TL;DR
Blood coagulation (clotting) disorders affect how blood clots, leading to either excessive bleeding or inappropriate clot formation within blood vessels.
Types ▾
- Inherited: Caused by genetic mutations affecting clotting factors (e.g., hemophilia A, hemophilia B, vWD).
- Acquired: Develop throughout life due to various factors (e.g., vitamin K deficiency, liver disease, DIC).
Consequences
- Bleeding complications: Easy bruising, prolonged bleeding after injuries or surgery, bleeding from mucous membranes.
- Thrombosis: Formation of blood clots in healthy blood vessels, potentially leading to stroke, heart attack, or pulmonary embolism.
Diagnosis ▾
- Clinical history: Symptoms, medical history, family history, medications.
- Physical examination: Signs of bleeding.
- Laboratory tests: PT, PTT, specific clotting factor assays, additional tests depending on suspected cause.
Management ▾
- Address the underlying cause.
- Prevent bleeding episodes: Lifestyle modifications, medications (factor replacement, medications to promote platelet function, but not anticoagulants for bleeding disorders).
Specific treatments ▾
- Hemophilia A/B: Factor replacement therapy.
- vWD: Depends on type/severity (desmopressin, factor replacement, medications for menstrual bleeding).
- Vitamin K deficiency: Supplementation.
- HDN: Prophylactic vitamin K to newborns.
- Liver disease: Manage underlying disease, consider blood product replacement.
- DIC: Address underlying cause, potentially anticoagulants and blood product replacement.
- Antibodies to clotting factors: Depends on cause (bypassing agents, immunosuppressants).
Early diagnosis and appropriate management are crucial to prevent complications and improve quality of life.
*Click ▾ for more information
Introduction
Coagulation disorders are a group of conditions that disrupt the body’s normal blood clotting process. This disruption can manifest in two main ways:
- Increased Bleeding Tendency: This occurs when the blood has difficulty forming clots or the clots formed are weak and easily broken down. This can lead to excessive bleeding after injuries, surgery, or even spontaneously.
- Thrombosis: In some cases, the coagulation system becomes overactive, leading to the formation of blood clots within healthy blood vessels. These clots can block blood flow and cause serious complications like stroke, heart attack, or pulmonary embolism (blood clot in the lung).
Coagulation (Hemostasis)
Coagulation, also known as clotting, is a complex biological process by which blood changes from a liquid state to a gel-like consistency, forming a blood clot. This clot acts as a plug at the site of a blood vessel injury, preventing further blood loss and promoting wound healing.
Hemostasis, on the other hand, is the entire process of stopping bleeding. It’s a multi-step cascade involving several mechanisms, with coagulation being a crucial component. Here’s how coagulation fits into the bigger picture of hemostasis:
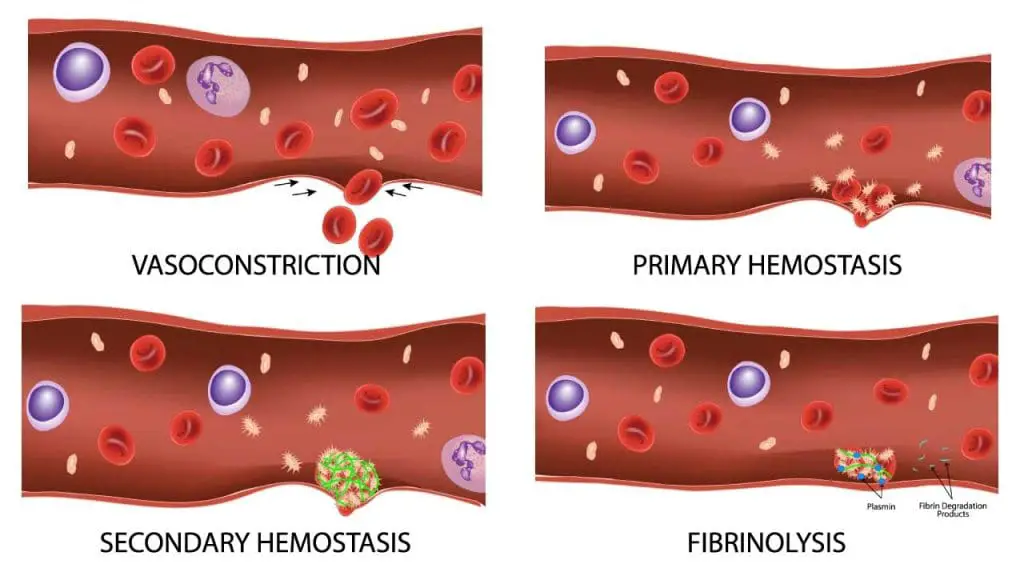
- Vascular Constriction: Upon injury to a blood vessel, the smooth muscle in the vessel wall contracts, immediately reducing blood flow to the area.
