TL;DR
Neutrophils are the most common type of white blood cell, serving as the body’s primary defense against bacterial and fungal infections.
- Function ▾: Neutrophils primarily function by engulfing and destroying invading microorganisms through a process called phagocytosis. They also release enzymes and reactive oxygen species to kill pathogens.
- Lifespan ▾: Neutrophils have a relatively short lifespan, circulating in the bloodstream for about a day before migrating to tissues.
- Production ▾: Neutrophils are produced in the bone marrow through a process called granulopoiesis.
- Disorders ▾: Abnormalities in neutrophil count or function can lead to conditions like neutrophilia (high neutrophil count), neutropenia (low neutrophil count), or disorders affecting neutrophil morphology and function.
- Laboratory tests ▾: Various blood tests, including CBC with differential, bone marrow aspiration, and neutrophil function tests, can evaluate neutrophil numbers and function.
*Click ▾ for more information
Introduction
Neutrophils are a type of white blood cell (leukocyte) that constitute the majority of the circulating granulocytes. They are essential components of the innate immune system, providing rapid and effective defense against bacterial and fungal infections.
Neutrophil Morphology
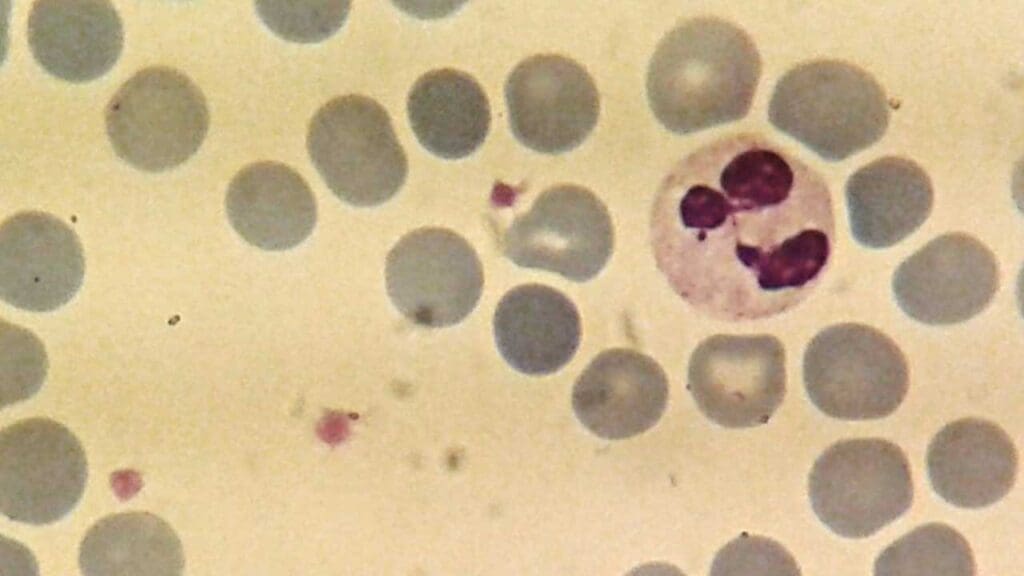
The average size of a mature neutrophil is approximately 12-15 micrometers in diameter.
Granules
Neutrophils contain various types of granules within their cytoplasm, each with specific functions:
- Primary granules (azurophilic granules): These are large, dense granules containing lysosomal enzymes, myeloperoxidase, and other antimicrobial substances. They are involved in the killing and degradation of ingested pathogens.
- Secondary granules (specific granules): Smaller than primary granules, these contain enzymes such as alkaline phosphatase, lactoferrin, and lysozyme, which play a role in bacterial killing and inflammation.
- Tertiary granules (gelatinase granules): These contain gelatinase enzymes, which are involved in tissue remodeling and degradation of extracellular matrix components.
