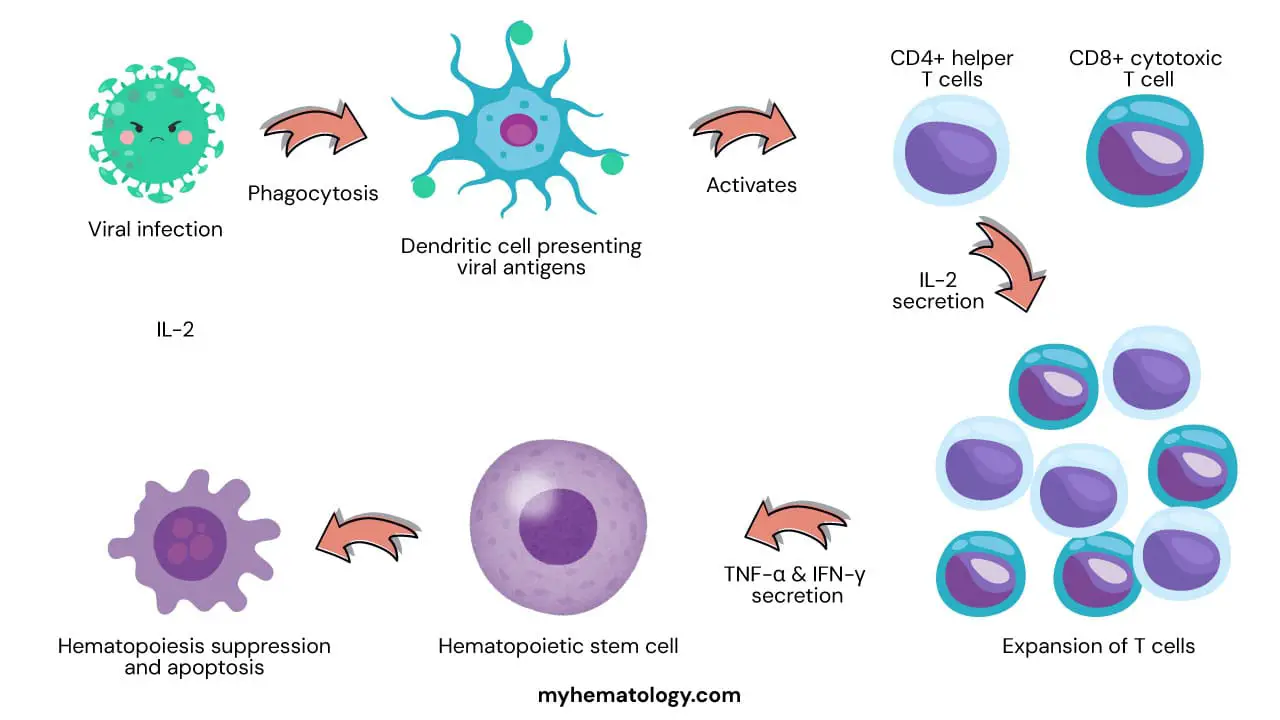
One of the leading hypotheses is that idiopathic aplastic anemia (IAA) is an autoimmune disorder in which the immune system mistakenly attacks and destroys hematopoietic stem pluripotent cells (HSPCs). This could be triggered by a viral infection or exposure to certain chemicals or medications. The immune system may also be activated by self-antigens, which are molecules that are normally found on the surface of HSPCs but are mistaken by the immune system as foreign invaders.
Another possibility is that idiopathic aplastic anemia (IAA) is caused by a defect in the HSPCs themselves. This could make them more susceptible to damage by the immune system or other factors. Additionally, there may be problems with the bone marrow microenvironment, which is the environment in which HSPCs develop and mature.
The exact mechanism by which HSPCs are destroyed in idiopathic aplastic anemia (IAA) is still unknown. However, it is thought to involve a combination of factors, including:
- Activation of cytotoxic T cells: These are a type of white blood cell that can kill cells that are infected with viruses or other pathogens. In IAA, cytotoxic T cells may mistakenly attack HSPCs.
- Production of cytokines: These are signaling molecules that can regulate the immune system. In IAA, the production of certain cytokines may be altered, which could lead to the activation of cytotoxic T cells and the destruction of HSPCs.
- Apoptosis: This is a programmed cell death process that is normally used to remove damaged or unwanted cells. In IAA, apoptosis of HSPCs may be increased, leading to a further reduction in their numbers.