- Platelet Plug Formation: Platelets, cell fragments in the blood, adhere to the damaged vessel wall and clump together, forming a preliminary plug.
- Coagulation Cascade Activation: Platelet activation triggers a series of enzymatic reactions known as the coagulation cascade. This cascade involves various clotting factors in the blood plasma, culminating in the formation of fibrin.
- Fibrin Mesh Formation: Fibrin is an insoluble protein that forms a mesh-like structure, trapping red blood cells and platelets within the clot, strengthening the plug and sealing the wound.
The Importance of Coagulation in Hemostasis
Coagulation plays a vital role in hemostasis by:
- Limiting Blood Loss: The fibrin mesh and platelet plug effectively prevent excessive blood loss from the injured vessel, allowing for proper healing.
- Facilitating Wound Healing: The clot serves as a scaffold for tissue repair and growth, promoting wound closure.
- Maintaining Blood Flow: While stopping blood loss is crucial, it’s equally important to maintain blood flow to healthy tissues. Coagulation is tightly regulated to ensure clot formation is localized at the injury site and doesn’t obstruct blood flow in unaffected vessels.
When coagulation malfunctions, either due to deficiencies in clotting factors or uncontrolled activation, it can lead to either excessive bleeding or inappropriate clot formation within blood vessels (thrombosis). This highlights the delicate balance that coagulation maintains within the body’s hemostatic system.
Coagulation Cascade
The coagulation cascade is a complex but fascinating series of enzymatic reactions that orchestrate blood clot formation. Imagine a waterfall where water tumbles down, picking up speed and volume with each step. The coagulation cascade functions similarly, with each step activating the next clotting factor, ultimately leading to a dramatic amplification of the clotting process.
Key Players
- Clotting Factors: These are plasma proteins designated with Roman numerals (e.g., Factor VIII, Factor IX). They circulate in inactive forms and become sequentially activated during the cascade.
- Platelets: These cell fragments play a dual role. They adhere to the damaged vessel wall and clump together, forming the initial plug. They also release chemicals that activate the coagulation cascade.
- Phospholipid Surface: Exposed collagen fibers in the damaged vessel wall provide a negatively charged surface for the clotting factors to bind and facilitate their activation.
- Calcium: This mineral acts as a cofactor, essential for the activity of many clotting factors.
