Procedure At a Glance
The Dithionite Solubility Test (Sickle Cell Disease Test) is a screening method used to detect the presence of abnormal Hemoglobin S (HbS), indicative of Sickle Cell Disease or Sickle Cell Trait.
- Ensure a freshly prepared dithionite solution is available.
- In a test tube, thoroughly mix 2.0 ml of dithionite solution with 20 µl of anticoagulated patient peripheral blood.
- Allow the mixture to stand at room temperature for 5-10 minutes.
- Observe the test tube for signs of turbidity.
- Place a single drop of the mixture onto a microscope slide.
- Cover the drop with a coverslip and seal the edges with petroleum jelly-paraffin wax or depex.
- Immediately examine the slide under a microscope (10x and 40x objectives).
- Negative Result: Clear, transparent solution; red blood cells appear normal (biconcave discs).
- Positive Result: Turbid or cloudy solution; red blood cells appear sickled.
What is the Sickle Cell Disease (SCD) Test (Dithionite Solubility Test)?
The Sickle Cell Disease (SCD) test, commonly known as the Dithionite Solubility Test or Hb S Sickling Test, is a rapid and inexpensive screening method used to detect the presence of Hemoglobin S (HbS) in a blood sample.
Understanding Sickle Cell Disease (SCD) and Hemoglobin S (HbS)