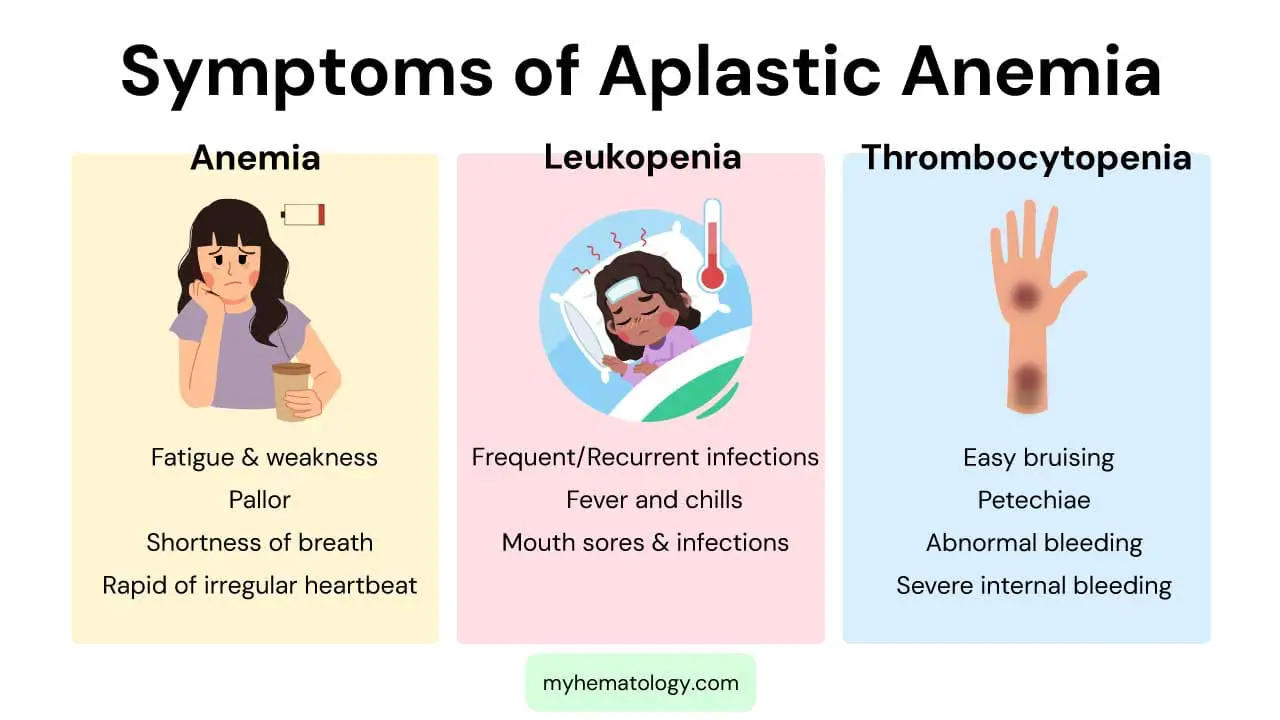
What are the signs and symptoms of aplastic anemia?
Aplastic anemia is characterized by a failure of the bone marrow to produce enough new blood cells, leading to a deficiency of red blood cells, white blood cells, and platelets. The signs and symptoms are a direct consequence of these low blood cell counts, a condition known as pancytopenia. The severity of the symptoms depends on which cell lines are most affected and how low the counts have fallen.
Symptoms of Anemia (Low Red Blood Cells)
A deficiency in red blood cells leads to a lack of oxygen being carried throughout the body, causing symptoms of anemia. These include:
- Fatigue and Weakness: This is one of the most common and prominent symptoms, as a lack of oxygen delivery to muscles and tissues results in a constant feeling of tiredness.
- Pallor: The skin and the lining of the eyelids may appear unusually pale due to the low number of red blood cells.
- Shortness of Breath: Patients may experience shortness of breath, especially with physical exertion, as the body struggles to get enough oxygen.
- Rapid or Irregular Heartbeat: To compensate for the low oxygen supply, the heart works harder and faster, which can lead to a racing heart or arrhythmias.
Symptoms of Leukopenia (Low White Blood Cells)
A low white blood cell count (leukopenia), particularly a deficiency in neutrophils (neutropenia), makes the body vulnerable to infections. Symptoms are related to the body’s inability to fight off pathogens effectively.
- Frequent or Recurrent Infections: Patients may experience a higher frequency of bacterial, fungal, or viral infections. These infections may be more severe or last longer than usual.
- Fever and Chills: Fevers, often unexplained, can be a sign of an underlying infection that the body is struggling to control.