The Cascade
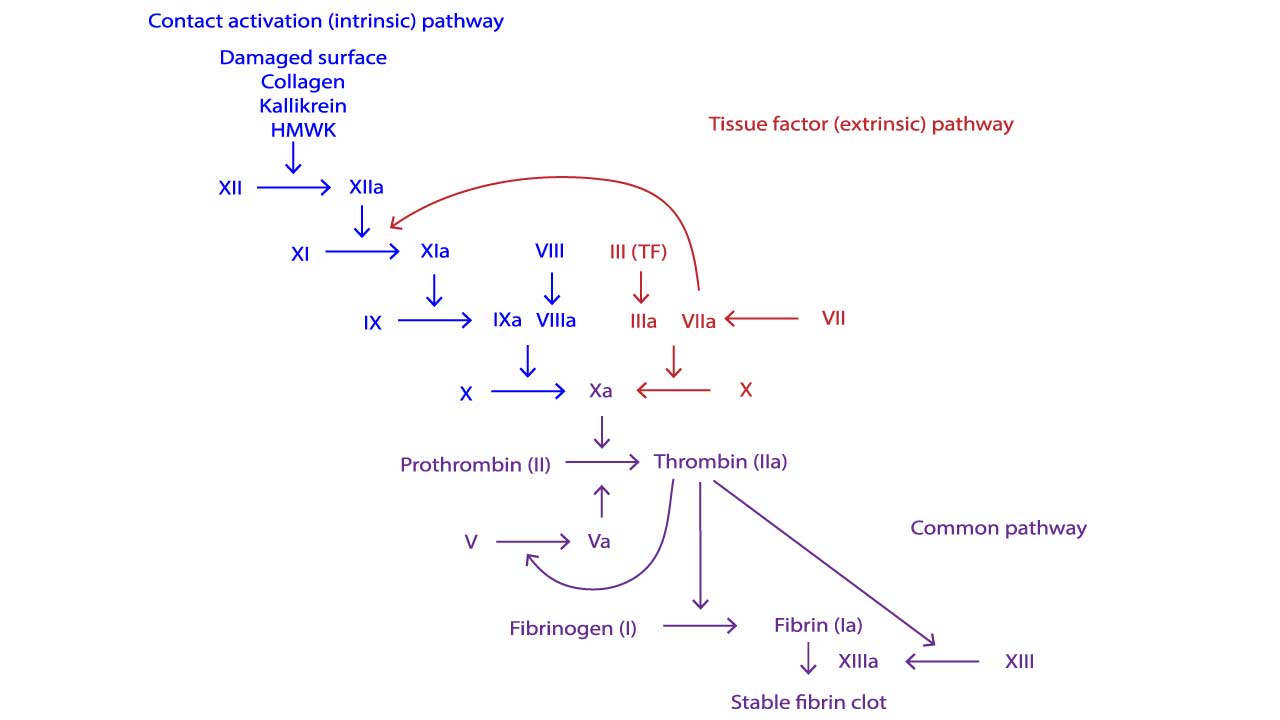
There are two main pathways that initiate the cascade, converging into a common pathway:
- Intrinsic Pathway: This pathway is activated within the blood itself, triggered by factors like contact with negatively charged surfaces or damaged tissues. It involves factors XII, XI, IX, and VIII.
- Extrinsic Pathway: This pathway is faster and is triggered by tissue factor, a protein exposed by damaged tissues outside the blood vessels. It involves factor VII.
The Common Pathway
Both pathways converge at the activation of factor X. From here, the common pathway takes over, involving factors X, V, II (prothrombin), I (fibrinogen), and XIII.
- Factor X Activation: Activated factor X, along with Factor Va on the platelet surface, cleaves prothrombin into thrombin.
- Thrombin Formation: Thrombin is the central enzyme of the cascade. It has two crucial functions:
- Converts fibrinogen, a soluble protein, into fibrin, an insoluble protein that forms the mesh of the clot.
- Activates factor XIII, which strengthens the fibrin mesh by cross-linking fibrin strands.
The Outcome
The end result of the coagulation cascade is the formation of a stable fibrin clot that seals the wound and prevents further blood loss. This clot is eventually broken down by the body’s plasminolytic system once healing is complete.
Regulation of the Cascade
The coagulation cascade is tightly regulated to ensure localized clot formation and prevent unwanted clotting within blood vessels. This regulation involves various natural anticoagulants (e.g., protein C, protein S, antithrombin) that inhibit specific clotting factors and prevent uncontrolled activation.
Types of Coagulation Disorders
Inherited Coagulation Disorders
Inherited coagulation disorders arise from genetic mutations that affect the production or function of proteins essential for blood clotting. These mutations are passed down from parents to children, and the severity of symptoms can vary depending on the specific disorder and the type of inheritance pattern.
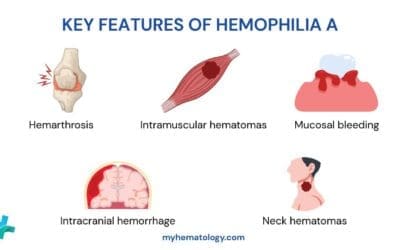
Hemophilia A
- Cause: Mutation in the FVIII gene located on the X chromosome. Males are more commonly affected as they only have one X chromosome (X-linked inherited disorder).
