Procedure At A Glance
The ABO RhD test (Blood group typing) tube method involves Forward Grouping (identifying antigens on red blood cells) and Reverse Grouping (identifying antibodies in plasma).
Key Steps ▾
- Prepare Red Cell Suspensions: Create a standardized suspension of the patient’s red blood cells (2 – 5%) in saline.
- Prepare Plasma: Obtain the patient’s plasma/serum.
- Forward Grouping:
- Mix patient’s red cell suspension with known anti-A, anti-B, anti-A,B (optional) and anti-D antisera.
- Incubate (as per protocol).
- Centrifuge the tubes.
- Observe for agglutination.
- Reverse Grouping:
- Mix patient’s plasma/serum with known A cells and B cells.
- Incubate (as per protocol).
- Centrifuge the tubes.
- Observe for agglutination.
- Interpret Results ▾:
- Determine ABO and RhD blood type (blood group typing) based on the agglutination patterns in both forward and reverse grouping.
- A positive reaction (agglutination) indicates the presence of the corresponding antigen/antibody.
- Troubleshoot any discrepancies.
*Click ▾ for more information
Introduction
The ABO and RhD blood groups are two of the most important systems for classifying human blood. They are determined by the presence or absence of specific antigens on the surface of red blood cells.
The ABO Blood Group System
The ABO blood group system is based on the presence or absence of two antigens, A and B, on the surface of red blood cells. There are four possible ABO blood groups: A, B, AB, and O.
- People with type A blood have the A antigen on their red blood cells.
- People with type B blood have the B antigen on their red blood cells.
- People with type AB blood have both the A and B antigens on their red blood cells.
- People with type O blood have neither the A nor the B antigen on their red blood cells.
The RhD Blood Group System
The RhD blood group system is based on the presence or absence of the RhD antigen on the surface of red blood cells. There are two possible RhD blood types: RhD positive and RhD negative.
- People with RhD positive blood have the RhD antigen on their red blood cells.
- People with RhD negative blood do not have the RhD antigen on their red blood cells.
Clinical Significance of ABO and Rh Blood Group Systems
Blood Transfusions
Preventing Transfusion Reactions
This is the most crucial role. If a patient receives blood that is incompatible with their ABO or Rh type (blood group typing), their immune system will recognize the donor’s red blood cell (RBC) antigens as foreign. Pre-existing antibodies (for ABO) or newly formed antibodies (for Rh after sensitization) will bind to these foreign RBCs, leading to agglutination (clumping) and hemolysis (destruction) of the transfused cells.
- ABO Incompatibility: Antibodies to ABO antigens (anti-A, anti-B) are naturally occurring and are usually of the IgM class, which are potent activators of the complement system. An acute hemolytic transfusion reaction (AHTR) due to ABO incompatibility can be severe and life-threatening, causing symptoms like fever, chills, back pain, hemoglobinuria, kidney failure, disseminated intravascular coagulation (DIC), and even death. This is why ABO Rh test (blood group typing) is the first and most critical step in pre-transfusion testing.
- Rh Incompatibility: Unlike ABO antibodies, anti-D antibodies (the most significant Rh antibody) are not naturally occurring. They develop only after an Rh-negative individual is exposed to Rh-positive blood (e.g., through transfusion or pregnancy). Once sensitized, subsequent transfusions of Rh-positive blood can lead to delayed hemolytic transfusion reactions, which can still be clinically significant. Therefore, Rh-negative patients should always receive Rh-negative blood, especially females of child-bearing age.
Universal Donors and Recipients
- O-negative: Considered the “universal red cell donor” because O-negative red cells lack A, B, and D antigens, making them compatible with most recipients’ plasma antibodies. Used in emergency situations when blood typing is unknown.
- AB-positive: Considered the “universal red cell recipient” because AB-positive individuals have both A and B antigens and the D antigen, and therefore do not have anti-A, anti-B, or anti-D antibodies.
- Plasma Transfusions: The compatibility rules for plasma are essentially reversed. AB plasma (lacking anti-A and anti-B antibodies) is the “universal plasma donor,” while O plasma (containing both anti-A and anti-B) can only be given to O recipients.
Blood Transfusion Compatibility
ABO and RhD blood group compatibility is important for blood transfusions. If a person receives a transfusion of blood that is incompatible with their ABO or RhD blood type, their immune system will attack the donor red blood cells. This can lead to a serious and potentially life-threatening reaction called a transfusion reaction.
- People with type A blood can safely receive blood from people with type A and type O blood.
- People with type B blood can safely receive blood from people with type B and type O blood.
- People with type AB blood can safely receive blood from people with all four blood types (A, B, AB, and O).
- People with type O blood can only safely receive blood from people with type O blood.
- People with RhD positive blood can safely receive blood from people with both RhD positive and RhD negative blood.
- People with RhD negative blood can only safely receive blood from people with RhD negative blood.
Hemolytic Disease of the Fetus and Newborn (HDFN)
Rh Incompatibility (Rh Disease)
This is the most common and historically severe form of HDFN. It occurs when an Rh-negative mother carries an Rh-positive fetus (inheriting the RhD antigen from the father). During pregnancy or at delivery, fetal Rh-positive red cells can enter the mother’s circulation, sensitizing her immune system to produce anti-D antibodies. In subsequent pregnancies with another Rh-positive fetus, these maternal anti-D antibodies (which are typically IgG and can cross the placenta) attack and destroy the fetal red blood cells. This can lead to fetal anemia, jaundice, kernicterus (bilirubin-induced brain damage), hydrops fetalis, and even fetal death.
The advent of Rh immunoglobulin (RhIG or RhoGAM) has dramatically reduced the incidence of Rh-HDFN. RhIG is administered to Rh-negative mothers during pregnancy and after delivery to “mop up” any fetal Rh-positive red cells, preventing maternal sensitization.
ABO Incompatibility
ABO incompatibility can also cause HDFN, but it is generally much milder than Rh-HDFN. It most commonly occurs when a Group O mother carries a Group A or Group B fetus. While anti-A and anti-B are typically IgM antibodies (which don’t cross the placenta), Group O individuals often produce some IgG anti-A and anti-B antibodies that can cross the placenta. The milder severity is partly because fetal RBCs express fewer ABO antigens, and ABO antigens are also widely expressed on other fetal tissues, which may absorb some of the maternal antibodies.