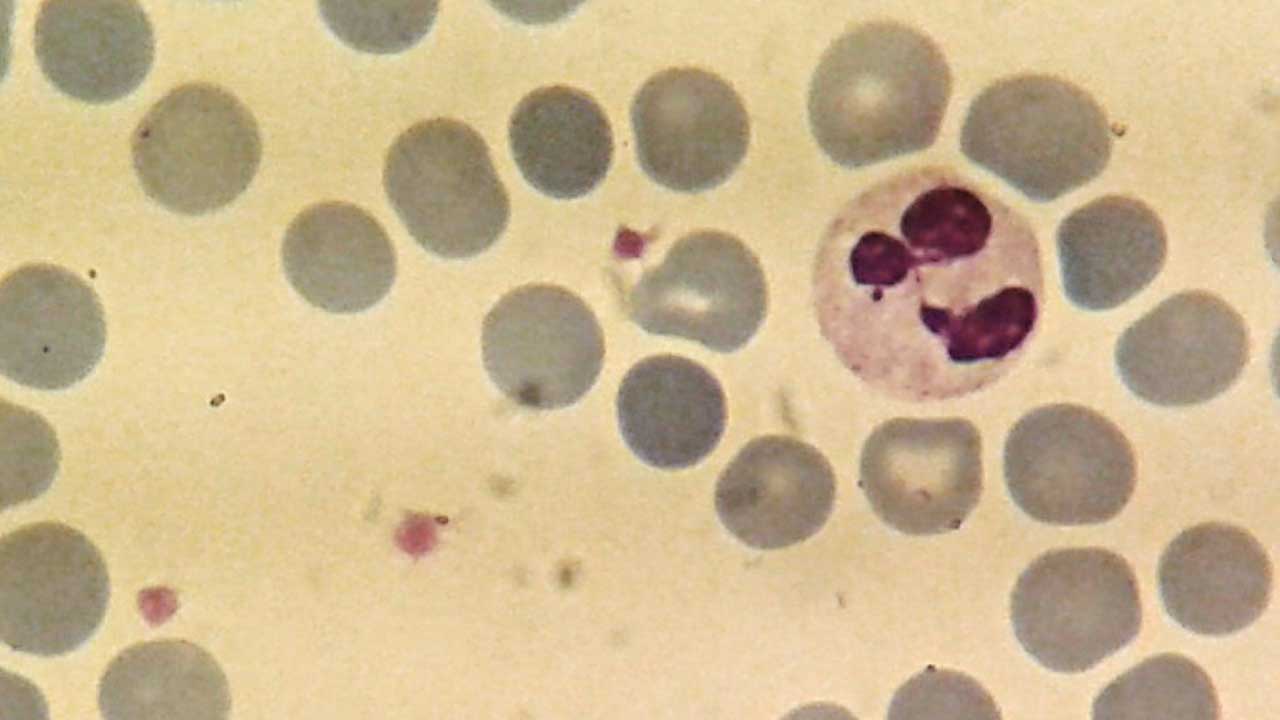
Nuclear Segmentation
The neutrophil nucleus is characteristically segmented, consisting of two to five lobes connected by thin nuclear strands. This unique shape is a hallmark of mature neutrophils. The number of nuclear lobes increases as the neutrophil ages.
Cytoplasm
The neutrophil cytoplasm is filled with granules and contains various organelles, including mitochondria, ribosomes, and the Golgi apparatus. The cytoplasm also contains glycogen stores, which provide energy for the cell’s metabolic activities.
Neutrophil Lifespan
Neutrophils have a relatively short lifespan.
The majority of their life cycle is spent in the bone marrow, where they mature. Once released into the bloodstream, their lifespan is significantly shorter.
- Bone marrow: Approximately 10 days.
- Bloodstream: Less than 24 hours.
- Tissue: 2-3 days.
Absolute Neutrophil Count (ANC)
The absolute neutrophil count (ANC) is a calculation that determines the total number of neutrophils in a sample of blood.