- Symptoms: Easy bruising, prolonged bleeding after injuries or surgery, joint bleeds (especially knees and elbows), deep tissue bleeds. Severity can range from mild to severe, with some individuals experiencing only occasional bleeding episodes.
- Diagnosis: Blood tests to measure factor VIII activity levels, family history.
- Treatment: Replacement therapy with recombinant factor VIII to replenish missing clotting factor, medications to prevent joint bleeds (prophylaxis), physical therapy to maintain joint health.
Hemophilia B
- Cause: Mutation in the FIX gene located on the X chromosome, similar inheritance pattern to hemophilia A.
- Symptoms: Similar to hemophilia A, but bleeding episodes may be less frequent or severe.
- Diagnosis: Blood tests to measure factor IX activity levels, family history.
- Treatment: Replacement therapy with recombinant factor IX, similar to hemophilia A treatment strategies.
von Willebrand Disease (vWD)
- Cause: Most common inherited bleeding disorder, caused by mutations in the VWF gene which codes for von Willebrand factor (vWF). This protein plays a crucial role in platelet adhesion and clot formation.
- Symptoms: Easy bruising, prolonged bleeding from mucous membranes (nosebleeds, heavy menstrual bleeding), menorrhagia (excessively heavy periods) in women. Symptoms can vary widely depending on the type and severity of vWD.
- Diagnosis: Blood tests to measure vWF levels and activity, bleeding time test.
- Treatment: Depends on the type and severity of vWD. Options include medications like desmopressin (DDAVP) to stimulate vWF release from storage sites, factor replacement therapy with concentrates containing vWF, medications to reduce menstrual bleeding.
Other Inherited Coagulation Disorders
- Factor XI Deficiency: This is a rare autosomal dominant or recessive disorder affecting factor XI, a clotting factor in the intrinsic pathway. Symptoms can range from mild bleeding to severe, depending on the level of deficiency. Treatment may involve factor XI replacement therapy or antifibrinolytic medications to prevent clot breakdown.
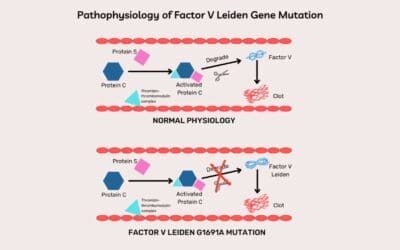
- Factor V Leiden Mutation: This is a common genetic variant that increases the risk of blood clots (thrombosis) rather than bleeding. Individuals with this mutation may not experience any symptoms but have a higher risk of developing deep vein thrombosis (DVT) or pulmonary embolism (PE) especially with additional risk factors like prolonged immobilization or surgery. Management focuses on preventing blood clots, with strategies like low-dose aspirin or anticoagulant medications in high-risk situations.
- Prothrombin Gene Mutation: Similar to Factor V Leiden, this mutation increases the risk of thrombosis rather than bleeding. Management focuses on preventing blood clots in individuals with this mutation and additional risk factors.
Acquired Coagulation Disorders
Acquired coagulation disorders develop later in life due to various factors that disrupt the normal blood clotting process. Unlike inherited disorders, these are not caused by genetic mutations but by external factors impacting the production or function of clotting factors.
Vitamin K Deficiency
- Cause: Insufficient intake or absorption of vitamin K, which is essential for the production of several clotting factors in the liver. This can occur due to malnutrition, malabsorption syndromes, or use of antibiotics that interfere with vitamin K production by gut bacteria. Newborns also have low vitamin K stores and are at risk for deficiency.
- Symptoms: Easy bruising, bleeding from various sites (nosebleeds, gums, gastrointestinal bleeding).
- Diagnosis: Blood tests to measure clotting times (PT, PTT) and assess vitamin K levels.
- Treatment: Vitamin K supplementation (oral or intravenous) to correct the deficiency and restore normal clotting function.