Organ and Hematopoietic Stem Cell Transplantation
Solid Organ Transplants (e.g., Kidney, Heart, Liver)
ABO compatibility is crucial for solid organ transplantation, similar to blood transfusions. ABO antigens are expressed not only on red blood cells but also on the endothelial cells lining the blood vessels of most organs. Transplanting an ABO-incompatible organ can lead to hyperacute rejection, where pre-existing antibodies in the recipient immediately attack the donor organ, causing rapid graft failure. Therefore, ABO matching is a primary consideration in organ allocation.
Hematopoietic Stem Cell Transplantation (HSCT)
ABO matching is less critical for HSCT than for solid organs because hematopoietic stem cells themselves do not express ABO antigens. However, ABO incompatibility can still cause complications, such as:
- Major Incompatibility: Recipient has antibodies against donor RBC antigens (e.g., Group A donor to Group O recipient). Requires removal of recipient antibodies or reduction of donor red cells.
- Minor Incompatibility: Donor has antibodies against recipient RBC antigens (e.g., Group O donor to Group A recipient). Requires removal of donor antibodies or transfusion of washed red cells to the recipient.
These incompatibilities can complicate post-transplant engraftment and lead to delayed red cell aplasia or hemolysis.
Forensic Medicine and Paternity Testing (Historical/Ancillary)
- Forensics: Blood typing can be used to exclude suspects or link individuals to crime scenes based on blood samples found, though DNA profiling has largely superseded this as a more definitive method.
- Paternity Testing: Historically, ABO and Rh blood groups could be used to exclude paternity (e.g., if a presumed father’s blood type makes it impossible for him to be the biological father of a child). Again, DNA testing has replaced this as the gold standard.
ABO Rh test (blood group typing) is a crucial laboratory procedure that determines an individual’s ABO and RhD blood group type based on the presence or absence of specific antigens on the surface of their red blood cells. The tube method is a widely used technique for ABO Rh test (blood group typing), offering simplicity, accuracy, and cost-effectiveness. Further investigations of the Rh system can be done using the Rh phenotyping protocol.
Principle of ABO Rh test (blood group typing)
In the ABO Rh test (blood group typing) tube method, both serum and red blood cells are utilized to identify the ABO and RhD blood group of an individual. Human serum contains antibodies that react with specific antigens on red blood cells.
Red blood cells possess antigens that are either present or absent, determining the individual’s blood group. The principle of ABO Rh test (blood group typing) involves two distinct processes: forward grouping and reverse grouping.
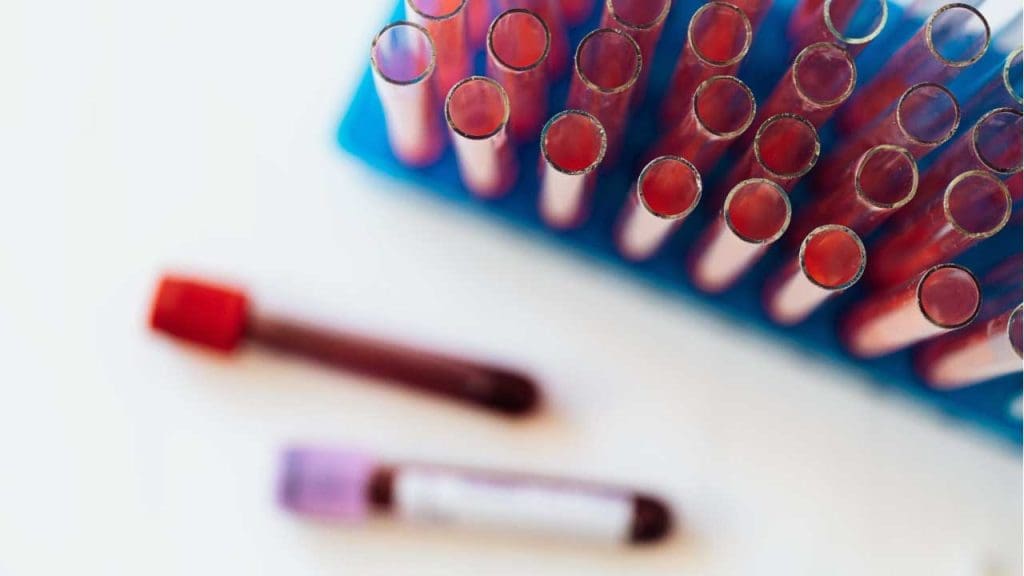
Forward Grouping
Forward grouping, also known as cell grouping, directly identifies the antigens present on an individual’s red blood cells. It utilizes antisera, which contain antibodies specifically directed against A and B antigens. These antisera are added to a suspension of the patient’s red blood cells. If the patient’s red blood cells possess the corresponding antigen (A or B), they will agglutinate or clump together due to the antigen-antibody reaction.