- Mouth Sores and Infections: The immune system’s weakness can manifest as sores in the mouth, throat, or on the skin.
Symptoms of Thrombocytopenia (Low Platelets)
Platelets are essential for blood clotting. A low platelet count (thrombocytopenia) impairs this process, leading to a tendency to bleed and bruise easily.
- Easy Bruising: Patients may develop bruises from minor bumps or injuries that would not normally cause bruising.
- Petechiae: These are tiny, pinpoint red or purple spots on the skin caused by bleeding under the skin. They are a classic sign of low platelet count.
- Abnormal Bleeding: This can include frequent nosebleeds, bleeding gums, or prolonged bleeding from small cuts. Women may also experience abnormally heavy menstrual periods.
- Severe Internal Bleeding: In severe cases, the platelet count can drop dangerously low, leading to life-threatening internal bleeding in the brain or gastrointestinal tract.
What are the laboratory investigations related to aplastic anemia (AA)?
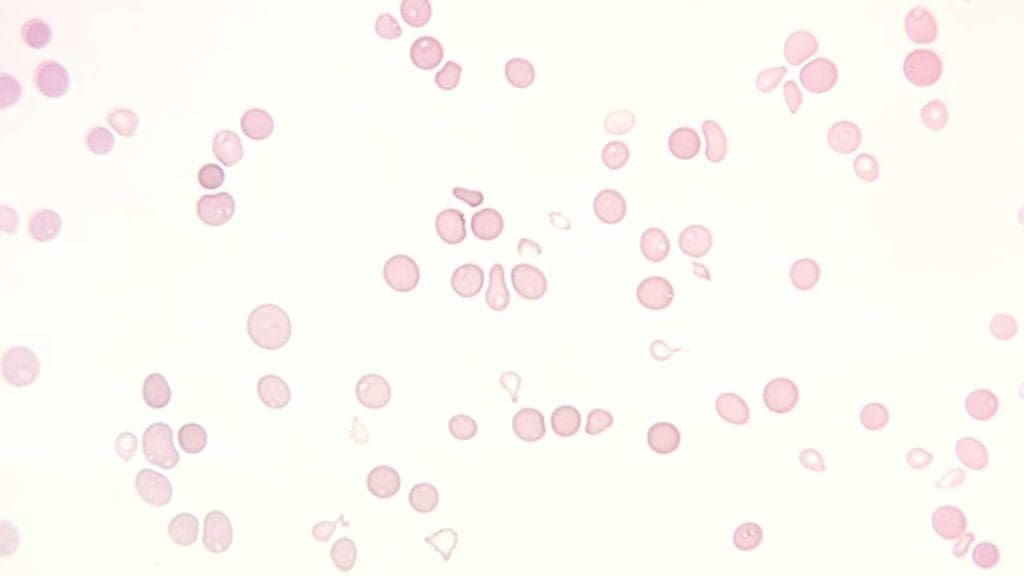
- Full blood count and peripheral blood smear: Normochromic normocytic or slight macrocytic anemia with extremely low reticulocytes count with leukopenia and thrombocytopenia
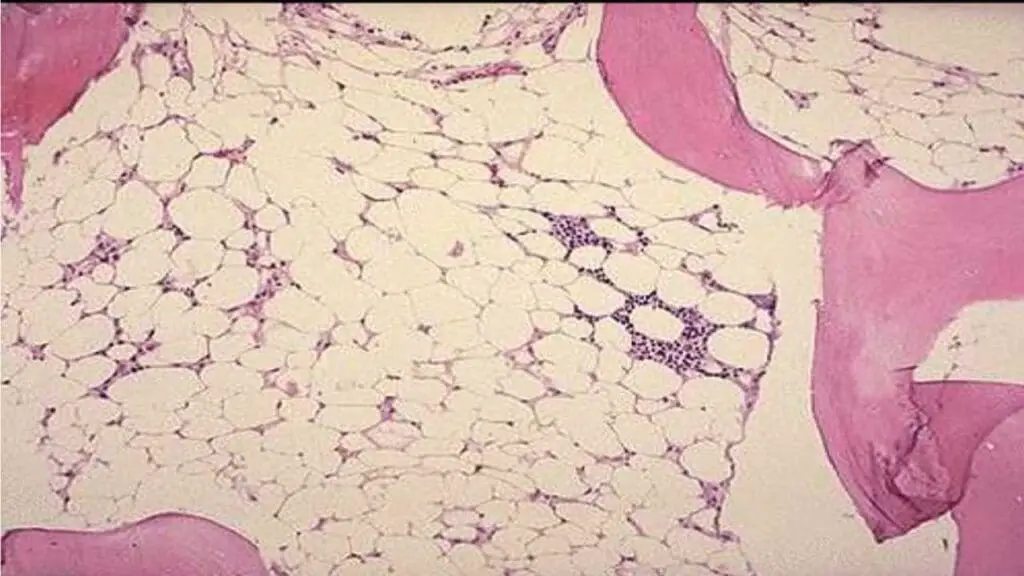
- Bone marrow examination: Hypoplasia especially trephine biopsy with patchy cellular areas with hypocellular backgrounds (more than 75% of marrow fat). The main cells present are lymphocytes and plasma cells.