Sickle Cell Disease (SCD) is a serious, inherited blood disorder that affects the shape of red blood cells. It is passed down genetically from the parents to their children recessively. It is not contagious.
SCD is caused by a mutation in the gene that produces the beta globin gene, which makes up 50% of the hemoglobin, the protein in red blood cells responsible for carrying oxygen throughout the body. This mutation leads to the production of an abnormal type of hemoglobin called Hemoglobin S (HbS).
Normally, red blood cells are round, flexible, and easily move through blood vessels. In SCD, when HbS gives up its oxygen, it polymerizes (sticks together), forming rigid, rod-like structures inside the red blood cells. This causes the red blood cells to become stiff, sticky, and take on a characteristic crescent or “sickle” shape.
Consequences of Sickled Cells
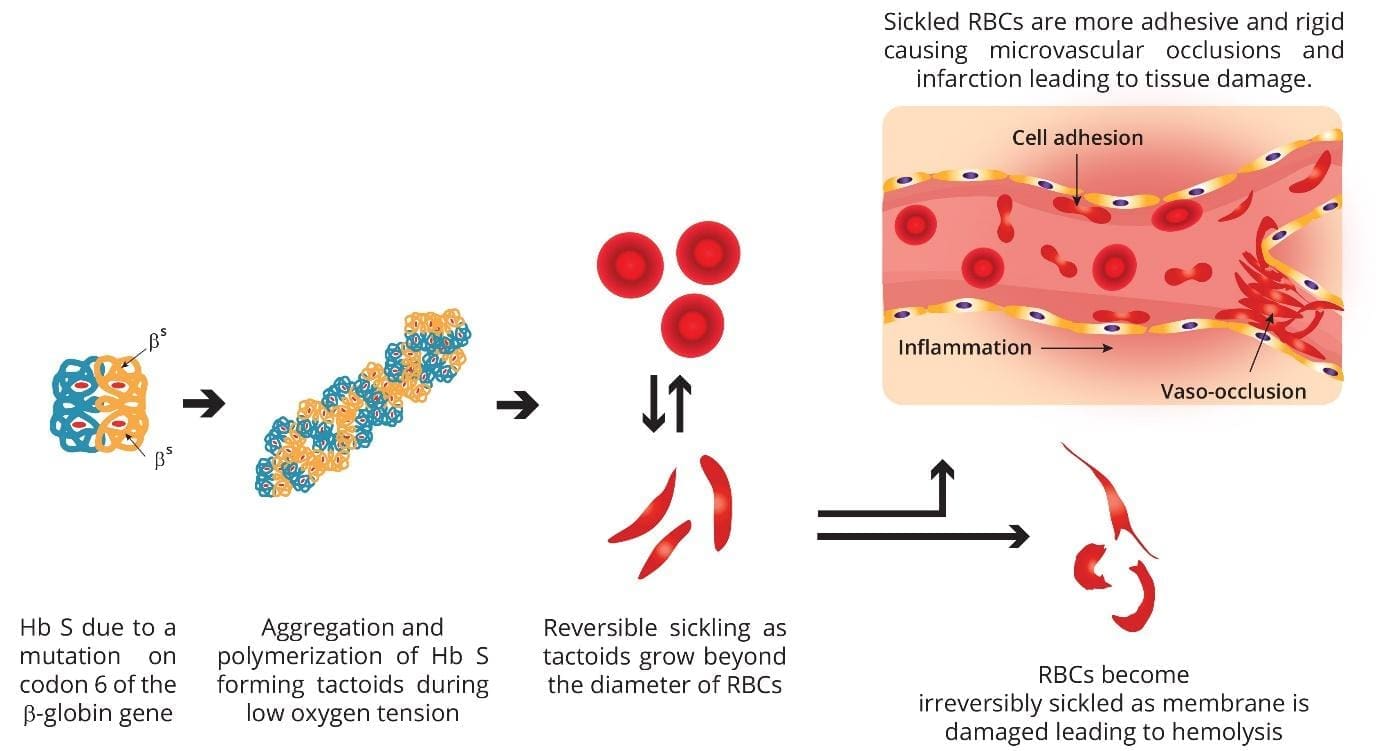
- Short Lifespan: Sickled cells are fragile and have a much shorter lifespan (10-20 days) compared to normal red blood cells (120 days). This leads to chronic anemia.
- Blood Vessel Blockage: Their rigid, sticky nature causes them to get stuck in small blood vessels, blocking blood flow to organs and tissues.
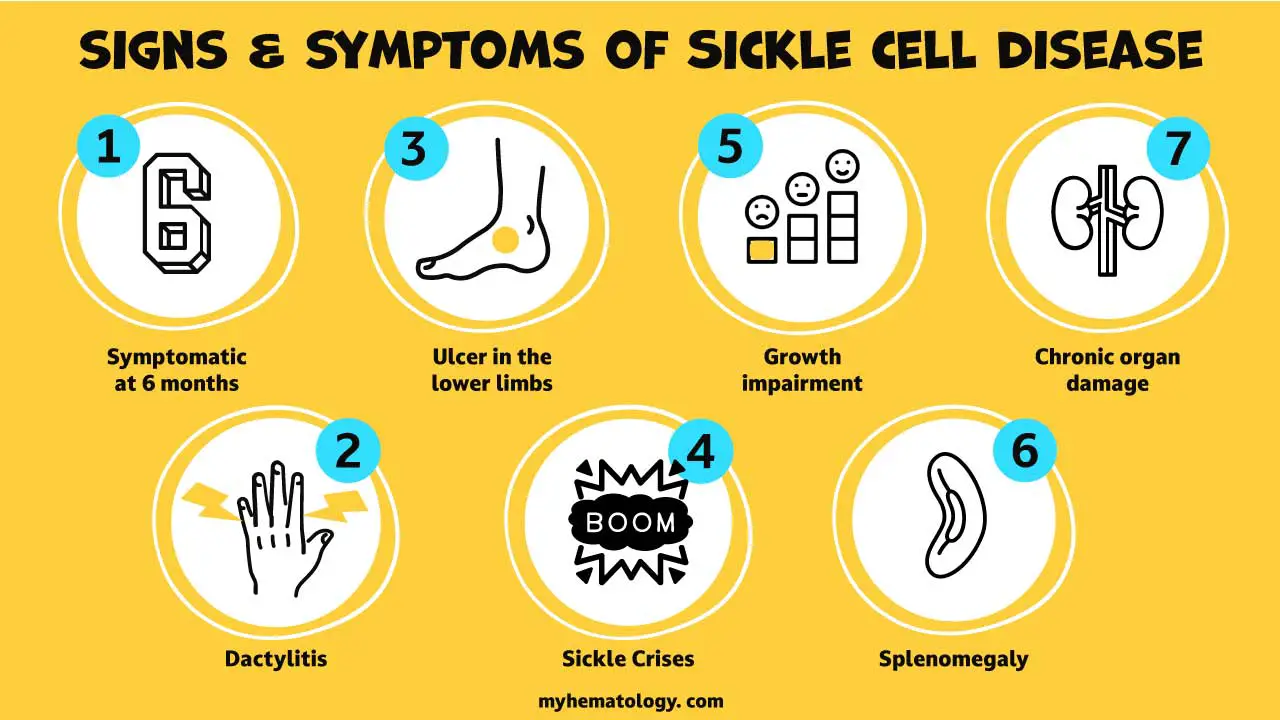
- Complications: This blockage can lead to a wide range of severe and chronic complications, including:
- Pain crises (vaso-occlusive crises): Sudden, severe pain in various parts of the body.