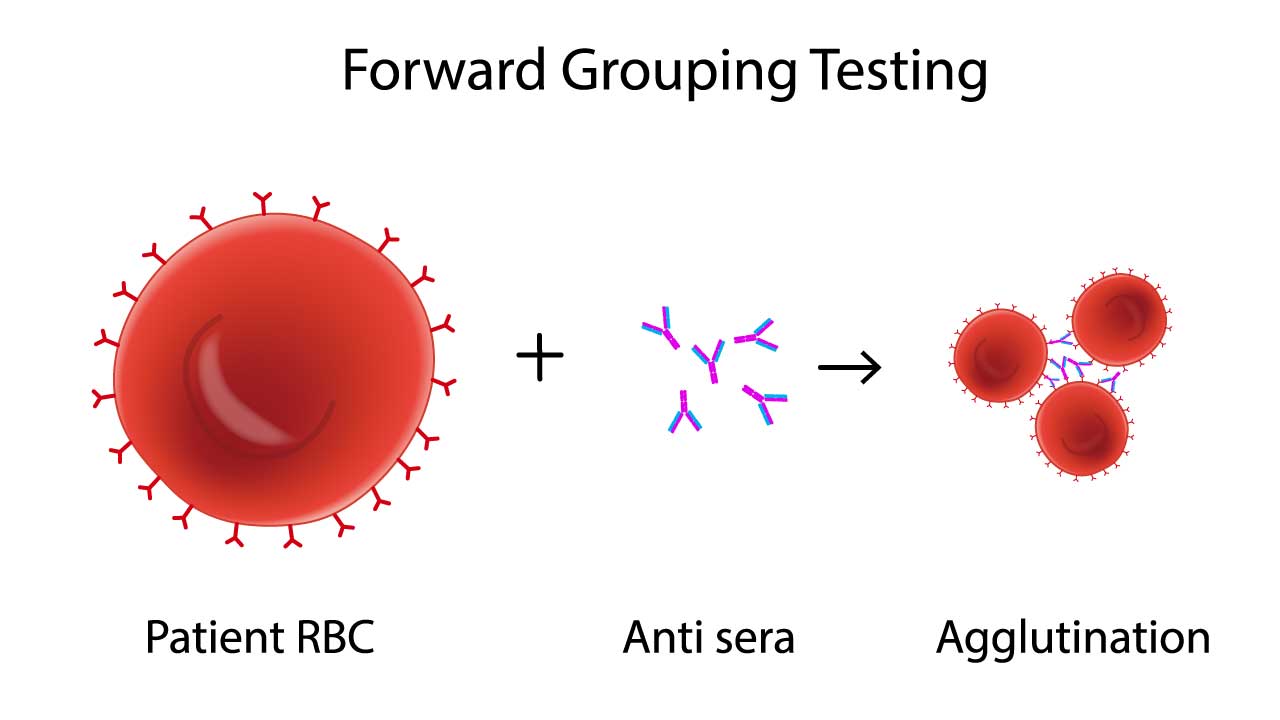
This image depicts the key principle involved in forward blood group testing, a process that determines an individual’s blood type. It illustrates how antibodies in the serum react with antigens on red blood cells, causing them to clump together or “agglutinate.” The pattern of agglutination reveals the person’s ABO blood group, ensuring safe and effective transfusions.
Reverse Grouping
Reverse grouping, also known as serum grouping, identifies the antibodies present in an individual’s serum, which are indicative of the antigens they lack on their red blood cells. It utilizes red blood cells of known A, B and O blood groups. The patient’s serum is added to each tube, and agglutination of the corresponding red blood cells indicates the presence of antibodies against that antigen (anti-A or anti-B).
In addition to ABO blood group determination, reverse grouping also serves as a quality control measure for forward grouping and helps identify individuals with irregular antibodies that may cause transfusion reactions.
Materials
- Polypropylene/glass tubes 10 mm x 75 mm
- Serofuge
- Refrigerated centrifuge
- Pasteur pipettes
- Commercially prepared anti-A, -B, -A,B and -D sera
- Commercially prepared known A, B and O cells
- Patient’s EDTA whole blood
- Normal saline
Protocol
Preparation of 2 – 5% red cell suspension and plasma
- Centrifuge patient’s whole blood at 1000 – 2000 x g for 10 minutes using refrigerated centrifuge.
- Label two tubes with the patient/donor’s name and ID, designating one for red cell suspension and the other for plasma.
- Use a Pasteur pipette to carefully extract the serum/plasma, avoiding touching the red blood cells layer. Transfer the extracted serum/plasma into the appropriately labeled plasma tube.
- Carefully insert the tip of the Pasteur pipette into the center of the red cell layer and aspirate a small quantity of red cells. Dispense one drop the aspirated red cells into the appropriately labeled tube for red cell suspension.
- Add saline solution to the tube containing the red cells until it is approximately three-quarters full (approximately 0.5 – 1 mL of saline). Thoroughly resuspend the red cells.
* For quality control purposes, visually compare the color of the prepared red cell suspension to that of a 3% commercial red cell suspension. Adjust the suspension strength if necessary. Alternatively, you can place one drop of the prepared red cell suspension and one drop of a commercial red cell suspension into separate test tubes, centrifuge the tubes, and compare the size of the resulting red cell buttons.
**Red cell suspensions should be used for testing on the day of preparation for more accurate results.
Forward Grouping (testing the patient’s red cells)
- Label four tubes with the patient/donor’s name and ID, assigning each tube a corresponding reagent label: anti-A, anti-B, anti-A,B, and anti-D.
- Dispense a drop of commercial anti-A serum into the tube labeled anti-A. Repeat this process for anti-B, anti-A,B, and anti-D, adding each serum to its respective labeled tube.
- Carefully add one drop of well-mixed prepared red cell suspension to each tube of the antiserum.
- Thoroughly mix the reagents and red cell suspension by gently flicking the base of each tube.
- Centrifuge the tubes according to the reagent manufacturer’s instructions. Usually, centrifugation is performed at 1500 rpm for 15-20 seconds using a serofuge.
- Gently resuspend the cell button in each tube and examine for agglutination.
- Interpret and record the test results. Compare the test results for the red cell suspension with those obtained from reverse grouping.
Reverse Grouping (testing the patient’s plasma)
- Label three tubes with the patient/donor’s name and ID, assigning each tube a corresponding cell type label: A cells, B cells, and O cells.
- Dispense a drop of commercially prepared known type A red cells into the tube labeled A cells. Repeat this process for known type B and type O red cells, adding each cell type to its respective labeled tube.
- Carefully add one drop of the patient’s plasma to each tube containing the corresponding known red cells.
- Thoroughly mix the contents of each tube by gently flicking.
- Centrifuge the tubes according to the reagent manufacturer’s instructions. Usually, centrifugation is performed at 1500 rpm (503 g) for 15-20 seconds using a serofuge.
- Examine the plasma overlying the cell buttons for any signs of hemolysis.
- Gently resuspend the cell buttons in each tube and examine for agglutination.
- Interpret and record the test results. Compare the test results for the plasma with those obtained from forward grouping.