Unlike the percentage of neutrophils, which is part of the differential white blood cell count, the absolute neutrophil count (ANC) provides a more accurate picture of the body’s ability to fight infection. This is because it takes into account both the total number of white blood cells and the percentage of neutrophils.
Why is it important? A low absolute neutrophil count (ANC), often referred to as neutropenia, increases the risk of infection. This is particularly important for patients undergoing chemotherapy or those with certain medical conditions.
How is it calculated?
Absolute neutrophil count (ANC) = (Total white blood cell count) x (% neutrophils + % bands) / 100
- Total white blood cell count: The total number of white blood cells in a sample of blood.
- % neutrophils: The percentage of neutrophils in the white blood cell count.
- % bands: The percentage of immature neutrophils (bands) in the white blood cell count.
While specific ranges should be obtained from the laboratory where the test was performed, the following provides a general estimate. Disclaimer: Reference ranges can vary significantly between laboratories, and it’s essential to consult with a healthcare professional for accurate interpretation of specific results.
| Group | Neutrophil Count (x10^9/L) |
| Adults (men and women) | 2.5 – 7.5 |
| Children (older than 2 years) | 2.0 – 8.0 |
| Infants | 1.8 – 8.0 |
| Newborns | 1.5 – 11.0 |
| Elderly | Can be slightly lower than adults |
Ethnic Variations
As mentioned, recent studies have indicated differences in neutrophil counts among different ethnic groups. For example, African Americans tend to have lower neutrophil counts compared to Caucasians. It’s essential to consider these variations when interpreting results, especially in populations with diverse ethnic backgrounds.
Factors Affecting Neutrophil Reference Ranges
Several factors influence neutrophil reference ranges, including:
- Age: Neutrophil counts vary significantly throughout life.
- Sex: There can be subtle differences between males and females.
- Ethnicity: Recent studies have shown variations in neutrophil counts among different ethnic groups.
- Laboratory methods: Different laboratories may use different methods to measure neutrophil counts, leading to variations in reference ranges.
Neutrophil Function
Phagocytosis: Process and Mechanism
Phagocytosis is the primary function of neutrophils. It involves the engulfment and destruction of pathogens.
Process
- Chemotaxis: Neutrophils are attracted to the site of infection by chemical signals released by bacteria and damaged tissues.
- Adherence: Neutrophils adhere to the surface of the pathogen.
- Ingestion: The neutrophil extends pseudopods, which surround and engulf the pathogen, forming a phagosome.
- Phagosome-lysosome fusion: The phagosome merges with lysosomes, which contain digestive enzymes.
- Killing and digestion: The enzymes within the phagosome kill and digest the pathogen.
Mechanism
- Oxidative burst: Neutrophils produce reactive oxygen species (ROS), such as superoxide and hydrogen peroxide, which damage the pathogen’s cell wall and DNA.
- Degranulation: Neutrophil granules release enzymes, such as lysozyme and elastase, which break down the pathogen’s cell wall.
Role in Inflammation
Neutrophils play a crucial role in the inflammatory response.
- Recruitment: Neutrophils are rapidly recruited to the site of inflammation in response to chemical signals.
- Vasodilation: Neutrophils contribute to vasodilation, increasing blood flow to the area.
- Tissue damage: While essential for fighting infection, neutrophils can also cause tissue damage if their response is excessive or prolonged.
Other Functions
Neutrophil Extracellular Traps (NETs) are fibrous networks composed primarily of DNA released from the nuclei of neutrophils during a process called NETosis. These sticky, web-like structures are coated with antimicrobial proteins and histones.
While degranulation and oxidative burst are primarily associated with phagocytosis, they can also occur independently.
Degranulation
- Phagocytosis: Degranulation is essential for releasing antimicrobial substances into the phagosome to kill the engulfed pathogen.
- Other situations: Neutrophils can also degranulate in response to other stimuli, such as:
- Inflammation: Release of inflammatory mediators to amplify the inflammatory response.