Hemorrhagic Disease of the Newborn (HDN)
- Cause: Vitamin K deficiency in newborns due to low vitamin K stores at birth and limited dietary intake initially.
- Symptoms: The most concerning symptom is bleeding, particularly intracranial hemorrhage (bleeding in the brain) which can have devastating consequences. Other bleeding manifestations like bloody stools or umbilical cord bleeding may also occur.
- Diagnosis: Blood tests to measure clotting times and assess for vitamin K deficiency.
- Treatment: Prophylactic administration of vitamin K to all newborns shortly after birth is standard practice to prevent HDN.
Liver Disease
- Cause: Severe liver damage can impair the production of clotting factors by the liver.
- Symptoms: Easy bruising, bleeding tendencies along with other liver disease symptoms like jaundice, ascites (fluid accumulation in the abdomen).
- Diagnosis: Blood tests to assess liver function and clotting times, imaging studies of the liver.
- Treatment: Treatment focuses on managing the underlying liver disease. In some cases, fresh frozen plasma or specific clotting factor replacement therapy may be necessary to control bleeding.
Disseminated Intravascular Coagulation (DIC)
- Cause: A complex disorder characterized by abnormal activation of the coagulation system, leading to widespread formation of microclots throughout the bloodstream. This can paradoxically cause both bleeding and thrombosis (clotting) complications.
- Causes: Severe infections, sepsis, trauma, burns, certain cancers.
- Symptoms: Easy bruising, bleeding from various sites, signs of organ dysfunction (depending on where clots are located – e.g., kidney failure, respiratory failure).
- Diagnosis: Blood tests to assess clotting times, fibrin degradation products (markers of clot breakdown), and other laboratory findings suggestive of the underlying cause (e.g., infection markers).
- Treatment: Treatment focuses on addressing the underlying cause of DIC. In some cases, anticoagulant medications may be used to prevent further clot formation, while blood product replacement therapy (platelets, plasma) may be necessary to manage bleeding.
Antibodies to Clotting Factors
- Cause: Development of antibodies against specific clotting factors, inhibiting their function. This can occur in autoimmune diseases or as a complication of certain medications (e.g., heparin-induced thrombocytopenia).
- Symptoms: Bleeding tendencies, severity depending on the targeted clotting factor and the degree of inhibition.
- Diagnosis: Blood tests to detect antibodies against specific clotting factors.
- Treatment: Treatment depends on the underlying cause. In some cases, bypassing agents (medications that activate clotting pathways independent of the inhibited factor) may be used to control bleeding. For autoimmune disorders, immunosuppressive medications may be necessary.
Massive Transfusion Syndrome
- Cause: During massive blood transfusions, the rapid administration of large volumes of blood can dilute clotting factors and platelets in the recipient’s circulation, increasing the risk of bleeding.
- Symptoms: Increased bleeding risk during and after transfusions.
- Diagnosis: Blood tests to assess clotting times and platelet count.
- Treatment: Strategies to minimize the risk of massive transfusion syndrome include using fresh whole blood or blood components with higher clotting factor and platelet content. Additionally, monitoring clotting times and administering blood products as needed to maintain adequate hemostasis is crucial.
Antiphospholipid Syndrome (APS)
- Cause: In APS, the immune system mistakenly produces antibodies that target phospholipids, which are essential components of cell membranes and are also involved in blood clotting. These antibodies can interfere with the normal clotting process, leading to:
- Increased risk of blood clots: APS can cause blood clots to form in both arteries and veins, potentially leading to complications like stroke, heart attack, or deep vein thrombosis (DVT).
- Pregnancy complications: APS can also increase the risk of miscarriage, premature birth, and other pregnancy problems.
- Symptoms: Antiphospholipid syndrome (APS) can present with a variety of symptoms, some related to blood clots and others more general. Blood clot symptoms depend on the location (e.g., chest pain for pulmonary embolism, leg pain and swelling for DVT) while general symptoms can include fatigue, headaches, migraines, miscarriage, or even memory problems.
- Diagnosis: Diagnosis of APS involves a combination of clinical criteria (symptoms and history of blood clots or pregnancy complications) and laboratory tests that detect the presence of antiphospholipid antibodies.