Interpretation of ABO Rh Test (Blood Group Typing)
Positive results in ABO Rh test (blood group typing) forward grouping and reverse grouping are indicated by agglutination of tested red cells and either hemolysis or agglutination in serum, respectively. A negative result is observed when there is a clear supernatant after resuspension in the red cell agglutination test.
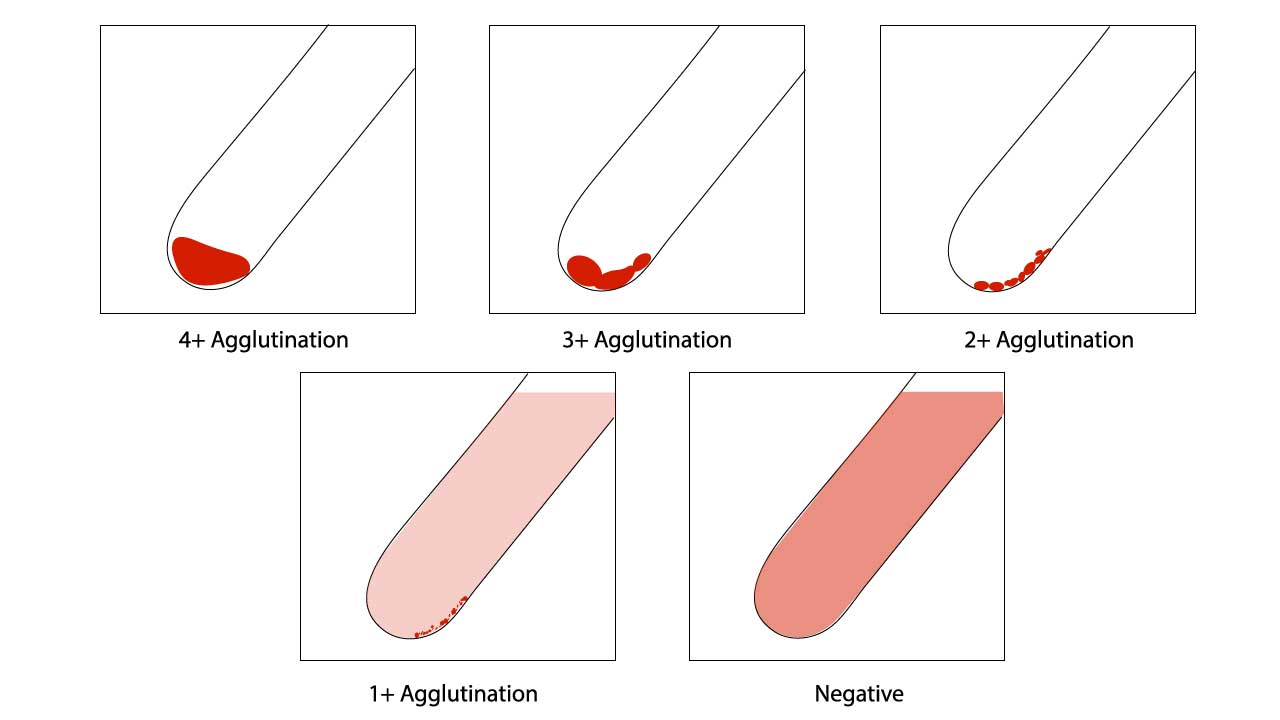
Assessment of red cell agglutination grading in ABO Rh test (Blood group typing)
| Symbol | Agglutination score | Description |
| 4+ / Complete (C) | 12 | Macroscopically visible cell button with a clear supernatant. |
| 3+ | 10 | Macroscopically visible large clumps of cell button with a clear supernatant. |
| 2+ | 8 | Macroscopically visible small clumps of cell button with a clear supernatant. |
| 1+ | 5 | Just macroscopically visible fine granular clumps of the cell button and the supernatant is turbid and reddish. |
| or weak (w) | 3 | Only microscopically visible fine granules of the cell button and the supernatant is turbid. |
| 0 | 0 | No agglutination seen. The supernatant is clear and reddish in color. |
| MF (mixed field) | MF | A mixture of agglutinated and unagglutinated red cells seen. |
| H | Complete hemolysis of the patient sample. The supernatant is grossly red with no evidence of red cells. |
In forward grouping, four distinct tubes are prepared:
- Tube 1 contains anti-A serum and patient’s red blood cells.
- Tube 2 contains anti-B serum and patient’s red blood cells.
- Tube 3 contains anti-A,B serum and patient’s red blood cells.
- Tube 4 contains anti-D serum and patient’s red blood cells.
Based on the agglutination patterns observed in these tubes, the ABO blood group is determined:
- Agglutination in tubes 1 and 3 (anti-A serum) indicates type A blood.
- Agglutination in tubes 2 and 3 (anti-B serum) indicates type B blood.
- Agglutination in tubes 1, 2 and 3 indicates type AB blood.
- Absence of agglutination in tubes 1, 2 and 3 indicates type O blood.
- Agglutination in tube 4 indicates RhD positive blood.
- Absence of agglutination in tube 4 indicates RhD negative blood.
In reverse grouping, based on the agglutination patterns observed in the tubes, the RhD blood group is determined:
- Agglutination in tube 1 indicates the presence of anti-A antibodies, corresponding to type B or type O blood.
- Agglutination in tube 2 indicates the presence of anti-B antibodies, corresponding to type A or type O blood.
- Absence of agglutination in both tubes indicates the absence of anti-A and anti-B antibodies, corresponding to type AB blood.
- Agglutination in both tubes indicates the presence of anti-A and anti-B antibodies, corresponding to type O blood.
- Agglutination in tube 3 could indicate presence of alloantibodies.
Interpreting weak agglutination in ABO Rh test (blood group typing)
Interpreting weak agglutination in ABO Rh test (blood group typing) is one of the most critical and challenging aspects of immunohematology. It signifies a potential discrepancy that must be thoroughly investigated and resolved before a patient’s or donor’s blood type can be accurately reported. Misinterpreting weak reactions can lead to serious consequences, including incompatible transfusions.
What Constitutes Weak Agglutination?
A weak reaction usually falls into the 1+ or 2+ range, sometimes described as “trace” or “microscopic” agglutination. Anything less than a strong (3+ or 4+) reaction should prompt further investigation. Mixed-field agglutination (small agglutinates in a sea of unagglutinated cells) is also a form of weak reaction that requires special attention.