- Organ damage: Affecting the spleen, liver, kidneys, lungs, heart, and brain (leading to strokes).
- Increased risk of infections: Especially in young children, due to spleen damage.
- Acute Chest Syndrome: A life-threatening lung complication.
- Delayed growth and development.
Sickle Cell Trait vs. Sickle Cell Disease
- Sickle Cell Trait: Occurs when a person inherits one copy of the HbS gene from one parent and one normal hemoglobin gene from the other. Individuals with the trait usually do not have symptoms but can pass the gene to their children.
- Sickle Cell Disease: Occurs when a person inherits two copies of the HbS gene (one from each parent) or one HbS gene and another abnormal hemoglobin gene (e.g., HbC or beta-thalassemia). These individuals experience the symptoms and complications of the disease.
Principle of the Dithionite Solubility Sickle Cell Disease Test
The principle of the Sickle Cell Disease test, specifically the Dithionite Solubility Test, hinges on a unique characteristic of abnormal hemoglobin S (HbS) when it is deprived of oxygen.
Normal red blood cells contain Hemoglobin A (HbA), which remains soluble whether it’s carrying oxygen (oxygenated) or not (deoxygenated). Individuals with Sickle Cell Disease or Sickle Cell Trait have Hemoglobin S (HbS). HbS differs from HbA by a single amino acid substitution (valine instead of glutamic acid at position 6 of the beta-globin chain). This seemingly small change has profound effects under specific conditions.
The sickle cell disease test uses a strong reducing agent, typically sodium dithionite (also known as sodium hydrosulfite), dissolved in a phosphate buffer.
When this solution in the sickle cell disease test is mixed with a blood sample, the saponin (a component often included in the reagent) causes the red blood cells to lyse (break open), releasing their hemoglobin.
Simultaneously, the sodium dithionite rapidly removes oxygen from the released hemoglobin.
Unlike HbA, deoxygenated HbS is much less soluble in the concentrated phosphate buffer solution of the sickle cell disease test. When deoxygenated, HbS molecules undergo a conformational change and begin to polymerize. This means they stick together and stack into long, rigid, rod-like fibers or aggregates, sometimes referred to as “tactoids” or “liquid crystals.”
Normal HbA, under the same deoxygenated conditions, remains soluble and does not polymerize.
Visible Turbidity
The formation of these insoluble HbS polymers (fibers) in the solution creates a turbid (cloudy or opaque) appearance. The more HbS present in the sickle cell disease test, the greater the turbidity. If no significant HbS is present (i.e., mostly normal HbA), the solution in the sickle cell disease test remains clear and transparent.
Microscopic Confirmation (Red Blood Cell Sickling)
While the turbidity is a macroscopic indicator, the underlying reason for it is the sickling process within the red blood cells before lysis, and the polymerization of released HbS.
The sickle cell disease test (Dithionite Solubility Test) also allows for microscopic examination, where the characteristic sickle (crescent) shapes of the red blood cells can be directly observed. This confirms that the turbidity is indeed due to the sickling phenomenon.
Materials
- Anticoagulated patient peripheral blood sample
- Dithionite solution: 10% sodium dithionite (Na2S2O4) in phosphate buffer, pH 6.8.
- Saponin (or other hemolytic agent)
- Pasteur pipette
- Microscope slides and coverslips
- Petroleum jelly-paraffin wax or depex
- Test tube
- Viewer/Reading Stand (for visual interpretation): Some kits include a stand with lines or text to aid in assessing turbidity.
Note: Commercial kits for the Dithionite Solubility Test (sickle cell disease test) are widely available and often contain pre-measured reagents in two parts (a buffer solution and a sodium dithionite powder) that need to be reconstituted just before use. These kits simplify the preparation process and ensure correct concentrations.
Protocol
- Make a fresh dithionite solution by dissolving 10 mg of sodium dithionite in 1 mL of phosphate buffer, pH 6.8. Mix well and use immediately.