- Cytogenetic testing: Is necessary to differentiate from other forms of pancytopenia for example hypoplastic myelodysplasia. Myelodysplasia will show qualitative abnormalities of the cells and clonal cytogenetic changes.
- For patients with a positive chromosome breakage study, next generation sequencing (NGS) is used to analyze multiple causative mutations, up to 22 genes. All pathogenic and likely pathogenic variants, as well as variants of unknown (indeterminate) significance, as determined bioinformatically, are confirmed by Sanger sequencing.
Differential Diagnosis of Aplastic Anemia
| Condition | Key Features | Diagnostic Distinctions from Aplastic Anemia |
| Aplastic Anemia | Pancytopenia; empty, fatty bone marrow (hypocellular); remaining hematopoietic cells are morphologically normal. | Confirmed by a bone marrow biopsy showing severely hypocellular marrow. |
| Myelodysplastic Syndromes (MDS) | Pancytopenia or bicytopenia; bone marrow can be hypocellular, normocellular, or hypercellular. | Bone marrow biopsy shows dysplastic (abnormal) cell morphology. Genetic analysis often reveals chromosomal abnormalities. |
| Paroxysmal Nocturnal Hemoglobinuria (PNH) | Pancytopenia and/or hemolytic anemia; can co-exist with aplastic anemia. | Flow cytometry detects the absence of specific surface proteins (e.g., CD55, CD59) on blood cells, which indicates the presence of a PNH clone. |
| Fanconi Anemia | Pancytopenia; often associated with congenital physical abnormalities (e.g., thumb defects, short stature) and increased skin pigmentation. | Diagnosed by chromosomal breakage testing, which shows increased sensitivity to DNA-damaging agents. Genetic testing confirms specific gene mutations. |
| Dyskeratosis Congenita | Pancytopenia; characteristic triad of physical features: abnormal nails, skin hyperpigmentation, and oral leukoplakia. | Diagnosis is based on clinical features and telomere length analysis, which shows abnormally short telomeres for the patient’s age. |
| Large Granular Lymphocytic (LGL) Leukemia | Pancytopenia, often with neutropenia; bone marrow can be hypocellular. | The presence of a clonal proliferation of large granular lymphocytes on peripheral blood smear and bone marrow biopsy is the key distinction. |
What are the treatment and management options for IAA?
The treatment and management of idiopathic aplastic anemia (IAA) depend on the severity of the condition and the patient’s overall health. The goal of treatment is to restore blood cell production and manage symptoms. For treatment purposes, the underlying cause like drugs or radiotherapy therapy treatment need to be identified and removed.
Supportive Care
Supportive care is essential for all patients with IAA. This includes:
- Blood and platelet concentrates transfusions: Blood transfusions are used to replace the low levels of red blood cells, white blood cells, or platelets. All blood products should be filtered to reduce the risk of alloimmunization and irradiated to prevent grafting of live donor lymphocytes.
- Iron supplementation: Iron supplementation may be given to patients with anemia to help the body produce new red blood cells.
- Antimicrobial prophylaxis: Antimicrobial prophylaxis may be given to patients with low white blood cell counts to prevent infections.
- Nutritional counseling: Nutritional counseling can help patients maintain their strength and overall health.
Immunosuppressive Therapy
Immunosuppressive therapy is the primary treatment for IAA. It involves using medications to suppress the immune system and prevent it from attacking the bone marrow. The most common immunosuppressive agents used for IAA include:
- Antithymocyte globulin (ATG): ATG is a protein that destroys certain types of white blood cells, including T cells, which are thought to play a role in the pathogenesis of IAA.
- Cyclosporine: Cyclosporine is a medication that suppresses the immune system by interfering with the production of T cells.