- Tissue damage: Release of enzymes to aid in tissue repair and remodeling.
- Allergic reactions: Release of histamine and other mediators contributing to allergic symptoms.
Oxidative Burst
- Phagocytosis: The oxidative burst is crucial for generating reactive oxygen species (ROS) to kill the ingested pathogen.
- Other situations: Neutrophils can also produce ROS in response to other stimuli, including:
- Bacterial or fungal components: Recognition of pathogen-associated molecular patterns (PAMPs) can trigger ROS production.
- Inflammatory cytokines: These signaling molecules can induce ROS generation.
- Physical stimuli: Certain physical stresses, such as exposure to ionizing radiation, can activate the oxidative burst.
It’s important to note that while degranulation and oxidative burst can occur independently, they often work together to enhance the neutrophil’s antimicrobial and inflammatory functions.
Granulopoiesis
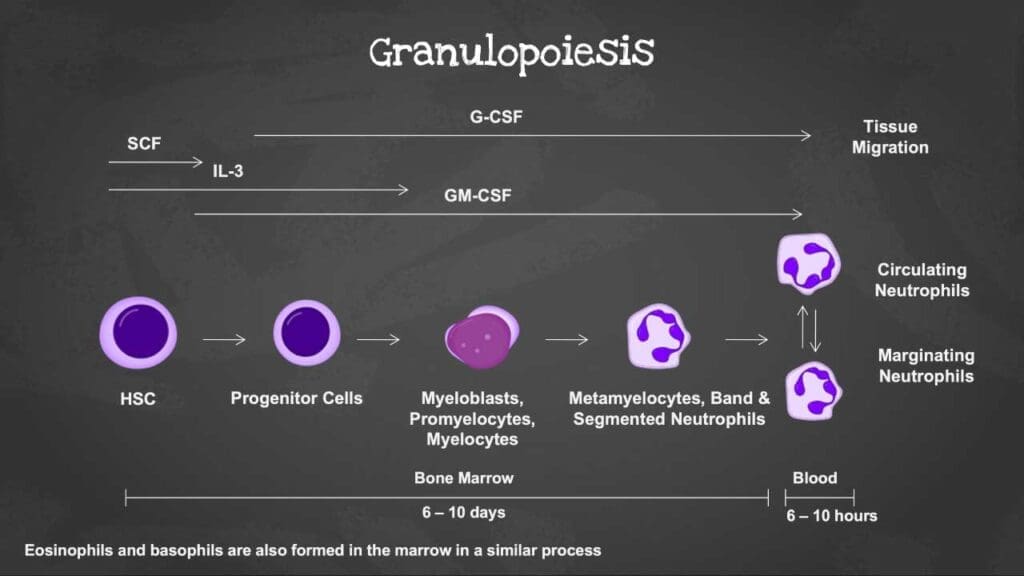
Overview of Hematopoiesis
Hematopoiesis is the process of blood cell formation. It occurs primarily in the bone marrow. All blood cells, including red blood cells, white blood cells, and platelets, originate from a single type of cell called a hematopoietic stem cell. This stem cell has the potential to differentiate into various cell lineages, including the myeloid and lymphoid lineages. Granulopoiesis is specifically the process of granulocyte (neutrophils, eosinophils, and basophils) production.
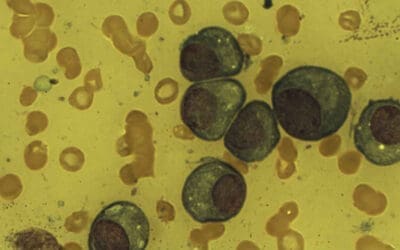
Stages of Neutrophil Development
Granulopoiesis is the process by which neutrophils develop from granulocyte-monocyte progenitor cells (GMPs). This development occurs in distinct stages, each characterized by specific morphological features and surface markers.
