TL;DR
Hypersensitivity is an exaggerated/inappropriate immune responses to innocuous antigens leading to tissue damage. Gell and Coombs system divides hypersensitivity reactions into four types (I, II, III, IV).
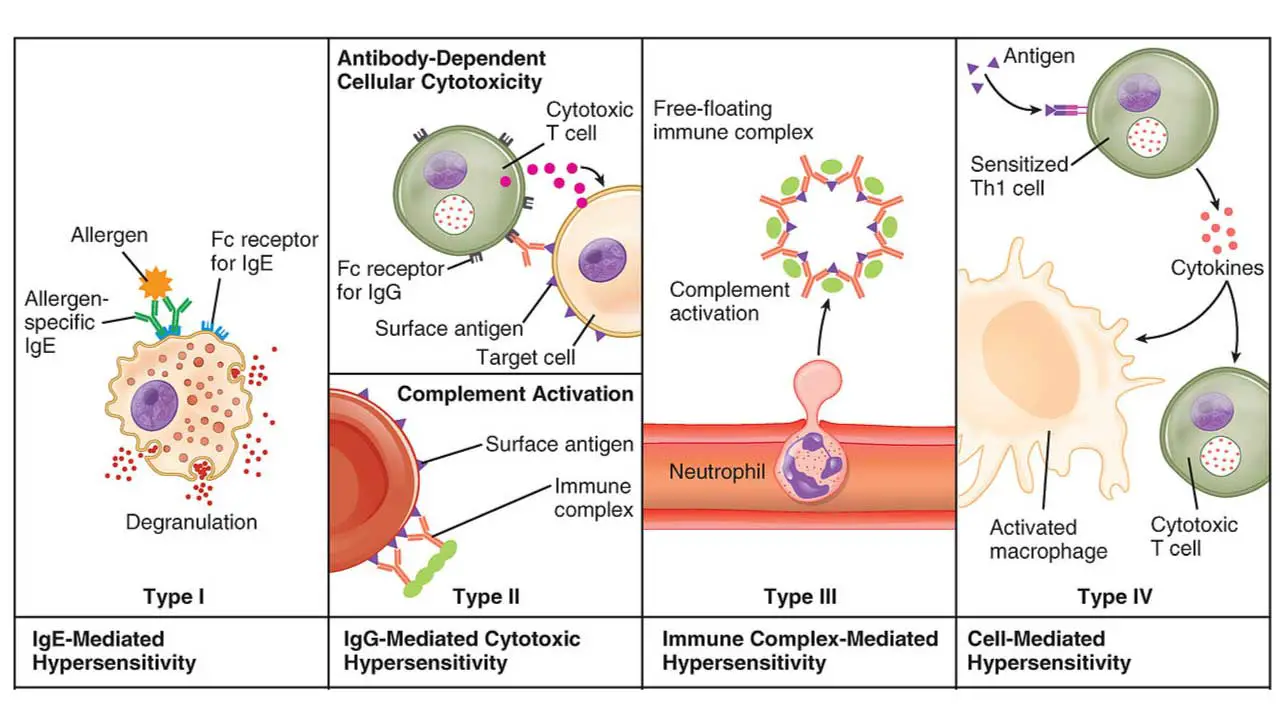
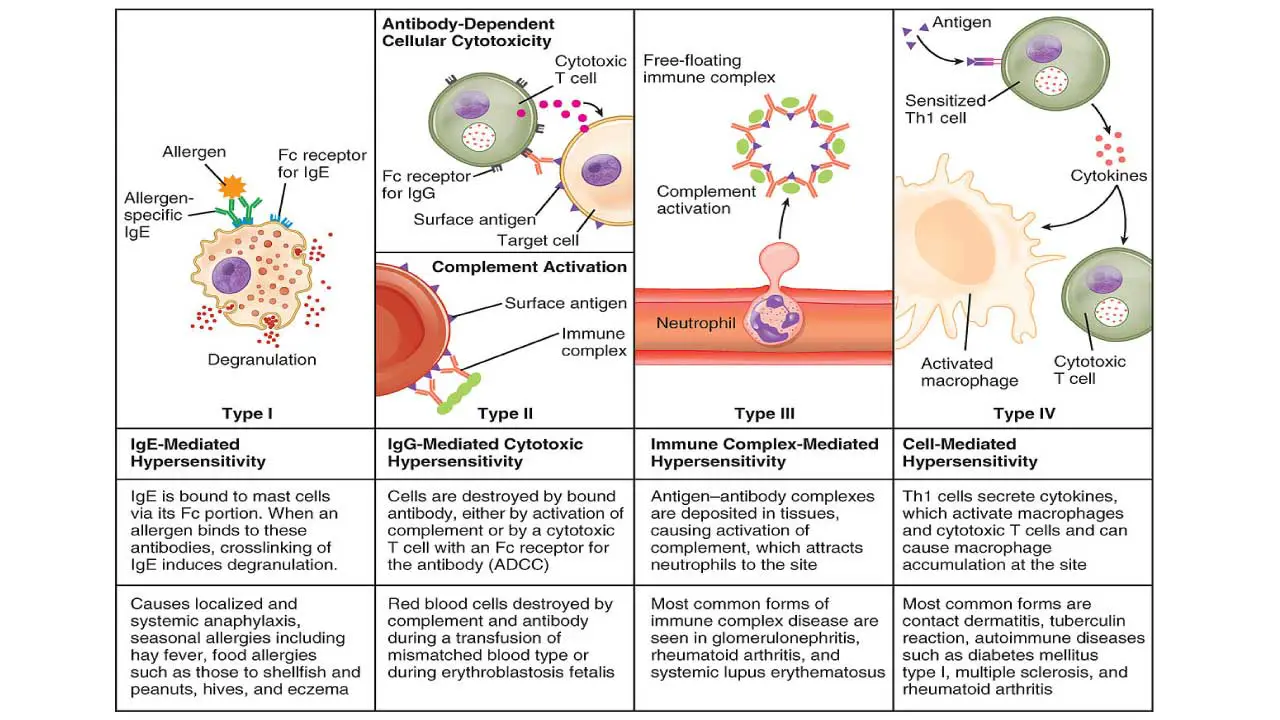
Type I (Immediate (IgE-mediated) Hypersensitivity) ▾
- Mechanism: Involves the production of IgE antibodies in response to an allergen. These IgE antibodies bind to the surface of mast cells and basophils. Upon re-exposure to the same allergen, the allergen cross-links the IgE on these cells, leading to rapid degranulation and the release of inflammatory mediators (like histamine, leukotrienes, and prostaglandins).
- Timeframe: Occurs very rapidly, within minutes of exposure.
- Examples: Allergic rhinitis (hay fever), asthma, urticaria (hives), anaphylaxis (a severe, life-threatening systemic reaction).
Type II (Cytotoxic (Antibody-mediated) Hypersensitivity) ▾
- Mechanism: Involves IgG or IgM antibodies binding directly to antigens on the surface of host cells or tissues. This binding leads to cell destruction through various mechanisms, including activation of the complement system, antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells, or opsonization and phagocytosis.
- Timeframe: Can occur within minutes to hours.
- Examples: Hemolytic transfusion reactions, hemolytic disease of the newborn (Rh incompatibility), autoimmune hemolytic anemia, Goodpasture’s syndrome.
Type III (Immune Complex-mediated Hypersensitivity) ▾
- Mechanism: Involves the formation of soluble antigen-antibody (IgG or IgM) immune complexes in the circulation. These complexes can deposit in various tissues (e.g., blood vessel walls, kidney glomeruli, joints), leading to complement activation and the recruitment of inflammatory cells (especially neutrophils), which cause tissue damage.