- Methotrexate: Methotrexate is a medication that interferes with the growth of cells, including T cells.
Immunosuppressive therapy is typically given for several months or even years. The goal is to achieve remission, which is when the bone marrow is producing enough blood cells on its own.
Stem Cell Transplant
A stem cell transplant is a potentially curative treatment for IAA. It involves replacing the patient’s damaged bone marrow with healthy stem cells from a donor. Stem cells can come from a matched unrelated donor, a sibling donor, or the patient’s own blood (autologous transplant).
A stem cell transplant is a complex procedure with significant risks, so it is only considered for patients with severe IAA who have not responded to other treatments.
Frequently Asked Questions (FAQs)
Can aplastic anemia (AA) be cured?
Yes, aplastic anemia (AA) can be cured in some cases. The most effective treatment for aplastic anemia is a stem cell transplant, also known as a bone marrow transplant. This procedure replaces damaged bone marrow with healthy stem cells from a donor.
However, not everyone with aplastic anemia (AA) is a candidate for a stem cell transplant. Other treatments, such as blood transfusions and medications to suppress the immune system, may be used to manage symptoms while waiting for a suitable donor or if a transplant is not possible.
Can aplastic anemia (AA) turn into leukemia?
No, aplastic anemia cannot turn into leukemia. They are two distinct diseases.
- Aplastic anemia (AA) is a condition where the bone marrow fails to produce enough blood cells.
- Leukemia is a type of cancer that affects the blood and bone marrow.
While both conditions involve the blood and bone marrow, they have different causes and treatments.
What is the 5 year survival for aplastic anemia (AA)?
The 5-year survival rate for aplastic anemia (AA) varies depending on several factors, including:
- Age: Younger individuals generally have better outcomes.
- Cause of aplastic anemia (AA): Some causes may have a different prognosis.
- Treatment: The effectiveness of treatment, such as stem cell transplantation, significantly impacts survival rates.
- Overall health: Underlying health conditions can influence survival.
Overall, the 5-year survival rate for aplastic anemia (AA) can range from 50% to 80% or higher, especially with effective treatments like stem cell transplants.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC; British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016 Jan;172(2):187-207. doi: 10.1111/bjh.13853. Epub 2015 Nov 16. Erratum in: Br J Haematol. 2016 Nov;175(3):546. PMID: 26568159.
- Bacigalupo A, Socié G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, Cybicka A, Sengelov H, Unal A, Beelen D, Locasciulli A, Dufour C, Passweg JR, Oneto R, Signori A, Marsh JC; Aplastic Anemia Working Party of the European Group for Blood Marrow Transplantation. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015 May;100(5):696-702. doi: 10.3324/haematol.2014.115345. Epub 2015 Jan 23. PMID: 25616576; PMCID: PMC4420220.
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Swathi D, Reddy PRK. A Quick Guide to Aplastic Anemia (Scholars’ Press). 2018.
- Williams S. Danielle’s Story: A Daughter’s Battle with Aplastic Anemia. 2006.
- Lande B. Beat Aplastic Anemia – With a Personal Wellness Plan: Diagnosed in 2001 – Thriving Today – Visit http://aplassticcentral.org. (2014).
- Nimmana BK, Penney SW. Aplastic Anemia. [Updated 2025 Jul 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534212/
- Schoettler, M. L., & Nathan, D. G. (2018). The Pathophysiology of Acquired Aplastic Anemia: Current Concepts Revisited. Hematology/oncology clinics of North America, 32(4), 581–594. https://doi.org/10.1016/j.hoc.2018.03.001
- Giudice, V., & Selleri, C. (2022). Aplastic anemia: Pathophysiology. Seminars in hematology, 59(1), 13–20. https://doi.org/10.1053/j.seminhematol.2021.12.002
- Shallis, R. M., Ahmad, R., & Zeidan, A. M. (2018). Aplastic anemia: Etiology, molecular pathogenesis, and emerging concepts. European journal of haematology, 101(6), 711–720. https://doi.org/10.1111/ejh.13153
- Wang, L., & Liu, H. (2019). Pathogenesis of aplastic anemia. Hematology, 24(1), 559–566. https://doi.org/10.1080/16078454.2019.1642548
- Medinger, M., Drexler, B., Lengerke, C., & Passweg, J. (2018). Pathogenesis of Acquired Aplastic Anemia and the Role of the Bone Marrow Microenvironment. Frontiers in oncology, 8, 587. https://doi.org/10.3389/fonc.2018.00587