Initially, the GMP matures into a myeloblast. Subsequent stages include the promyelocyte, marked by the emergence of azurophilic granules. As the cell progresses to the myelocyte stage, specific granules begin to form and fully develop in the metamyelocyte. Importantly, the cell’s ability to divide ceases at the metamyelocyte stage. The final stages involve the formation of a band-shaped nucleus and the development of gelatinase granules and secretory vesicles, marking the mature neutrophil.
Myeloblast
- The earliest recognizable stage of neutrophil development.
- Large cell with a large nucleus that occupies most of the cell.
- Fine chromatin and nucleoli are present.
- Cytoplasm is basophilic (blue-staining) and agranular.
Promyelocyte
- Larger than the myeloblast.
- Nucleus is large and round or oval.
- Chromatin becomes coarser.
- Primary (azurophilic) granules appear in the cytoplasm.
Myelocyte
- Smaller than the promyelocyte.
- Nucleus becomes indented or kidney-shaped.
- Chromatin becomes condensed.
- Secondary (specific) granules appear in the cytoplasm.
Metamyelocyte
- Smaller than the myelocyte.
- Nucleus is horseshoe-shaped or indented.
- Chromatin becomes more condensed.
- Cytoplasm contains both primary and secondary granules.
Band Neutrophil
- Nucleus is elongated and band-shaped with no or minimal segmentation.
- Chromatin is condensed.
- Cytoplasm contains both primary and secondary granules.
Segmented Neutrophil
- Mature neutrophil.
- Nucleus is divided into two to five lobes connected by thin nuclear strands.
- Chromatin is highly condensed.
- Cytoplasm contains primary, secondary, and tertiary granules.
These stages represent a progressive maturation process, with cells becoming smaller, the nucleus becoming more segmented, and the cytoplasm acquiring specific granules. The maturation of a neutrophil in the bone marrow takes approximately 5-6 days. Once mature, neutrophils are released from the bone marrow into the bloodstream to perform their immune functions.
Benign Disorders of Neutrophils
Neutrophils are a type of white blood cell that is essential for fighting infections. Neutrophil disorders can be broadly categorized into two main groups: neutrophilia (high neutrophil count) and neutropenia (low neutrophil count).
Neutrophilia (High Neutrophil Count)
Neutrophilia refers to an abnormally high number of neutrophils in the blood.
| Cause | Description |
| Infections | Bacterial, fungal, or parasitic infections can stimulate the bone marrow to produce more neutrophils. |
| Inflammation | Chronic inflammatory conditions like rheumatoid arthritis can cause neutrophilia. |
| Tissue necrosis | Tissue death due to injury or other causes can trigger the release of neutrophils from the bone marrow. |
| Medications | Certain medications, such as corticosteroids, can transiently increase neutrophil count. |
| Physiological factors | Stress, exercise, and pregnancy can cause mild neutrophilia. |
Clinical Significance
While neutrophilia (high neutrophil count) often indicates an underlying infection or inflammatory process, it’s not always a cause for concern. The clinical significance depends on the severity of the neutrophilia (high neutrophil count) and the context of other clinical findings. For example, a mild neutrophilia (high neutrophil count) in response to a minor infection is usually not a cause for concern. However, a severe neutrophilia (high neutrophil count) may be a sign of a more serious condition.
Neutropenia (Low Neutrophil Count)
Neutropenia refers to a low neutrophil count in the blood, increasing susceptibility to infections.