Why is Weak Agglutination a Concern?
- Risk of Misclassification: A weak reaction, if not correctly identified and resolved, could lead to misclassifying a blood type (e.g., an A subgroup being typed as O, or a weak D positive being typed as D negative).
- Transfusion Safety: Misclassification poses a significant risk for transfusion reactions. Transfusing incompatible blood, even due to a weakly expressed antigen, can lead to red blood cell destruction (hemolysis).
- HDFN Risk: In the case of RhD typing, a weak D individual, if misclassified as Rh-negative, could be sensitized by Rh-positive blood during transfusion or pregnancy, leading to the formation of anti-D antibodies and potential HDFN in future pregnancies.
Common Causes of Weak Agglutination (Discrepancies) in ABO Rh Test (Blood Group Typing)
Weak agglutination often indicates an ABO discrepancy (when forward and reverse grouping don’t match) or a weak D phenotype.
Weak or Missing Antigens (Forward Grouping Problems)
- ABO Subgroups: This is a very common cause. Individuals can have ABO antigens that are weakly expressed on their red blood cells due to genetic variations (e.g., A3, Ax, Aend, B3, Bx, Bel). These may react weakly or even negatively with routine antisera in the ABO Rh test (blood group typing).
- Disease States: Certain medical conditions can temporarily weaken antigen expression:
- Leukemia and other hematological malignancies.
- Patients undergoing chemotherapy.
- Acquired B phenomenon: In some severe bacterial infections (especially colon cancer), bacterial enzymes can modify the A antigen to resemble the B antigen, leading to a weak “acquired B” reaction.
- Transfusion of Large Volumes of Type O Blood: If a non-O patient receives a massive transfusion of O blood, their own (e.g., A or B) red cells may be diluted, leading to weaker antigen reactions and misinterpretation in the ABO Rh test (blood group typing).
- Very Young Age (Neonates): ABO antigens are not fully developed at birth, leading to weaker reactions in forward grouping of the ABO Rh test (blood group typing) in newborns.
- Weak D Phenotype: The D antigen on RhD-positive red cells can be weakly expressed due to genetic variations. This often requires additional testing (Indirect Antiglobulin Test – IAT) to detect.
- Antigen Loss/Deterioration: Deterioration of antigens on stored blood samples.
Weak or Missing Antibodies (Reverse Grouping Problems)
- Age Extremes:
- Newborns/Infants (under 4-6 months): Their immune system has not yet produced naturally occurring ABO antibodies. Any antibodies present are usually maternal IgG antibodies passively acquired. Reverse grouping is unreliable.
- Elderly Patients: May have a generalized decrease in antibody production, leading to weaker or absent isoagglutinins.
- Immunosuppression/Hypogammaglobulinemia: Patients with immune deficiencies, those on immunosuppressive drugs, or those with conditions like multiple myeloma or chronic lymphocytic leukemia (CLL) may have reduced or absent antibody production.
- Dilution: Patients receiving large volumes of intravenous fluids, plasma exchange, or plasma transfusions may have diluted antibody titers.
- ABO Subgroups: Some rare ABO subgroups may produce unexpected antibodies (e.g., A2 individuals with anti-A1).
- “Bombay” Phenotype (Oh): Individuals lacking the H antigen (and thus A and B antigens) will produce strong anti-H, anti-A, and anti-B antibodies, resulting in forward typing as Group O but strong reactions in reverse grouping with A, B, and O cells.
Technical Errors
These are often the first to be ruled out:
- Improper red cell suspension (too concentrated or too dilute).
- Incorrect reagent-to-cell ratio.
- Insufficient or excessive centrifugation.
- Inadequate mixing.
- Failure to add reagents.
- Use of expired or contaminated reagents.
- Improper reading technique (e.g., shaking too vigorously).
- Inadequate incubation time or temperature.
Other Issues
- Rouleaux Formation: Due to abnormal plasma proteins (e.g., in multiple myeloma), red cells can stack like coins, mimicking agglutination but dispersing with saline.
- Cold Autoantibodies: Non-specific antibodies that react at room temperature or colder, causing false positive reactions, particularly in reverse grouping or forward grouping with patient’s own cells (autocontrol).
- Unexpected Alloantibodies: Antibodies to low-incidence antigens present in reagent antisera, or unexpected antibodies to high-incidence antigens on reagent cells.
Resolution Strategies for Weak Agglutination in ABO Rh Test (Blood Group Typing)
Whenever weak agglutination or an ABO discrepancy is noted, it must be resolved before reporting the blood group. The general approach involves:
- Repeat the Test: Always the first step, ensuring strict adherence to the protocol and checking for technical errors. Use a fresh sample if possible.
- Review Patient History: Check age, diagnosis, medications, recent transfusions, transplant history, and pregnancy status. This provides critical clues.
- Adjust Incubation Conditions:
- Prolonged Incubation: Increasing the incubation time (e.g., 15-30 minutes at room temperature) can enhance weak antigen-antibody reactions in the ABO Rh test (Blood group typing).
- Lower Temperature (4°C or “cold” incubation): For suspected weak antibodies in reverse grouping (Type I discrepancy), incubating at 4°C can strengthen IgM antibody reactions. An autocontrol with O cells must be run concurrently to rule out cold autoantibodies.
- Use Anti-A,B Antisera: This can help detect weak A or B subgroups when used the ABO Rh test (Blood group typing).
- Perform Weak D Test (Indirect Antiglobulin Test – IAT): If an RhD-negative result is obtained, especially in donors or pregnant women, further testing with the IAT (incubation at 37°C followed by addition of anti-human globulin) is necessary to detect weak D individuals.
- Wash Red Cells: For forward grouping, washing patient red cells thoroughly with saline can remove interfering substances (like plasma proteins causing rouleaux or medications) that might cause false agglutination or mask true reactions.
- Saliva Secretor Status Test: For suspected ABO subgroups, testing saliva for soluble A, B, and H substances can help classify the subgroup.