- Treatment: There’s no cure for APS, but treatment focuses on preventing blood clots and managing pregnancy complications.
Other acquired causes of coagulation disorders
- Warfarin toxicity: This blood-thinning medication can cause excessive bleeding if the dose is too high.
- Severe burns: Extensive burns can damage blood vessels and activate the coagulation system, leading to bleeding and clotting complications.
- Certain cancers: Some cancers can release substances that interfere with normal blood clotting.
- Oral Contraceptive Use: Certain types of birth control pills containing estrogen and progestin can increase the risk of blood clots, especially in women with additional risk factors like smoking, obesity, or a family history of thrombosis.
- Pregnancy and Postpartum Period: During pregnancy and the postpartum period, physiological changes occur in a woman’s body, including increased levels of clotting factors and decreased blood flow velocity. This creates a hypercoagulable state, a natural state with an increased risk of thrombosis to prevent excessive bleeding during childbirth. However, in some cases, this increased risk can become pathological, leading to blood clots.
Diagnosis of Coagulation Disorders
Diagnosing coagulation disorders involves a combination of strategies to identify the underlying cause and assess the severity of the bleeding problem.
Clinical History
- A detailed medical history plays a crucial role in pinpointing potential causes. This includes:
- Symptoms: The nature and frequency of bleeding episodes (easy bruising, prolonged bleeding after injuries, bleeding from mucous membranes) are important clues.
- Past medical history: Previous diagnoses of bleeding disorders, surgeries with excessive bleeding, liver disease, or autoimmune conditions can be informative.
- Family history: A family history of bleeding disorders suggests a potential inherited cause.
- Medications: Use of medications that affect blood clotting, such as warfarin or heparin, should be documented.
Physical Examination
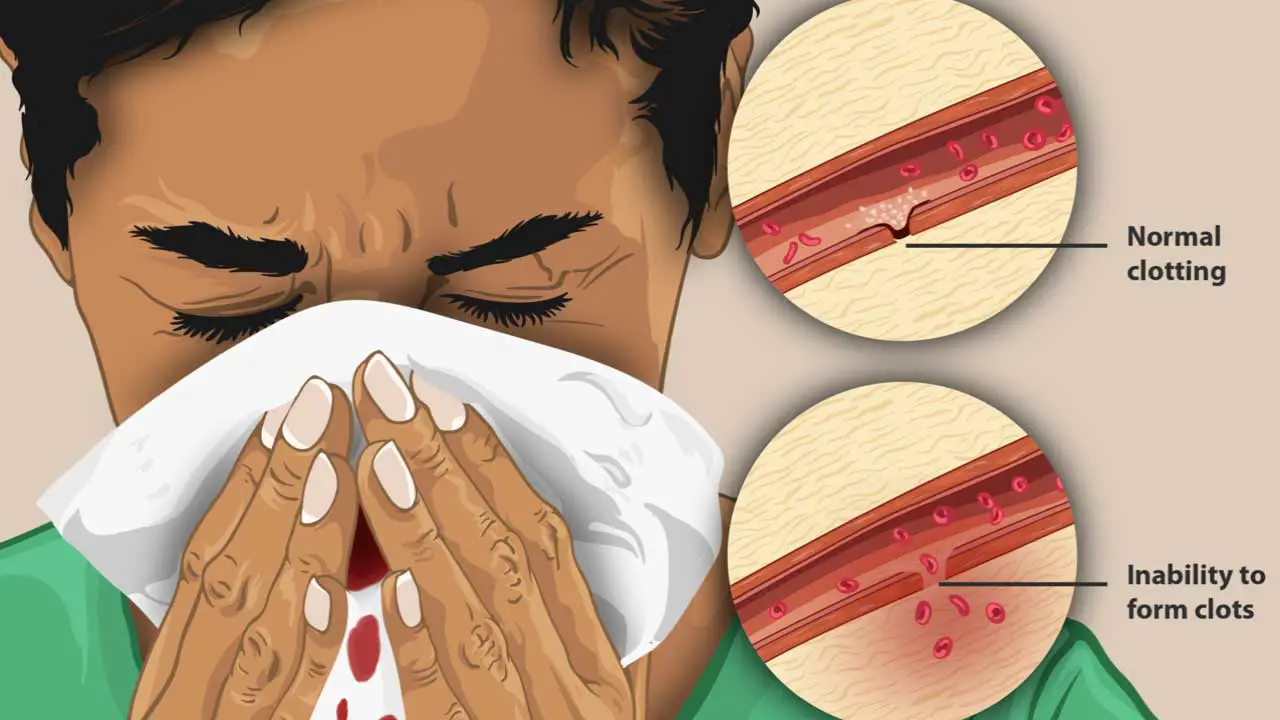
- A thorough physical examination can reveal signs suggestive of bleeding disorders, such as:
- Easy bruising or ecchymoses (large, purplish bruises)
- Petechiae (tiny red spots caused by bleeding under the skin)
- Signs of bleeding from mucous membranes (nosebleeds, bloody gums)
- Joint swelling or tenderness (in cases of repeated bleeding into joints)
Laboratory Tests
- Several laboratory tests play a vital role in diagnosing coagulation disorders:
- Prothrombin time (PT): This test measures the time it takes for blood to clot after the addition of tissue factor and other clotting factors. An abnormal PT may indicate a deficiency in factors involved in the common pathway of coagulation (factors I, II, V, X).
- Activated partial thromboplastin time (aPTT): This test assesses the intrinsic pathway and common pathway of coagulation. An abnormal aPTT may suggest deficiencies in factors VIII, IX, XI, XII, or other clotting factors involved in these pathways.