- Label a clean, dry test tube appropriately for the patient sample.
- Add 2.0 ml of the freshly prepared dithionite solution into the labeled test tube.
- Add 20 µl of the patient peripheral blood sample to the dithionite solution in the test tube. *Important: Avoid air bubbles during pipetting the blood.
- Mix the blood and dithionite solution immediately and thoroughly. This is typically done by inverting the tube several times or using a vortex mixer, ensuring complete lysis of red blood cells and homogenous mixing.
- Allow the mixture to stand undisturbed at room temperature for 5 to 10 minutes depending on manufacturer’s protocol.
- After incubation, observe the test tube for turbidity using the viewer/reading stand.
- Immediately after macroscopic observation, proceed with microscopic confirmation.
- Place a drop of dithionite solution and a drop of anticoagulated blood into a test tube.
- Mix the blood and dithionite gently to ensure thorough mixing.
- Place a drop of the mixture on a clean microscope slide.
- Cover the slide with a coverslip and avoid trapping air bubbles.
- Cover all edges of the cover slip with petroleum jelly-paraffin wax or depex.
- Observe under the microscope.
Interpretation
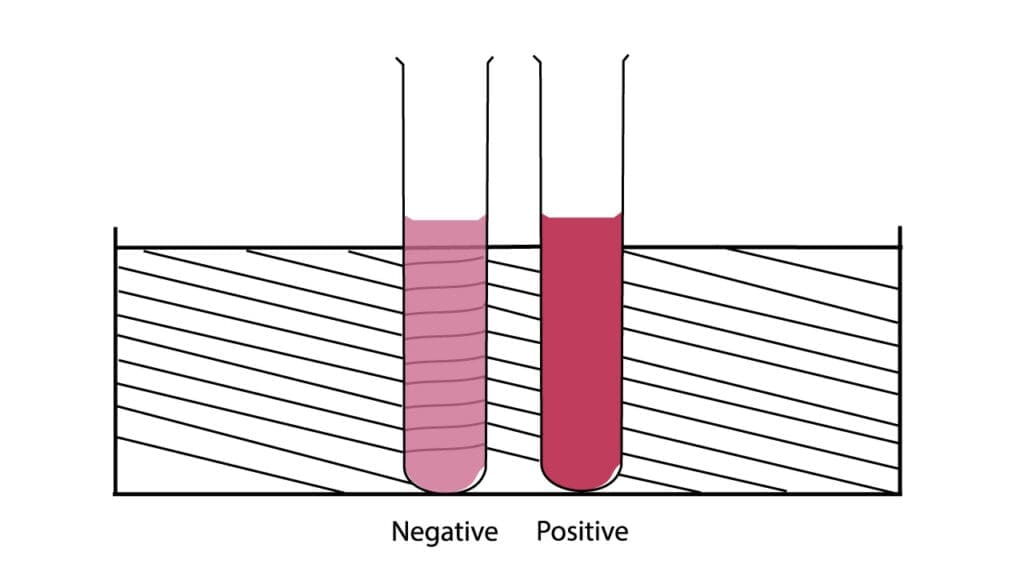
The interpretation of the Dithionite Solubility Test (sickle cell disease test) relies on both the macroscopic appearance of the solution in the test tube and the microscopic examination of the red blood cells.
Negative Result
A negative result in the sickle cell disease test (Dithionite Solubility Test) indicates that there is no significant amount of Hemoglobin S (HbS) present in the blood sample. This sickle cell disease test result suggests the absence of Sickle Cell Trait or Sickle Cell Disease.
- Macroscopic Appearance (Test Tube): The solution in the sickle cell disease test tube will remain clear and transparent after the incubation period. You should be able to easily read fine print or lines (e.g., from a test tube rack, or a standardized reading card) through the solution.