- Adsorption and Elution Studies: Advanced techniques used to concentrate and identify weak antibodies or antigens.
- Enzyme Treatment: Using enzymes (e.g., papain, ficin) can enhance or destroy certain antigens, aiding in identification.
- Referral to Reference Laboratory: For complex cases that cannot be resolved in-house, the sample should be sent to a specialized immunohematology reference laboratory.
- Molecular Testing: DNA-based typing can definitively determine ABO and Rh genotypes, especially for very weak or silent alleles, resolving serological discrepancies.
Troubleshooting
While accurate and highly reliable, the tube method of ABO Rh test (blood group typing) can sometimes have false positive reactions. These can lead to incorrect blood typing, potentially posing risks in blood transfusions or other blood-related procedures.
Technical errors
Pre-analytical errors
- Patient misidentification: Mistakes in labeling or identifying patients can lead to testing the wrong blood sample.
- Improper sample collection: Contaminated samples due to improper collection technique or storage can interfere with the ABO Rh test (blood group typing).
- Incorrect sample type: Using the wrong type of anticoagulant tube or collecting insufficient blood volume can impact results.
Analytical errors
- Improper centrifugation: Insufficient or excessive centrifugation can impact the visibility of agglutination reactions, leading to misinterpretation of the ABO Rh test (Blood group typing).
- Incorrect incubation: Deviation from recommended temperature or timeframes can affect how antibodies and antigens interact, causing false positives or negatives.
- Contamination: Contamination of reagents, glassware, or the sample itself with other blood products or even microorganisms can trigger non-specific agglutination.
- Pipetting errors: Using the wrong volume of antiserum or blood sample can distort the antigen-antibody ratio, impacting reaction sensitivity.
Post-analytical errors
- Transcription errors: Mistakes in recording or transcribing test results can lead to miscommunication and errors in patient care.
- Interpretation errors: Improper interpretation of agglutination reactions, especially faint or borderline reactions, can lead to incorrect blood group typing.
- Communication errors: Failure to effectively communicate results to healthcare providers or misinterpreting their requests can lead to errors in blood transfusions or other procedures.
Biological factors
- Weak antigens or antibodies: Weak antigens might not cause visible agglutination in forward grouping, but be detected by stronger antigens in reverse grouping, leading to misinterpretation. Similarly, weak antibodies might produce faint clumping, appearing positive.
- Rouleaux formation: This non-specific stacking of red blood cells can mimic agglutination, especially in low protein environments like saline. Careful microscopic examination is crucial to differentiate.
- Polyagglutination: Some rare antisera can react with a wider range of red blood cells, causing false positives regardless of the ABO type.
- Cold agglutinins: These are antibodies that activate at cooler temperatures, potentially causing nonspecific agglutination, especially in blood samples collected in cold environments.
Patient-specific factors
- Recent immunizations or transfusions: Exposure to foreign antigens can trigger antibody production, leading to unexpected antibodies detectable in reverse grouping, potentially causing false positives.
- Certain medications: Drugs like some antibiotics or intravenous immunoglobulins can interfere with red blood cell agglutination reactions, impacting test results.
- Underlying medical conditions: Autoimmune diseases or certain infections can alter antigen or antibody expression, potentially contributing to false positives.
Prevention and Management
- Strict adherence to standardized protocols minimizes technical errors.
- Experienced technicians can better recognize and troubleshoot false positives.
- Quality control measures for reagents and equipment ensure accuracy.
- Repeating tests with fresh samples or different antisera can confirm or refute questionable results.
- Consulting specialists in transfusion medicine or immunology might be necessary for complex cases.
Frequently Asked Questions (FAQs)
What are the five steps in the ABO Rh test (blood group typing) procedure?
The five main steps in the ABO Rh test (blood group typing) using the tube method are:
1. Sample Preparation
2. Forward Grouping
3. Observation and Recording
4. Reverse Grouping
5. Blood Type Determination
How do antigens and antibodies interact in ABO Rh test (blood group typing)?
In ABO Rh test (blood group typing), antigens and antibodies play a key role in determining the blood type.
Antigens: These are molecules present on the surface of the red blood cells (RBCs). In the ABO system, there are two main antigens: A and B. Individuals with type A blood have A antigens, those with type B have B antigens, those with type AB have both A and B, and those with type O have neither A nor B.
Antibodies: These are proteins produced by the immune system in response to foreign substances. In ABO Rh test (blood group typing), antibodies are present in the plasma and can react with specific antigens they recognize. For instance, someone with type A blood has anti-B antibodies, while someone with type B has anti-A antibodies. Type O individuals have both anti-A and anti-B antibodies, and type AB individuals have neither.
Agglutination Reaction: This is the key phenomenon observed in ABO Rh test (blood group typing). When an antigen on an RBC encounters its corresponding antibody in the plasma, they bind together, causing the RBCs to clump together in a visible mass called an agglutinate. This reaction occurs because the antigen and antibody have specific binding sites that fit together like a lock and key.
How it works in ABO Rh test (blood group typing)
- Forward grouping: RBCs are tested with antisera containing known antibodies (anti-A, anti-B, and anti-Rh). If an antigen on an RBC encounters its corresponding antibody in the antiserum, agglutination occurs, indicating a positive reaction. The absence of agglutination indicates the antigen is not present on the RBC.
- Reverse grouping: Plasma is tested with known RBCs (type A and B). If an antibody in the plasma encounters its corresponding antigen on the RBC, agglutination occurs, indicating a positive reaction. The absence of agglutination indicates the antibody is not present in the plasma.
Importance of agglutination
- By observing agglutination patterns in both forward and reverse grouping, the specific antigen and antibody profile can be determined, ultimately revealing the individual’s blood type.
- This information is crucial for safe blood transfusions, ensuring compatibility between donor and recipient blood. Incompatible blood types can cause severe reactions.
What is the significance of performing both forward and reverse grouping in ABO Rh test (blood group typing)?
Performing both forward and reverse grouping in ABO Rh test (blood group typing) is crucial for ensuring accurate and reliable determination of an individual’s blood type.