- Specific clotting factor assays: These tests measure the activity level of individual clotting factors (e.g., factor VIII assay, factor IX assay). They are helpful in identifying the specific factor deficiency responsible for the bleeding disorder.
- Fibrinogen assay: This test measures the level of fibrinogen, the protein that forms the clot structure. A low fibrinogen level can contribute to bleeding problems.
- Bleeding time: This test assesses platelet function by measuring how long it takes for a small skin puncture to stop bleeding. While not routinely used, it can be helpful in some cases.
Additional Tests (Depending on the Suspected Cause)
- Vitamin K levels: Measured to assess for vitamin K deficiency.
- Liver function tests: Assessed to evaluate liver function, as liver disease can affect clotting factor production.
- Autoimmune tests: Performed if an autoimmune disorder is suspected as a cause of antibody formation against clotting factors.
Management of Coagulation Disorders
The management of coagulation disorders focuses on two main goals:
- Addressing the underlying cause: This involves treating the underlying condition that is causing the disruption in blood clotting. For example, if vitamin K deficiency is the culprit, supplementation will be the primary intervention. If a specific autoimmune disease is identified, immunosuppressive medications may be necessary.
- Preventing bleeding episodes: This is crucial to minimize complications and improve quality of life. Strategies for preventing bleeding episodes can be broadly categorized as follows:
- Lifestyle modifications: Avoiding activities with a high risk of injury, maintaining a healthy weight, and following a balanced diet are important.
- Medications: Depending on the specific disorder, medications may be used to:
- Replace missing clotting factors: This is the mainstay of treatment for hemophilia A and B, where factor VIII or IX concentrates are infused intravenously to replenish the deficient clotting factor and prevent bleeding episodes.
- Prevent clot formation: In some cases, such as with Factor V Leiden mutation where there’s an increased risk of thrombosis, low-dose aspirin or other anticoagulant medications may be used to prevent blood clots. However, these medications are not used for bleeding disorders as they can worsen bleeding.
- Promote platelet function: Medications like desmopressin (DDAVP) can be used in some cases to stimulate the release of von Willebrand factor from storage sites in the body, which can be helpful in vWD.
Specific treatments for some common coagulation disorders
- Hemophilia A and B: Factor replacement therapy with recombinant factor VIII or IX concentrates, respectively, is the cornerstone of treatment. Prophylactic regimens (regular infusions) are often used to prevent bleeding episodes, especially in severe cases.