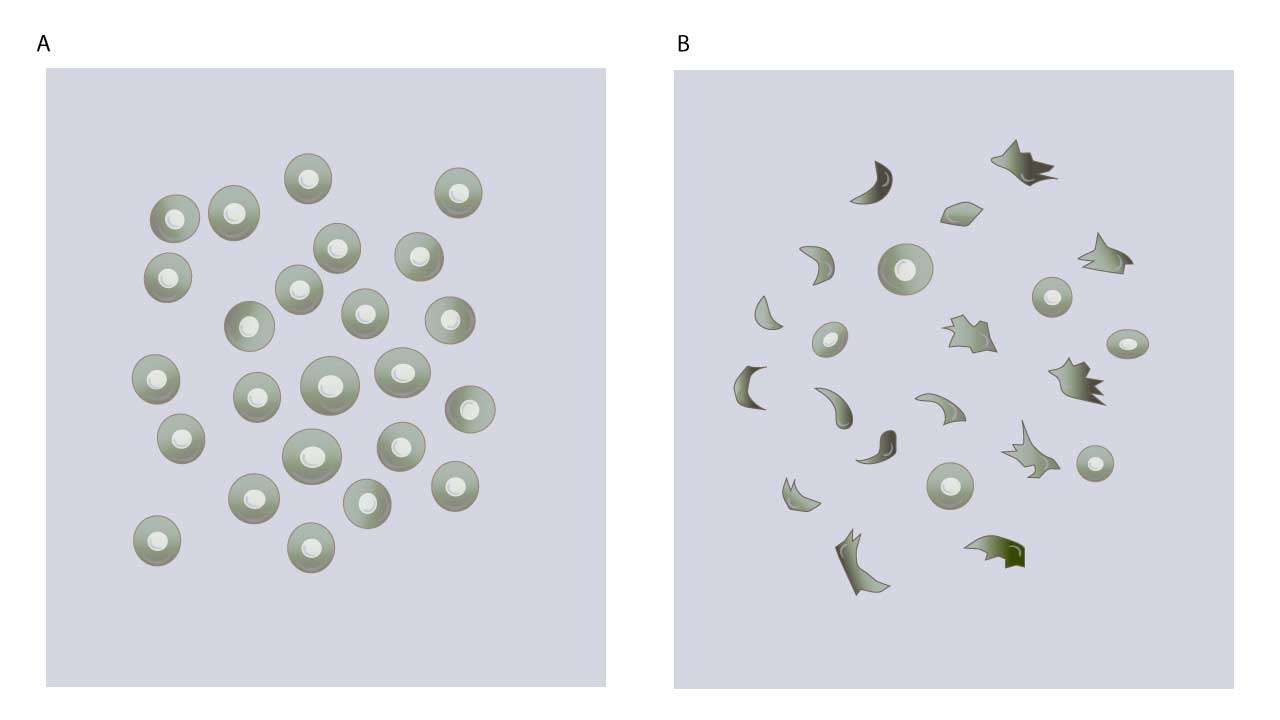
- Microscopic Examination (Slide): Under the microscope, the red blood cells will primarily maintain their normal biconcave disc shape. You will not observe sickled or otherwise abnormally shaped red blood cells.
Positive Result
A positive result in the sickle cell disease test (Dithionite Solubility Test) indicates the presence of Hemoglobin S (HbS) in the blood sample, suggesting either Sickle Cell Trait or Sickle Cell Disease.
- Macroscopic Appearance (Test Tube): The solution in the sickle cell disease test tube will become turbid (cloudy or opaque) after the incubation period. The turbidity will be dense enough to obscure fine print or lines when viewed through the solution. The degree of turbidity can sometimes correlate with the amount of HbS present (more HbS, more turbidity).
- Microscopic Examination (Slide): Under the microscope, you will observe the presence of sickled-shaped erythrocytes. These cells will appear as characteristic crescent, elongated, holly leaf, or other irregular, pointed forms, deviating significantly from the normal round, biconcave disc shape.
This sickle cell disease test result indicates the presence of HbS. However, it does not differentiate between Sickle Cell Trait (heterozygous HbAS) and Sickle Cell Disease (homozygous HbSS or other compound heterozygotes like HbSC, HbS-beta thalassemia). Further confirmatory tests are required.
Indeterminate or Weak Positive Results
Indeterminate or weak positive results in the sickle cell disease test occur when the results are ambiguous and do not clearly fall into the “negative” or “strong positive” categories. This ambiguity in the sickle cell disease test can be challenging for technicians and necessitates careful interpretation and follow-up.
An indeterminate or weak positive result suggests the presence of some HbS or an interfering factor, but the reaction is not strong enough for a definitive positive diagnosis via the screening test.
Visual Characteristics
- Macroscopic Appearance: The solution in the sickle cell disease test tube may show only partial turbidity or a faint cloudiness, rather than the dense opacity seen in a strong positive result. It may appear slightly hazy, making it difficult to read lines through the solution, but not completely obscured.