Forward Grouping
- Identifies antigens on red blood cells (RBCs): RBCs are tested with antisera containing known antibodies (anti-A, anti-B, and anti-Rh). If an antigen on an RBC encounters its corresponding antibody, agglutination occurs, confirming the presence of that antigen in the blood group typing.
- Direct testing: This confirms what antigens are present on the RBC surface, which directly determines the blood group typing.
Reverse Grouping
- Detects antibodies present in the plasma: Plasma is tested with known RBCs (type A and B). If an antibody in the plasma encounters its corresponding antigen on the RBC, agglutination occurs, indicating the presence of that antibody.
- Indirect testing: This confirms what antibodies are present in the plasma, which should logically correspond to the missing antigens on the RBCs for compatibility.
Significance of Both
- Verification: Comparing the results of both methods provides internal verification. Compatibility between forward and reverse grouping strengthens the accuracy of the blood type determination.
- Identifying Discrepancies: Inconsistent results between forward and reverse grouping (e.g., agglutination in both forward and reverse) can indicate potential issues like technical errors, weak antigens/antibodies, or rare blood types. This alerts the lab to investigate further before confirming the blood type.
- Ensuring Safety: Accurate blood type identification is crucial for safe blood transfusions. Matching donor and recipient blood based on both antigen and antibody presence minimizes the risk of transfusion reactions.
What are the three rules of ABO Rh test (blood group typing)?
There aren’t universally agreed-upon “three rules” for ABO Rh test (blood group typing), as it’s a more nuanced process than a set of simple rules. However, here are three key principles that underpin the entire ABO Rh test (blood group typing) procedure:
1. Antigen-Antibody Specificity: Each antigen (A, B, or Rh) can only react with its corresponding antibody (anti-A, anti-B, or anti-Rh). This specific binding occurs due to unique shapes on the antigen and antibody molecules that fit together like a lock and key. This forms the basis for identifying antigens and antibodies present in an individual’s blood.
2. Agglutination as Confirmation: When an antigen and its corresponding antibody meet, they bind together, causing the red blood cells (RBCs) to clump together in a visible mass called an agglutinate. This visible reaction confirms the presence of both the antigen and antibody, allowing for interpretation of the blood type.
3. Compatibility for Transfusions: Safe blood transfusions rely on matching the donor’s blood with the recipient’s based on both antigen and antibody compatibility. This means:
- Antigen compatibility: The recipient’s plasma shouldn’t contain antibodies that can react with the donor’s RBC antigens. For example, a recipient with type A blood cannot receive type B blood because their plasma has anti-B antibodies.
- Antibody compatibility: The donor’s plasma shouldn’t contain antibodies that can react with the recipient’s RBC antigens. For example, a type O donor can donate to anyone because their plasma lacks anti-A and anti-B antibodies.
What are the methods of determining the ABO type?
The most common method for determining ABO blood type through ABO Rh test (blood group typing) is the tube agglutination technique. This method has two main components:
- Forward grouping: Red blood cells (RBCs) are tested with known antibodies (anti-A, anti-B, and anti-Rh) to identify antigens present on the RBC surface. Agglutination (clumping of RBCs) indicates a positive reaction and the presence of a specific antigen.
- Reverse grouping: Plasma is tested with known RBCs (type A and B) to detect antibodies present in the plasma. Agglutination again indicates a positive reaction and the presence of a specific antibody.
However, there are a few other methods for determining ABO blood type, although these are less commonly used:
Gel test cards: These pre-made cards contain wells filled with antisera and indicator gel. Adding blood and incubating the card allows for visual interpretation of agglutination reactions within the gel.
Microfluidic devices: These automated systems use microfluidic channels to mix blood samples with antisera and analyze agglutination patterns electronically.
Molecular methods: Techniques like DNA sequencing can analyze the genes responsible for ABO antigens, but this is not yet routinely used in clinical settings due to cost and complexity.
Choosing the appropriate method depends on several factors, including:
- Availability of resources: Tube agglutination is the most widely available and cost-effective method.
- Throughput needs: Microfluidic devices might be preferred for high-volume testing in blood banks.
- Patient factors: In rare cases, specialized techniques like molecular methods might be necessary for individuals with unusual blood types.
What are the different types of antisera used in the tube method, and how do they target specific antigens?
In the tube method of ABO Rh test (blood group typing), several types of antisera are used, each targeting specific antigens on red blood cells.
Anti-A and Anti-B Sera
- Source: These are typically produced by immunizing animals (e.g., rabbits, horses) with purified A or B antigens from human red blood cells.
- Specificity:
- Anti-A serum contains IgM antibodies that specifically bind to A antigens on red blood cells.
- Anti-B serum contains IgM antibodies that specifically bind to B antigens on red blood cells.
- Mechanism: Antibodies have specific recognition sites that bind to corresponding structures on antigens. When anti-A encounters A antigens, or anti-B encounters B antigens, they bind together, causing agglutination of red blood cells, visible as a clumping reaction.
Anti-Rh (D) Serum
- Source: Typically obtained from Rh positive humans who have naturally formed antibodies against the Rh (D) antigen.
- Specificity: Contains IgG antibodies that specifically bind to the D antigen on red blood cells.
- Mechanism: Similar to Anti-A/B, IgG antibodies in Anti-Rh(D) bind to D antigens, causing agglutination if present.
Additional Types
- Subgroup Antisera: These are used to further differentiate within A and B groups (e.g., A1, A2, B subgroups). They are less commonly used in routine ABO Rh test.
- Anti-Human Globulin (AHG): Used in the indirect antiglobulin test (IAT) to detect weak antigens or antibodies not readily visible in standard agglutination tests.
Each antiserum is produced to target a specific antigen and won’t react with other antigens. The type of antibody in the antiserum (IgM vs. IgG) affects the reaction kinetics and temperature requirements for optimal agglutination. Strict quality control and standardization of antisera are essential for accurate ABO Rh test (blood group typing) results.
What are the critical steps and considerations for ensuring accurate results in tube method ABO Rh test (blood group typing)?
Ensuring accurate results in ABO Rh test (blood group typing) using the tube method requires adhering to critical steps and considerations throughout the process. Here are some key aspects to highlight:
Pre-analytical phase
- Patient identification: Double-check patient identifications meticulously at every step to avoid testing the wrong sample.