- von Willebrand Disease (vWD): Treatment depends on the type and severity of vWD. Options include desmopressin (DDAVP) to stimulate vWF release, factor replacement therapy with concentrates containing vWF, and medications to reduce menstrual bleeding.
- Vitamin K Deficiency: Vitamin K supplementation (oral or intravenous) is the main treatment to correct the deficiency and restore normal clotting function.
- Hemorrhagic Disease of the Newborn (HDN): Prophylactic administration of vitamin K to all newborns shortly after birth is standard practice to prevent HDN.
- Liver Disease: Treatment focuses on managing the underlying liver disease. In some cases, fresh frozen plasma or specific clotting factor replacement therapy may be necessary to control bleeding.
- Disseminated Intravascular Coagulation (DIC): Treatment focuses on addressing the underlying cause of DIC. In some cases, anticoagulant medications may be used to prevent further clot formation, while blood product replacement therapy (platelets, plasma) may be necessary to manage bleeding.
- Antibodies to Clotting Factors: Treatment depends on the underlying cause. In some cases, bypassing agents (medications that activate clotting pathways independent of the inhibited factor) may be used to control bleeding. For autoimmune disorders, immunosuppressive medications may be necessary.
Frequently Asked Questions (FAQs)
How do you know if you have coagulation issues?
Recognizing potential blood coagulation disorders can be tricky because symptoms can vary depending on the specific disorder and whether it causes excessive bleeding or an increased risk of blood clots. Here’s a breakdown of some signs and symptoms to be aware of:
Excessive Bleeding
- Easy bruising or excessive bruising after minor injuries
- Prolonged bleeding from cuts or scrapes
- Frequent nosebleeds
- Bleeding gums, especially after brushing teeth
- Heavy menstrual bleeding
- Blood in the stool (dark red or black stools)
- Blood in the urine (pink or reddish urine)
Increased Risk of Blood Clots (Thrombosis)
- Unexplained swelling, pain, and redness in one leg (deep vein thrombosis)
- Sharp chest pain or shortness of breath (pulmonary embolism)
- Sudden severe headache, weakness, or confusion (stroke)
- Vision problems or difficulty speaking (stroke)
- Repeated miscarriages or pregnancy complications
It’s important to note that these symptoms can also be caused by other conditions.
Can blood clotting disorders be cured?
While there isn’t a permanent cure for inherited disorders, advancements in gene therapy are being explored as a potential future option for correcting the underlying genetic mutations.
Overall, the management strategies for blood clotting disorders have significantly improved, allowing individuals to live full and active lives. Early diagnosis and proper management are essential for optimal outcomes.
Can blood clotting disorders cause miscarriage?
Yes, some blood clotting disorders can increase the risk of miscarriage. When a blood clot forms in the tiny vessels of the developing placenta, it can disrupt the vital blood and oxygen supply to the fetus, leading to miscarriage. Conditions like inherited deficiencies in clotting factors or antiphospholipid syndrome, where the immune system attacks clotting factors, can contribute to this risk. Early diagnosis and management of these disorders with medications or blood product replacement therapy can improve pregnancy outcomes.
Are blood clotting disorders life-threatening and fatal?
Blood clotting disorders can be life-threatening, but the risk of death depends on several factors:
- Type of disorder: Inherited bleeding disorders like hemophilia are rarely fatal on their own, but complications from severe bleeding episodes can be life-threatening if not treated promptly. Conversely, some acquired clotting disorders, like uncontrolled DIC (disseminated intravascular coagulation), can lead to widespread blood clots throughout the body, causing organ damage and potentially death.
- Severity of the disorder: The severity of the clotting deficiency or the tendency to form clots significantly impacts the risk. Inherited disorders can range from mild to severe, with severe cases posing a higher risk of life-threatening complications.
- Presence of complications: Complications like uncontrolled bleeding, stroke, heart attack, or pulmonary embolism (blood clot in the lung) due to blood clots can be fatal if not treated promptly.
- Access to treatment: Early diagnosis and proper management with medications or blood product replacement therapy can significantly reduce the risk of life-threatening complications.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Kaushansky K, Levi M. Williams Hematology Hemostasis and Thrombosis (McGraw-Hill). 2017.