Causes
| Cause | Description |
| Decreased Production | Bone marrow suppression (Chemotherapy, radiation therapy, certain medications): Certain medical treatments, such as chemotherapy and radiation therapy used to treat cancer, can damage the bone marrow and suppress its ability to produce neutrophils. Some medications can also have this side effect. Aplastic anemia (Bone marrow disorder): Aplastic anemia is a serious bone marrow disorder that can cause pancytopenia, which is a decrease in all three types of blood cells, including neutrophils. Nutritional deficiencies (Vitamin B12, folic acid, copper): Deficiencies in certain vitamins and minerals, such as vitamin B12, folic acid, and copper, can impair the production of neutrophils. |
| Increased Destruction | Immune-mediated destruction (Autoimmune diseases like lupus): In some autoimmune diseases, such as lupus, the immune system mistakenly attacks healthy tissues, including neutrophils. Severe infections: Severe infections can overwhelm the bone marrow’s ability to produce neutrophils, leading to neutropenia. |
| Other Causes | Splenomegaly (Enlarged spleen): An enlarged spleen can trap neutrophils, leading to a falsely low count in the blood. |
Clinical Significance
Neutropenia (low neutrophil count) is a serious condition that increases the risk of developing infections. The severity of the risk depends on the degree of neutropenia (low neutrophil count) and the duration. People with severe neutropenia (low neutrophil count) may need to take precautions to avoid infections, such as avoiding crowds and people who are sick.
Pelger-Huët anomaly
Pelger-Huët anomaly is a rare benign inherited condition characterized by an abnormality in the shape of neutrophil nuclei. Normally, neutrophil nuclei have two to five lobes connected by thin strands. In Pelger-Huët anomaly, the nuclei are typically bilobed or only slightly indented, resembling a “dumbbell” shape.
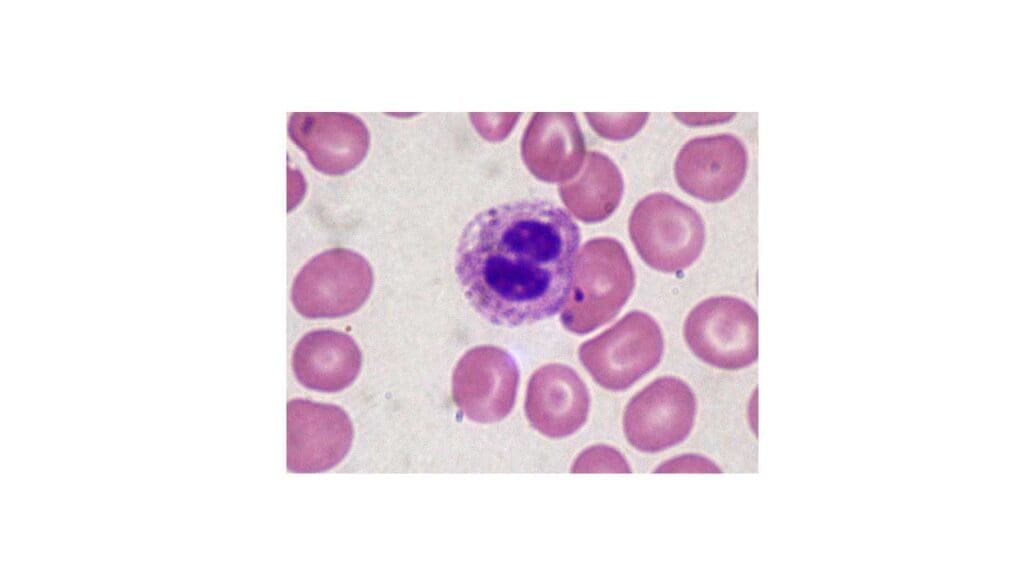
Cause
In most cases, Pelger-Huët anomaly is inherited as an autosomal dominant trait, meaning that only one copy of the mutated gene is needed for an individual to develop the condition. However, it can also occur as an acquired condition in certain bone marrow disorders, infections, or as a drug-induced effect.
Clinical Significance
The majority of individuals with Pelger-Huët anomaly experience no health problems. The condition is often discovered incidentally during a routine blood test. It’s essential to differentiate between inherited and acquired Pelger-Huët anomaly, as the latter may indicate an underlying medical condition.