- Timeframe: Can take several hours to days, or even weeks, to develop after antigen exposure.
- Examples: Serum sickness, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), post-streptococcal glomerulonephritis.
Type IV (Delayed-Type (Cell-mediated) Hypersensitivity (DTH)) ▾
- Mechanism: Unlike the other types, this type is not antibody-mediated. Instead, it is mediated by T lymphocytes, specifically T helper 1 (Th1) cells and cytotoxic T lymphocytes (CTLs). Sensitized T cells, upon re-exposure to the antigen, release cytokines that activate macrophages and other inflammatory cells, leading to a localized inflammatory response and tissue damage.
- Timeframe: The reaction is “delayed,” typically developing 24 to 72 hours after antigen exposure.
- Examples: Contact dermatitis (e.g., poison ivy, nickel allergy), tuberculin skin test (Mantoux test for tuberculosis), chronic transplant rejection, some autoimmune diseases like Type 1 diabetes.
*Click ▾ for more information
Introduction
A hypersensitivity reaction is an exaggerated or inappropriate immune response to an antigen that would normally be harmless. Instead of providing protection, this overreaction leads to tissue damage and disease. These reactions are classified into four main types (Type I, II, III, and IV) based on their underlying immunological mechanisms.
The Gell and Coombs classification is the most widely used system for categorizing hypersensitivity reactions. It divides these immune responses into four main types based on the specific immunological mechanisms involved and the speed of the reaction.
Factors Influencing Hypersensitivity Reactions
Hypersensitivity reactions are multifactorial conditions arising from a complex interplay of an individual’s genetic makeup, their lifelong environmental exposures, and the specific characteristics of the antigen encountered.