- Microscopic Examination: While some sickled cells might be observed, their number may be very low, or the sickling might appear subtle and not the characteristic, pronounced sickling seen in Sickle Cell Disease.
Common Causes of Indeterminate or Weak Positive Results
These ambiguous sickle cell disease test results are often linked to factors that reduce the concentration of HbS or interfere with the sickling process.
- Low Concentration of HbS: Individuals with Sickle Cell Trait (Hb AS) have about 25%–45% HbS. While they usually yield a positive result, in some cases, particularly if the HbS concentration is at the lower end, the solubility test may show a weaker reaction.
- Infants and Fetal Hemoglobin (HbF): The presence of high levels of fetal hemoglobin (HbF) significantly inhibits the polymerization of HbS. Because newborns and infants naturally have high levels of HbF, the dithionite solubility test may yield a false-negative or a weak positive result in infants with SCD, even if HbS is present.
- Recent Blood Transfusion: If a patient with SCD has recently received a blood transfusion with normal (HbA) blood, their percentage of HbS will be diluted. This can lead to a weak positive or even a false-negative result, as the concentration of HbS may fall below the detection threshold of the sickle cell disease test.
- Very Low Hemoglobin (Severe Anemia): In cases of severe anemia, the reduced concentration of hemoglobin overall can lead to a weak reaction in the sickle cell disease test.
- Reagent or Technical Issues:
- Aged Reagents: If the sodium dithionite reagent is old or improperly stored, its potency may be reduced, leading to incomplete deoxygenation and a weak reaction.
- Incorrect Mixing Ratios: Errors in measuring the blood-to-reagent ratio can affect the sickle cell disease test’s sensitivity.
Required Next Steps for Indeterminate Results
An indeterminate or weak positive result is considered inconclusive and requires immediate follow-up. It should never be interpreted as a definitive negative.
- Repeat the Test: The first step is to repeat the sickle cell disease test (Dithionite Solubility Test) with a fresh sample and fresh reagents, ensuring strict adherence to the protocol.
- Confirmatory Testing: Regardless of the results of the repeat test, any weak or indeterminate result strongly indicates the need for definitive diagnostic testing. The patient must be referred for:
These advanced tests can accurately identify the hemoglobin types present and quantify the HbS percentage, providing a clear diagnosis of Sickle Cell Trait, Sickle Cell Disease, or ruling out HbS altogether.
Factors Affecting Sickle Cell Disease Test Results
The sickle cell disease test (Dithionite Solubility Test) is a highly useful screening tool, but like all laboratory tests, it is not 100% perfect and can be subject to factors that lead to incorrect results, commonly categorized as false negatives and false positives. Understanding these factors is crucial for accurate interpretation and appropriate follow-up.
False Negative Results
A false negative result occurs when the sickle cell disease test indicates the absence of HbS (a clear, non-turbid solution and no sickled cells), but HbS is, in fact, present in the patient’s blood.
Causes of False Negative Results
- High Levels of Fetal Hemoglobin (HbF): HbF (Hemoglobin F) is the predominant hemoglobin in newborns and gradually declines over the first 4-6 months of life. HbF strongly inhibits the polymerization of HbS. If the HbF concentration is very high, it can prevent sickling even if HbS is present, leading to a false negative result. This is why the dithionite solubility test is often considered unreliable for newborns and infants under 6 months of age, and specific newborn screening programs use other methods (like HPLC or electrophoresis).
- Recent Blood Transfusion with Normal Blood: If a person with Sickle Cell Disease (HbSS) or Sickle Cell Trait (HbAS) has recently received a significant transfusion of normal (HbA) red blood cells, the concentration of HbS in their blood can be diluted below the test’s detection threshold. The HbA from the transfused blood can also interfere with sickling.
- Severely Low Hemoglobin Levels (Severe Anemia): If the patient has severe anemia (very low red blood cell count or hemoglobin concentration, typically less than 8 g/dL), there may not be enough HbS in the sample to produce a detectable turbidity or sufficient sickling, leading to a weak or false negative result.
- Improper Sample Handling/Storage:
- Aged Blood Samples: Red blood cells gradually lose their ability to sickle over time, especially if the sample is not fresh (e.g., older than 24-48 hours depending on storage).