While the neutrophil morphology is abnormal, the function of these neutrophils is typically normal. This means that individuals with Pelger-Huët anomaly do not have an increased risk of infections.
It’s crucial to note that the differential diagnosis of Pelger-Huët anomaly includes other conditions that can affect neutrophil morphology, such as myelodysplastic syndromes. Therefore, further investigations might be necessary to rule out other potential causes.
Neutrophils in Specific Diseases
Chronic Granulomatous Disease (CGD)
CGD is a genetic disorder characterized by the inability of neutrophils and other white blood cells to produce reactive oxygen species (ROS), essential for killing bacteria and fungi. This defect results in recurrent and severe infections.
May-Hegglin Anomaly
This is a rare inherited disorder characterized by large platelets, thrombocytopenia (low platelet count), and the presence of Döhle bodies (cytoplasmic inclusions) in neutrophils. While typically benign, it can sometimes be associated with bleeding tendencies due to thrombocytopenia.
Chediak-Higashi Syndrome
This is a rare genetic disorder affecting multiple organ systems, including the immune system. It is characterized by giant lysosomes in various cells, including neutrophils. This defect impairs phagocytosis and intracellular killing, leading to recurrent infections and other complications.
In summary, while these conditions involve neutrophils, they are not considered benign due to their significant impact on health and potential life-threatening complications. They are typically classified as primary immunodeficiency disorders.
Laboratory Tests for Neutrophil Evaluation
Laboratory tests play a crucial role in assessing neutrophil numbers, function, and morphology.
Basic Tests
- Complete Blood Count (CBC) with Differential: This is the initial test to evaluate neutrophil count, whether high neutrophil or low neutrophil count, percentage, and morphology.
- Peripheral Blood Smear: A microscopic examination of blood to assess neutrophil morphology in detail, including size, shape, and presence of inclusions.
Further Evaluation
- Bone Marrow Aspiration and Biopsy: This is performed to evaluate bone marrow cellularity and neutrophil maturation. It helps determine if there is a problem with neutrophil production, such as aplastic anemia or myelodysplastic syndrome.
- Flow Cytometry: This technique analyzes the expression of specific markers on the surface of neutrophils. It can be used to identify specific cell populations, detect abnormal cell markers, and assess neutrophil function.
- Neutrophil Function Tests: These specialized tests evaluate various aspects of neutrophil function, such as phagocytosis, oxidative burst, and chemotaxis. They are primarily used to diagnose primary neutrophil disorders, such as chronic granulomatous disease.
- Genetic Testing: In some cases, genetic testing may be necessary to identify inherited neutrophil disorders, such as chronic granulomatous disease or Chediak-Higashi syndrome.
Specific Tests for Neutrophil Disorders
- Granulocyte Immunofluorescence Test (GIFT): Detects antibodies against neutrophil antigens (HNA) associated with transfusion-related acute lung injury (TRALI).
- Granulocyte Agglutination Test (GAT): Another test to detect HNA antibodies.
Note: The choice of tests depends on the specific clinical presentation and suspected diagnosis
Frequently Asked Questions (FAQs)
What does it mean when neutrophils are high?
When neutrophils are high (neutrophilia), it often indicates an infection, inflammation, or stress on the body. These immune cells are working overtime to fight off a threat.
What does it mean if neutrophils are low?
Low neutrophils (neutropenia) means the body has fewer infection-fighting cells. This increases the risk of getting sick, especially from bacteria. It can be caused by infections, autoimmune diseases, or certain medications like chemotherapy.
What infection has high neutrophil count?
Bacterial infections are most commonly associated with high neutrophil counts. The body produces more of these white blood cells to fight off the bacterial invaders.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Blumenreich MS. The White Blood Cell and Differential Count. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 153.
- Tahir N, Zahra F. Neutrophilia. [Updated 2023 Apr 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- Justiz Vaillant AA, Rout P, Reynolds SB, et al. Neutropenia. [Updated 2024 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.