Genetic Predisposition
- Atopy: A strong genetic predisposition to develop Type I hypersensitivity (allergies) is known as atopy. Individuals with a family history of allergic conditions (asthma, eczema, hay fever) are more likely to develop allergies themselves. This is not a simple Mendelian inheritance but rather a complex trait influenced by multiple genes.
- HLA Genes (Human Leukocyte Antigen): For Type II, III, and IV hypersensitivity reactions, specific HLA alleles are often associated with increased susceptibility. HLA molecules are crucial for presenting antigens to T cells.
Certain HLA types can make individuals more prone to mounting an immune response against specific self-antigens (leading to autoimmunity) or drug antigens (leading to drug hypersensitivity). For example, certain HLA-B alleles are strongly linked to severe delayed drug hypersensitivity reactions.
- Immune Regulatory Genes: Polymorphisms (variations) in genes encoding for cytokines, cytokine receptors, immune cell signaling molecules, and enzymes involved in immune responses can influence the balance between pro-inflammatory and regulatory immune responses, thereby affecting susceptibility to hypersensitivity. For instance, variations in genes related to the Th1/Th2 balance can influence the tendency towards allergic (Th2-mediated) or cell-mediated (Th1-mediated) reactions.
- Enzyme Deficiencies/Variations: Genetic variations in enzymes involved in drug metabolism can lead to the accumulation of reactive drug metabolites, which can then act as haptens and trigger hypersensitivity reactions.
Environmental Factors
- Allergen Exposure: The type, frequency, and concentration of exposure to environmental allergens (pollen, dust mites, pet dander, mold, certain foods) significantly impact the development of Type I hypersensitivity. Early life exposure patterns can be particularly influential.
- Hygiene Hypothesis: This widely discussed theory suggests that reduced exposure to microbes and infections during early childhood in modern, hygienic societies may lead to an underdeveloped immune system that is more prone to overreacting to innocuous substances (allergens), thus increasing the risk of allergic diseases.
- Air Pollution: Exposure to air pollutants (e.g., diesel exhaust particles, ozone, nitrogen dioxide) can act as adjuvants, enhancing the allergenicity of environmental antigens and exacerbating allergic responses. Pollutants can also directly irritate airways and promote inflammation.
- Diet: Dietary patterns, including the timing of introduction of certain foods in infants, and the presence of specific food additives or processing methods, are being investigated for their role in allergy development.
- Microbial Environment/Microbiome: The composition of the gut and respiratory microbiome is increasingly recognized as a critical factor in shaping immune development and influencing susceptibility to allergies and autoimmune diseases. Alterations in the microbiome can lead to dysregulation of immune tolerance.
- Infections: Certain infections can prime the immune system in ways that either increase or decrease the risk of developing hypersensitivity. For example, some viral infections might trigger autoimmune reactions, while early life exposure to certain microbes might be protective against allergies.
- Chemicals/Toxins: Exposure to various chemicals in the environment, including occupational exposures, can act as sensitizers, particularly for contact dermatitis (Type IV) or drug-like hypersensitivity reactions.
Dose and Route of Antigen Exposure
Dose
- High Doses: Can sometimes lead to immune tolerance (anergy or deletion of reactive lymphocytes) rather than hypersensitivity, or in some cases, overwhelm clearance mechanisms leading to excessive immune complex formation (Type III).
- Low Doses: May be insufficient to induce a strong immune response, or in some cases, repeated low-dose exposure can lead to sensitization.
- Intermediate Doses: Often optimal for inducing sensitization and subsequent hypersensitivity reactions.
Route
The way an antigen enters the body profoundly influences the type and intensity of the immune response.
- Inhalation: Commonly leads to Type I reactions (allergic rhinitis, asthma) to airborne allergens.
- Ingestion: Can cause food allergies (Type I) or, less commonly, systemic reactions (Type III).
- Skin Contact: A common route for Type IV (contact dermatitis) and Type I (e.g., latex allergy) reactions.
- Intravenous Injection: More likely to lead to systemic reactions, including anaphylaxis (Type I) or serum sickness (Type III) if the antigen is widespread.
- Subcutaneous/Intramuscular Injection: Can induce localized Type I reactions (e.g., insect sting allergy) or, if repeated, Arthus reactions (Type III) in sensitized individuals.
Adjuvants
Adjuvants are substances that enhance the immunogenicity of an antigen when administered together. They typically work by prolonging antigen persistence, stimulating innate immune cells (like dendritic cells and macrophages), and promoting the uptake and presentation of antigens.