- Sample collection: Use appropriate tubes (e.g., red-topped for no additive) and follow proper collection techniques to avoid contamination or hemolysis.
- Sample handling: Maintain proper storage and transport temperatures to prevent sample deterioration.
Analytical phase
- Standardized protocols: Strictly follow established procedures and quality control measures for all steps, including reagent preparation, pipetting, centrifugation, and incubation times and temperatures.
- Technical proficiency: Ensure technicians are well-trained and experienced in recognizing and interpreting agglutination reactions.
- Reagent quality: Regularly check the validity and performance of antisera and other reagents according to manufacturer’s instructions.
- Microscopic examination: Carefully examine tube reactions under a microscope to differentiate true agglutination from rouleaux or other artifacts.
- Control tests: Include positive and negative controls for each test run to verify the validity of the procedure and reagents.
Post-analytical phase
- Result interpretation: Use clear and standardized criteria for interpreting agglutination patterns, avoiding subjective judgments.
- Double-checking: Two qualified personnel should independently review and document results before finalizing reports.
- Discrepancy management: Investigate and resolve any discrepancies between forward and reverse grouping or inconsistent results with documented history.
- Communication: Clearly communicate results to healthcare providers, highlighting any potential concerns or interpretations.
Additional considerations
- Patient factors: Be aware of potential influences like recent immunizations, transfusions, or medications that could affect antigen or antibody expression.
- Rare blood types: Consider using additional tests or consulting specialists for individuals with suspected rare blood types or complex cases.
- Continuing education: Stay updated on the latest guidelines and advancements in ABO Rh test blood typing practices.
How does the concentration of antisera and red blood cell suspensions affect the sensitivity of the test?
The concentration of both antisera and red blood cell (RBC) suspensions plays a crucial role in determining the sensitivity of the ABO Rh test (blood group typing) using the tube method.
Antisera Concentration
- Higher concentration: Generally increases the sensitivity of the ABO Rh test. More antibody molecules are available to bind with antigens on RBCs, potentially detecting even weak antigens that might not be visible with lower concentrations. However, excessively high concentrations can lead to non-specific agglutination, causing false positives.
- Lower concentration: Decreases the sensitivity of the ABO Rh test. Fewer antibody molecules are available, potentially missing weak antigens and leading to false negatives. However, a very low concentration is necessary to differentiate weak from strong reactions, crucial for accurate interpretation.
RBC Suspension Concentration
- Higher concentration: Can decrease the sensitivity of the test. With more RBCs, there are fewer antibody molecules per cell, potentially masking weak antigen expression and leading to false negatives. However, a certain minimum concentration is necessary for optimal agglutination to be visible.
- Lower concentration: May increase the sensitivity of the test, especially for detecting weak antigens. However, excessively low concentrations can make it difficult to visualize agglutination, even with positive reactions.
Therefore, finding the optimal balance between antisera and RBC suspension concentrations is crucial for achieving a sensitive and specific ABO Rh test (blood group typing). This balance is achieved through:
- Standardized protocols: Laboratories use established guidelines and quality control procedures to ensure optimal concentrations are used in each test.
- Reagent adjustments: Manufacturers specify recommended dilution factors for their antisera based on desired sensitivity. Technicians might adjust concentrations slightly based on experience or specific testing conditions.
- Microscopic examination: Careful observation under a microscope allows technicians to distinguish true agglutination from artifacts even with subtle reactions, ensuring accurate interpretation despite concentration variations.
What happens if there is no time for ABO Rh test (blood group typing)?
In extremely critical situations when there is absolutely no time for ABO Rh test blood typing, medical professionals may resort to administering universal donor blood, also known as O negative (O Rh-) blood. This is because:
- O negative blood lacks both A and B antigens: As a result, individuals with any blood type (A, B, AB, or O) can safely receive O negative blood without triggering an immune response due to incompatibility.
- Rh-negative blood: Additionally, O negative blood is Rh-negative, meaning it lacks the Rh factor antigen. While most individuals (over 85%) are Rh-positive, Rh-negative individuals can also safely receive Rh-negative blood without facing complications.
However, using universal donor blood is not an ideal situation and should only be considered as a last resort due to several potential drawbacks:
- Limited availability: O negative blood is relatively rare, accounting for only about 7% of the population. Relying on it for emergency transfusions can deplete blood bank reserves quickly.
- Increased risk of hemolytic reactions: Although rare, individuals with other blood types might develop delayed hemolytic reactions if they receive multiple transfusions of O negative blood due to the development of antibodies against A and B antigens.
- Potential sensitization: While less likely with a single transfusion, repeated exposure to foreign antigens in O negative blood can sensitize the recipient’s immune system, making it harder to find compatible blood for future transfusions.
Therefore, whenever possible, medical professionals prioritize ABO Rh test (blood group typing) and matching the specific blood type of the recipient for transfusions. This ensures maximum compatibility and minimizes the risk of adverse reactions.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- American Association of Blood Banks (AABB). Technical Manual, 21st Edition, 2023.
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005.
- Harmening DM. Modern Blood Banking & Transfusion Practices (FA Davis Company). 2018.
- Li HY, Guo K. Blood Group Testing. Front Med (Lausanne). 2022 Feb 11;9:827619. doi: 10.3389/fmed.2022.827619. PMID: 35223922; PMCID: PMC8873177.
- Yousuf, R., Abdul Ghani, S. A., Abdul Khalid, N., & Leong, C. F. (2018). Study on ABO and RhD blood grouping: Comparison between conventional tile method and a new solid phase method (InTec Blood Grouping Test Kit). The Malaysian journal of pathology, 40(1), 27–32.
- Younes, R., Spinella, P. C., Shea, S. M., Bailey-Kroll, L., Neal, M. D., Leeper, C., & Yazer, M. H. (2023). A rapid ABO and RhD test demonstrates high fidelity to blood bank testing for RhD typing. Transfusion, 63 Suppl 3, S208–S212. https://doi.org/10.1111/trf.17326
- Fathima S, Killeen RB. ABO Typing Discrepancies. [Updated 2025 Feb 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK585061/