- Improper Anticoagulant/Storage: Inadequate or incorrect anticoagulation, or storage at inappropriate temperatures, can affect cell integrity and test reactivity.
- Technical Errors:
- Incorrect Reagent Concentration: If the dithionite solution is not prepared correctly (e.g., too dilute) or has deteriorated (common as dithionite is unstable), it may not effectively deoxygenate the HbS.
- Insufficient Blood Volume: Adding too little blood to the reagent will lead to a diluted HbS concentration.
- Inadequate Incubation Time: Not allowing enough time for the reaction to occur can result in incomplete sickling/polymerization.
- Improper Mixing: Insufficient mixing of blood and reagent can lead to localized areas where the reaction doesn’t proceed as expected.
False Positive Results
A false positive result occurs when the sickle cell disease test indicates the presence of HbS (a turbid solution and/or sickled cells observed), but HbS is not actually present, or the turbidity is due to another substance.
Causes of False Positive Results
- High Red Blood Cell Counts (Polycythemia): In conditions with extremely high red blood cell counts, the sheer volume of cells in the solution can sometimes cause a non-specific turbidity, mimicking a positive reaction.
- Extremely High White Blood Cell or Platelet Counts: Very high levels of white blood cells (leukocytosis, as in some leukemias) or platelets (thrombocytosis) can also interfere with the clarity of the solution and cause non-specific turbidity.
- Abnormal Plasma Proteins (Hyperglobulinemia, Hyperlipidemia): Increased levels of certain plasma proteins (e.g., paraproteins in multiple myeloma) or very high lipid levels (hyperlipidemia) can cause the solution to appear cloudy, leading to a false positive.
- Presence of Other Sickling Hemoglobin Variants: While the sickle cell disease test is designed to detect HbS, some other rare hemoglobin variants can also cause sickling or turbidity under the test conditions. Examples include HbC-Harlem, HbC-Georgetown, and HbC-Ziquinchor. These are much less common than HbS but would yield a positive solubility test.
- Presence of Many Heinz Bodies: In certain conditions where red blood cells contain numerous Heinz bodies (e.g., some cases of unstable hemoglobinopathies, or after splenectomy), these insoluble inclusions can precipitate and cause turbidity.
- Technical Errors:
- Too Much Blood Added: Adding an excessive amount of blood to the reagent can overwhelm the system and cause non-specific turbidity.
- Contamination: Contamination of reagents or glassware can lead to spurious turbidity.
Due to the possibility of both false negative and false positive results, especially in specific clinical scenarios (like newborns or recently transfused patients), it is imperative that any positive or ambiguous result from a sickle cell disease test be confirmed by more definitive methods such as hemoglobin electrophoresis or HPLC.
Limitations of the Dithionite Solubility Sickle Cell Disease Test
The sickle cell disease test, while a valuable screening tool for Hemoglobin S (HbS), has several important limitations that must be understood to prevent misdiagnosis or incomplete patient evaluation:
- It is a Screening Test, Not a Definitive Diagnostic Test: This is the most crucial limitation. A positive result from the Dithionite Solubility Sickle Cell Disease Test only indicates the presence of HbS. It cannot definitively diagnose Sickle Cell Disease or Sickle Cell Trait on its own. All positive or ambiguous results require confirmation by more specific methods.
- Cannot Differentiate Between Sickle Cell Trait and Sickle Cell Disease: The sickle cell disease test will be positive whether an individual has Sickle Cell Trait (HbAS, carrying one copy of the gene and usually asymptomatic) or Sickle Cell Disease (HbSS, HbSC, HbS-beta thalassemia, etc., experiencing symptoms).
- Does Not Quantify the Amount of HbS: The sickle cell disease test provides a qualitative (positive/negative) or semi-quantitative (turbidity strength) result. It does not tell the percentage of HbS relative to other hemoglobins in the blood.
- Requires Manual Interpretation and Technique Sensitivity: The interpretation relies on subjective visual assessment of turbidity and microscopic observation, which can be influenced by the technician’s experience and adherence to precise technique.
Confirmatory Tests for Sickle Cell Disease and Trait
When a screening test like the sickle cell disease test indicates the possible presence of Hemoglobin S (HbS), or if there’s a strong clinical suspicion of Sickle Cell Disease (SCD), confirmatory tests are essential to provide a definitive diagnosis and differentiate between Sickle Cell Trait and various forms of SCD.