While primarily used in vaccines to boost protective immunity, adjuvants can also inadvertently influence hypersensitivity.
Some environmental pollutants (e.g., diesel exhaust particles) can act as environmental adjuvants, increasing the likelihood of developing allergic sensitization to co-exposed allergens. They can modify allergen penetration, interact with immune processes, and promote IgE production.
In allergen-specific immunotherapy (AIT), adjuvants are sometimes added to allergen extracts to modulate the immune response, aiming to shift it away from a pathogenic Th2 response (responsible for allergies) towards a more tolerant Th1 or regulatory T cell response. This is a complex area of research, with ongoing efforts to find adjuvants that can specifically promote tolerance.
Type I Hypersensitivity (Immediate Hypersensitivity/Allergy)
Type I hypersensitivity, also known as immediate or allergic hypersensitivity, is an IgE-mediated immune reaction that develops rapidly, typically within minutes of exposure to an allergen. It’s characterized by an exaggerated immune response to otherwise harmless substances.
Causes/Allergens of Type I Hypersensitivity
- Pollen
- Dust mites
- Animal dander
- Certain foods (like nuts, eggs, shellfish)
- Insect venoms
- Latex
- Certain drugs (like penicillin)
Mechanism of Type I Hypersensitivity
Sensitization phase: Initial exposure to the allergen triggers the production of IgE antibodies by B cells. These IgE antibodies then bind to Fc receptors on mast cells and basophils.
Effector phase: Upon re-exposure to the same allergen, the allergen cross-links the IgE on these cells, leading to rapid degranulation and the release of inflammatory mediators (like histamine, leukotrienes, and prostaglandins). These mediators cause vasodilation, increased vascular permeability, smooth muscle contraction, and inflammation.
This results in both an early-phase reaction (immediate symptoms) and potentially a late-phase reaction (hours later, involving inflammatory cell infiltration).
Timeframe: Occurs very rapidly, within minutes of exposure.
Examples of Type I Hypersensitivity Reaction
Local reactions: Allergic rhinitis (hay fever), allergic conjunctivitis, asthma, urticaria (hives), eczema, angioedema.
Systemic reactions: Anaphylaxis (a severe, potentially life-threatening reaction involving airway swelling, breathing difficulties, hypotension, and shock).
Diagnosis of Type I Hypersensitivity
- Clinical history and physical examination.
- Skin prick tests: Small amounts of allergens are introduced into the skin to observe for a localized reaction (wheal and flare).
- Specific IgE blood tests (RAST or ImmunoCAP): Measure the levels of IgE antibodies specific to particular allergens.
Management Principles of Type I Hypersensitivity
- Allergen avoidance: Identifying and avoiding the specific allergen is crucial.
- Pharmacological treatments
- Antihistamines: To block histamine effects.
- Corticosteroids: To reduce inflammation.
- Bronchodilators: For asthma symptoms.
- Epinephrine (adrenaline): For anaphylaxis (to reverse airway constriction and hypotension).
- Immunotherapy (desensitization): Gradual exposure to increasing doses of the allergen to reduce sensitivity.
Type II Hypersensitivity (Cytotoxic Hypersensitivity)
Type II hypersensitivity reactions are antibody-mediated, primarily involving IgG or IgM antibodies. These antibodies bind to antigens that are expressed on the surface of host cells or are part of the extracellular matrix. This binding typically leads to the destruction of the target cells or tissues, making them “cytotoxic.”
Causes/Antigens of Type II Hypersensitivity
The antigens involved are usually intrinsic to the cell surface or tissue (autoantigens) or are exogenous antigens (like drugs) that attach to cell membranes.
Examples:
- Drug-induced reactions: Certain drugs, like penicillin, can bind to the surface of red blood cells, making them targets for antibody attack.
- Incompatible blood transfusions: Antibodies in the recipient’s plasma bind to antigens on donor red blood cells.
- Rh incompatibility (Hemolytic Disease of the Newborn): Maternal antibodies attack fetal red blood cells.
- Autoimmune diseases: Conditions where the immune system mistakenly targets the body’s own cells or tissues (e.g., autoimmune hemolytic anemia, Goodpasture’s syndrome).
Mechanism
When IgG or IgM antibodies bind to antigens on the target cell surface, they initiate cell damage or destruction through several pathways:
- Complement Activation: The antibody-antigen complex activates the classical complement pathway. This leads to the formation of the Membrane Attack Complex (MAC), which punctures holes in the target cell membrane, causing osmotic lysis. Complement activation also generates anaphylatoxins (C3a, C5a) that recruit inflammatory cells, and opsonins (C3b) that tag cells for phagocytosis.
- Antibody-Dependent Cell-mediated Cytotoxicity (ADCC): Cells such as Natural Killer (NK) cells, macrophages, and eosinophils have Fc receptors that bind to the Fc portion of antibodies coating target cells. Once bound, these effector cells release cytotoxic substances (e.g., perforins, granzymes) that induce apoptosis or lysis of the target cell.
- Opsonization and Phagocytosis: Antibodies and/or complement components (like C3b) coat the target cells, acting as opsonins. Phagocytic cells (macrophages, neutrophils) recognize these opsonized cells via their Fc receptors or complement receptors and engulf them, leading to their destruction.
- Antibody-mediated Cellular Dysfunction: In some cases, antibodies do not directly destroy the cell but interfere with its normal function by binding to receptors. For example, in Graves’ disease, antibodies stimulate thyroid hormone production, or in Myasthenia Gravis, antibodies block acetylcholine receptors, impairing nerve-muscle communication.
Timeframe: Can occur within minutes to hours.
Clinical Manifestations of Type II Hypersensitivity Reaction
- Hemolytic Transfusion Reactions: Fever, chills, back pain, hemoglobinuria, renal failure due to rapid destruction of transfused red blood cells.
- Hemolytic Disease of the Newborn (Erythroblastosis Fetalis): Anemia, jaundice, and in severe cases, hydrops fetalis in the newborn due to maternal antibodies destroying fetal red blood cells.
- Autoimmune Hemolytic Anemia: Anemia due to the immune system attacking the body’s own red blood cells.
- Drug-induced Cytopenias: Thrombocytopenia (low platelets) or agranulocytosis (low white blood cells) caused by drug-antibody complexes.
- Goodpasture’s Syndrome: Antibodies target antigens in the basement membranes of the kidneys and lungs, leading to glomerulonephritis and pulmonary hemorrhage.
- Myasthenia Gravis: Antibodies block acetylcholine receptors at the neuromuscular junction, causing muscle weakness.
- Graves’ Disease: Antibodies stimulate thyroid-stimulating hormone (TSH) receptors, leading to hyperthyroidism.
Diagnosis of Type II Hypersensitivity
- Direct Coombs Test (Direct Antiglobulin Test – DAT): Detects antibodies or complement components already attached to the surface of red blood cells. This is crucial for diagnosing autoimmune hemolytic anemia and hemolytic disease of the newborn.
- Indirect Coombs Test (Indirect Antiglobulin Test – IAT): Detects unbound antibodies in the serum that can react with specific red blood cells. Used for cross-matching blood transfusions and screening for anti-Rh antibodies in pregnant women.
- Detection of specific autoantibodies: For conditions like Goodpasture’s syndrome (anti-GBM antibodies) or Myasthenia Gravis (anti-acetylcholine receptor antibodies).
- Biopsy and immunofluorescence: To visualize antibody deposition in tissues (e.g., kidney biopsy in Goodpasture’s).
Management Principles of Type II Hypersensitivity
- Remove or discontinue the offending agent: If a drug is the cause, stopping it is the first step.
- Immunosuppression: Corticosteroids are commonly used to suppress the immune response and reduce antibody production. Other immunosuppressants may be used in severe or refractory cases.
- Plasma exchange (plasmapheresis): To remove circulating antibodies, especially in acute and severe cases (e.g., severe Goodpasture’s syndrome).
- Intravenous Immunoglobulin (IVIg): Can be used to block Fc receptors on phagocytes, thus reducing antibody-mediated destruction, or to modulate the immune response.
- Supportive care: Blood transfusions for severe anemia, dialysis for kidney failure, etc., as needed.
- Splenectomy: In some cases of autoimmune hemolytic anemia, removal of the spleen (where much of the red blood cell destruction occurs) can be beneficial.
Type III Hypersensitivity (Immune Complex-Mediated Hypersensitivity)
Type III hypersensitivity reactions are caused by the formation and deposition of antigen-antibody immune complexes in various tissues and organs. These complexes activate the complement system and recruit inflammatory cells, leading to localized inflammation and tissue damage.
Unlike Type II reactions where antibodies bind to fixed antigens on cell surfaces, in Type III, the complexes are typically formed in the circulation and then deposited. The reactions can be systemic or localized and usually manifest several hours to days after antigen exposure.
Causes/Antigens of Type III Hypersensitivity
The antigens involved can be exogenous (from outside the body) or endogenous (self-antigens).
- Persistent Infections: Chronic infections (e.g., bacterial, viral, parasitic) can lead to sustained antigen presence and continuous immune complex formation (e.g., chronic viral hepatitis, malaria).