- Hemoglobin Electrophoresis (Hb Electrophoresis): This is a widely used and reliable method that separates different types of hemoglobin based on their electrical charge and size. An electric current is passed through a blood sample placed on a gel or support medium (like cellulose acetate or agarose).
- High-Performance Liquid Chromatography (HPLC): This automated method separates different hemoglobin variants based on their chemical properties (charge interactions) as they pass through a specialized column. It uses precise pumps to pass a liquid solvent containing the sample through a column packed with a solid absorbent material.
- Genetic Testing (DNA Analysis): This involves analyzing the patient’s DNA to directly identify the specific genetic mutations in the beta-globin gene (HBB gene) that cause HbS or other hemoglobinopathies.
These confirmatory tests are crucial for an accurate diagnosis, which is vital for proper medical management, genetic counseling, and family planning for individuals and families affected by SCD.
Clinical Significance and Importance of Early Detection
Early detection of SCD, often facilitated by newborn screening programs that include the sickle cell disease test (followed by confirmatory tests), is paramount and can dramatically alter the course of the disease and a patient’s life.
Early identification of an affected child or carrier status within a family provides an opportunity for other family members to be screened. This knowledge empowers families with genetic counseling, helping them understand inheritance patterns and make informed decisions about future pregnancies. Negative sickle cell disease (dithionite) test, it should be confirmed with a more specific test like hemoglobin electrophoresis.
Prevention of Life-Threatening Complications
Infections: Infants and young children with SCD are highly susceptible to severe bacterial infections (e.g., pneumonia, meningitis), which can be fatal. Early diagnosis allows for prophylactic penicillin administration, significantly reducing the risk of these infections.
Stroke: Children with SCD are at a higher risk of stroke. Early detection allows for regular screening (e.g., Transcranial Doppler ultrasound) and, if needed, preventive therapies like regular blood transfusions, which can drastically lower stroke risk.
Timely Medical Management
Comprehensive Care: Early diagnosis ensures that affected individuals are promptly enrolled in comprehensive sickle cell care programs. These programs involve regular check-ups, vaccinations, monitoring for complications, and educational support for families.
Pain Management: Early identification allows for education on recognizing and managing pain crises, providing pain medication, and potentially starting therapies like hydroxyurea to reduce crisis frequency.
Improved Quality of Life and Life Expectancy
By preventing or mitigating the most severe complications (like stroke, severe infections, and organ damage), early detection and intervention lead to a significant improvement in the patient’s overall health, quality of life, and often, an increased life expectancy.
Family Planning and Genetic Counseling
Early identification of an affected child or carrier status within a family provides an opportunity for other family members to be screened. This knowledge empowers families with genetic counseling, helping them understand inheritance patterns and make informed decisions about future pregnancies.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print (Elsevier) 12th Edition. 2016.
- Mendez-Marti, S., Zik, C., Alan, S., Wang, H., & Ershler, W. (2024). Sickle Cell Screening in Adults: A Current Review of Point-of-Care Testing. Journal Of Hematology, 13(3), 53-60.
- Nalbandian R. M. (1972). Dithionite test for sickle-cell hemoglobin. The New England journal of medicine, 287(5), 254. https://doi.org/10.1056/nejm197208032870515
- Torabian, K., Lezzar, D., Piety, N. Z., George, A., & Shevkoplyas, S. S. (2017). Substituting Sodium Hydrosulfite with Sodium Metabisulfite Improves Long-Term Stability of a Distributable Paper-Based Test Kit for Point-of-Care Screening for Sickle Cell Anemia. Biosensors, 7(3), 39. https://doi.org/10.3390/bios7030039
- Arishi, W. A., Alhadrami, H. A., & Zourob, M. (2021). Techniques for the Detection of Sickle Cell Disease: A Review. Micromachines, 12(5), 519. https://doi.org/10.3390/mi12050519
- Williams TN, Thein SL. Sickle Cell Anemia and Its Phenotypes. Annu Rev Genomics Hum Genet. 2018 Aug 31;19:113-147. doi: 10.1146/annurev-genom-083117-021320. Epub 2018 Apr 11. PMID: 29641911; PMCID: PMC7613509.