- Autoimmune Diseases: In these conditions, the body produces antibodies against its own soluble antigens, leading to chronic immune complex formation and deposition (e.g., Systemic Lupus Erythematosus – SLE, Rheumatoid Arthritis – RA).
- Inhaled Antigens: Repeated inhalation of certain antigens can lead to localized Type III reactions in the lungs (e.g., farmer’s lung, pigeon fancier’s disease).
- Drug Reactions: Some drugs can act as haptens, combining with host proteins to form antigens, leading to immune complex formation (e.g., serum sickness from antivenoms or antitoxins).
Mechanism of Type III Hypersensitivity
- Immune Complex Formation: Antigens (soluble) combine with antibodies (typically IgG or IgM) to form circulating immune complexes. The size and ratio of antigen to antibody are critical; intermediate-sized complexes are generally the most pathogenic because they are less efficiently cleared by phagocytes and are more prone to deposition.
- Deposition: These circulating immune complexes become trapped in small blood vessels or filter membranes, particularly in sites of turbulent blood flow or high filtration pressure, such as the glomeruli of the kidneys, joint synovium, and vascular endothelium.
- Complement Activation: Once deposited, the immune complexes activate the classical complement pathway. This generates various mediators, including:
- Anaphylatoxins (C3a, C5a): These cause mast cell degranulation (leading to increased vascular permeability) and act as powerful chemotactic agents, attracting neutrophils and other inflammatory cells to the site of deposition.
- Opsonins (C3b): While relevant in Type II, their role here is more about immune complex clearance, but if clearance is inefficient, it contributes to deposition.
- Inflammation and Tissue Damage: The recruited neutrophils attempt to phagocytose the immune complexes. However, since the complexes are often deposited on surfaces (e.g., basement membranes), the neutrophils cannot fully engulf them. Instead, they release potent lysosomal enzymes, reactive oxygen species, and other inflammatory mediators (e.g., proteases, cytokines) directly into the surrounding tissue. This uncontrolled inflammatory response causes significant localized tissue damage, vasculitis, thrombosis, and necrosis.
Timeframe: Can take several hours to days, or even weeks, to develop after antigen exposure.
Clinical Manifestations of Type III Hypersensitivity Reaction
The symptoms often depend on where the immune complexes are deposited.
Systemic Reactions
- Serum Sickness: A classic example, usually occurring 7-10 days after exposure to a large dose of foreign protein (e.g., antivenom, diphtheria antitoxin). Symptoms include fever, rash (urticarial or vasculitic), arthralgia (joint pain), arthritis, lymphadenopathy, and glomerulonephritis.
- Systemic Lupus Erythematosus (SLE): A chronic autoimmune disease where immune complexes containing DNA and other nuclear antigens deposit in multiple organs, leading to symptoms in joints, skin, kidneys (lupus nephritis), heart, lungs, and brain.
- Rheumatoid Arthritis (RA): Immune complexes containing rheumatoid factor (an antibody against IgG) deposit in the joints, causing chronic inflammation, pain, swelling, and joint destruction.
- Post-streptococcal Glomerulonephritis: Develops after a streptococcal infection, where immune complexes deposit in the kidney glomeruli, causing inflammation, hematuria, proteinuria, and kidney dysfunction.
Localized Reactions
- Arthus Reaction: A localized skin reaction that occurs when an antigen is injected intradermally into an already sensitized individual with high levels of circulating antibody. It manifests as localized redness, swelling, edema, and sometimes necrosis within hours.
Diagnosis of Type III Hypersensitivity
- Clinical presentation: History and symptoms are crucial.
- Laboratory tests:
- Detection of circulating immune complexes (CICs): Although not routinely performed, methods exist to detect CICs.
- Complement levels: Decreased levels of complement components (e.g., C3, C4, CH50) are often observed during active disease, as complement is consumed during immune complex activation.
- ESR (Erythrocyte Sedimentation Rate) and CRP (C-reactive protein): Elevated levels indicate systemic inflammation.
- Detection of specific autoantibodies: For autoimmune diseases (e.g., anti-dsDNA antibodies in SLE, rheumatoid factor in RA).
- Biopsy and Immunofluorescence: Biopsies of affected tissues (e.g., kidney, skin, synovium) can show characteristic histopathological changes (vasculitis, inflammatory cell infiltration) and, importantly, granular deposits of immune complexes (IgG, IgM, complement components) along basement membranes or in vessel walls when stained with fluorescent antibodies.
Treatment and Management of Type III Hypersensitivity
The primary goals are to remove the source of antigen (if possible), suppress the immune response, and manage symptoms.
- Identify and remove the offending antigen: If the cause is known (e.g., a drug or chronic infection), eliminating it is paramount.
- Anti-inflammatory drugs:
- Corticosteroids: The mainstay of treatment, reducing inflammation and suppressing the immune response (e.g., prednisone).
- NSAIDs (Non-Steroidal Anti-Inflammatory Drugs): For pain and inflammation, particularly in arthralgia/arthritis.
- Immunosuppressants: For severe or chronic conditions, other immunosuppressive drugs may be used to reduce antibody production and immune cell activity (e.g., azathioprine, cyclophosphamide, mycophenolate mofetil).
- Plasma exchange (plasmapheresis): In acute, severe cases (e.g., severe serum sickness, rapidly progressive glomerulonephritis), plasmapheresis can be used to rapidly remove circulating immune complexes and antibodies from the blood.
- Biologics: For specific autoimmune diseases, targeted therapies (e.g., TNF inhibitors for RA, B-cell depleting agents for SLE) may be used to modulate the immune response.
- Supportive care: Management of organ-specific complications (e.g., dialysis for kidney failure, pain management for arthritis).
Type IV Hypersensitivity (Delayed-Type Hypersensitivity – DTH)
Type IV hypersensitivity is unique among the hypersensitivity reactions because it is cell-mediated, not antibody-mediated.
It involves the activation of T lymphocytes (primarily T helper 1 (Th1) cells and cytotoxic T lymphocytes (CTLs)) upon re-exposure to an antigen.
The reaction is characterized by a delayed onset, typically appearing 24 to 72 hours (or even longer) after antigen exposure, which gives it its “delayed-type” designation. This delay is due to the time required for T cells to migrate to the antigen site, proliferate, and release cytokines.
Causes/Antigens of Type IV Hypersensitivity
The antigens that trigger Type IV reactions are typically intracellular pathogens or haptens that modify host proteins.
- Intracellular Pathogens: Bacteria (e.g., Mycobacterium tuberculosis, Mycobacterium leprae), fungi (e.g., Histoplasma capsulatum), viruses (e.g., herpes simplex virus, measles virus). The immune response aims to clear these pathogens but can also cause tissue damage.
- Contact Antigens (Haptens): Small molecules that penetrate the skin and bind to self-proteins, making them antigenic. Examples include nickel (jewelry), urushiol (from poison ivy/oak/sumac), chromate (leather), cosmetics, and certain chemicals in latex.
- Self-Antigens: In autoimmune diseases, T cells can mistakenly target self-antigens, leading to chronic inflammation and tissue destruction (e.g., in Type 1 diabetes, multiple sclerosis, rheumatoid arthritis).
- Transplant Antigens: In chronic transplant rejection, recipient T cells attack donor tissue antigens.
Mechanism of Type IV Hypersensitivity
Type IV hypersensitivity proceeds in two main phases.
Sensitization Phase (Initial Exposure)
An antigen-presenting cell (APC), such as a dendritic cell (DC), captures the antigen (e.g., a contact allergen, a protein from an intracellular pathogen).
The APC processes the antigen and presents peptide fragments on MHC class II molecules to naive CD4+ T helper cells (specifically directing them towards a Th1 phenotype) in the lymph nodes. For some antigens, presentation on MHC class I can activate CD8+ cytotoxic T lymphocytes (CTLs).
The activated Th1 cells proliferate and differentiate into memory T cells, sensitizing the individual to that particular antigen.
Effector Phase (Re-exposure)
Upon subsequent exposure to the same antigen, the sensitized memory T cells (primarily Th1 cells) are rapidly activated at the site of antigen entry.
Activated Th1 cells release a variety of cytokines, including:
- Interferon-gamma (IFN-γ): The most crucial cytokine, which activates macrophages, increasing their phagocytic and microbicidal activity, and enhancing MHC expression.
- Tumor Necrosis Factor-alpha (TNF-α) and TNF-beta (lymphotoxin): Promote inflammation, activate endothelial cells, and attract other immune cells.
- Chemokines: Recruit macrophages and other leukocytes to the site of inflammation.
The recruited and activated macrophages form the bulk of the inflammatory infiltrate and contribute significantly to tissue damage by releasing lysosomal enzymes, reactive oxygen species, and pro-inflammatory mediators.
In some instances, particularly with intracellular pathogens or altered self-cells (e.g., viral infections, tumor cells), activated CD8+ cytotoxic T lymphocytes (CTLs) directly kill target cells presenting the antigen on MHC class I molecules.
Over time, if the antigen persists, chronic macrophage activation can lead to granuloma formation, where macrophages and lymphocytes wall off the antigen in a nodular lesion.
Clinical Manifestations of Type IV Hypersensitivity Reaction
- Contact Dermatitis: The most common form, characterized by itchy, erythematous (red), vesicular, or papular rash at the site of contact with an allergen (e.g., poison ivy rash, nickel allergy, reaction to cosmetics).
- Tuberculin-Type Hypersensitivity (e.g., Mantoux Test for TB): A localized induration (hard swelling) and erythema at the site of intradermal injection of tuberculin protein, peaking at 48-72 hours, indicating previous exposure to Mycobacterium tuberculosis.
- Granulomatous Reactions: Chronic Type IV reactions where the body walls off persistent antigens that cannot be cleared, forming granulomas. Examples include tuberculosis, sarcoidosis, Crohn’s disease (in part).
- Chronic Transplant Rejection: T-cell mediated destruction of grafted tissues over months or years.
- Some Autoimmune Diseases: T cells play a significant role in the pathogenesis of conditions like Type 1 diabetes (destruction of pancreatic beta cells), multiple sclerosis (destruction of myelin sheath), and Hashimoto’s thyroiditis.
Diagnosis of Type IV Hypersensitivity
- Patch Testing: For contact dermatitis. Patches containing suspected allergens are applied to the skin for 48 hours, and the site is then examined for a delayed eczematous reaction.
- Tuberculin Skin Test (PPD/Mantoux test): Intradermal injection of tuberculin purified protein derivative (PPD) to assess for a DTH response, indicating previous exposure to Mycobacterium tuberculosis.
- Biopsy and Histopathology: Examination of affected tissue often reveals a perivascular cuffing of lymphocytes and macrophages, and in chronic cases, granuloma formation.
- In vitro assays: Lymphocyte proliferation assays or cytokine release assays can sometimes be used to detect T cell sensitization to specific antigens, though these are less common in routine clinical practice for DTH.
Treatment and Management of Type IV Hypersensitivity
- Identify and avoid the antigen: This is the most effective treatment for contact dermatitis (e.g., avoiding nickel, poison ivy).
- Corticosteroids (anti-inflammatory): Topical corticosteroids are the primary treatment for contact dermatitis to reduce inflammation and itching. Systemic corticosteroids may be used for severe, widespread reactions or systemic manifestations of DTH.
- Immunosuppressants: For chronic or severe autoimmune Type IV reactions or transplant rejection, broader immunosuppressive agents (e.g., azathioprine, cyclosporine, methotrexate) may be necessary to suppress T cell activity.
- Biologics: In some autoimmune diseases, targeted biologic therapies (e.g., anti-TNF-α agents for RA) can be used to block key cytokines involved in DTH.
- Supportive care: Symptomatic relief, wound care for skin lesions, and management of underlying diseases.
Frequently Asked Questions (FAQs)
What is the difference between hypersensitivity reaction and allergy?
A hypersensitivity reaction is a broad term referring to any exaggerated, undesirable, and often damaging immune response to an antigen that would normally be harmless. This encompasses all four types classified by Gell and Coombs (Type I, II, III, and IV).
An allergy, on the other hand, is a more specific term that is primarily defined as a disease resulting from a Type I hypersensitivity reaction, which is mediated by IgE antibodies reacting to an otherwise innocuous antigen (an allergen).
Therefore, while all allergies are a form of hypersensitivity reaction (specifically Type I), not all hypersensitivity reactions are considered allergies (e.g., autoimmune diseases like lupus or contact dermatitis are HSRs but not typically called allergies).
What is serum sickness in Type III hypersensitivity reaction?
Serum sickness is a Type III hypersensitivity reaction that occurs when the immune system reacts to foreign proteins (antigens), typically from medications or animal-derived products.
It’s characterized by the formation of antigen-antibody immune complexes in the bloodstream, which then deposit in various tissues like blood vessels, joints, and kidneys. This deposition triggers inflammation, leading to a classic triad of symptoms: fever, rash (often urticarial or hive-like), and joint pain (arthralgia or arthritis).
Historically, it was commonly seen after the administration of animal-derived antisera (e.g., antivenoms, antitoxins) to treat infections.
Today, it’s more frequently associated with certain medications, including antibiotics (like penicillin and cefaclor), monoclonal antibodies (e.g., rituximab), and other protein-based therapies.
The symptoms usually develop 7 to 21 days after initial exposure, but can appear sooner with re-exposure, and generally resolve once the offending agent is discontinued.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Abbas M, Moussa M, Akel H. Type I Hypersensitivity Reaction. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560561/
- Bajwa SF, Mohammed RH. Type II Hypersensitivity Reaction. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563264/
- Usman N, Annamaraju P. Type III Hypersensitivity Reaction. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559122/
- Marwa K, Goldin J, Kondamudi NP. Type IV Hypersensitivity Reaction. [Updated 2025 May 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562228/