TL;DR
Lymphocytes are a type of white blood cell crucial for adaptive immunity.
- Three main types ▾: B cells, T cells, and natural killer (NK) cells.
- Functions ▾:
- B cells: Produce antibodies to fight infections.
- T cells: Directly attack infected or abnormal cells.
- NK cells: Destroy damaged or abnormal cells.
- Development ▾: Lymphocytes develop in the bone marrow and thymus.
- Involved in various diseases ▾: Lymphocytes play roles in cancer, allergies, autoimmune diseases, and infectious diseases.
- Therapeutic applications ▾: Immunotherapy often targets lymphocytes for treating diseases like cancer.
- Aging and function ▾: Lymphocyte function declines with age, contributing to increased susceptibility to infections and diseases in older adults.
*Click ▾ for more information
Introduction
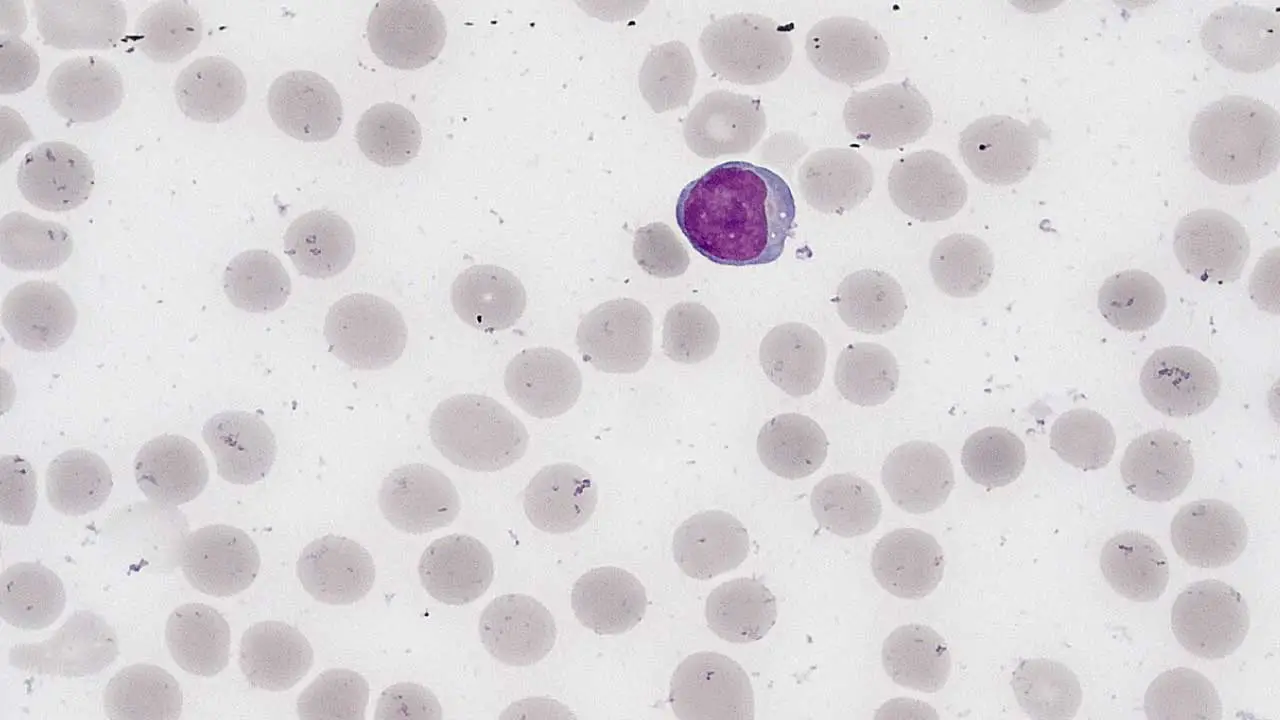
Lymphocytes are a type of white blood cell that play a crucial role in the immune system. They are small, round cells with a large nucleus. Lymphocytes are responsible for recognizing and attacking foreign invaders, such as bacteria, viruses, and cancer cells.
There are three main types of lymphocytes:
- B cells: Produce antibodies to fight off infections.
- T cells: Directly attack infected or abnormal cells.
- Natural killer (NK) cells: Destroy damaged or abnormal cells.
Overview of the Immune System
The immune system is a complex network of cells, tissues, and organs that work together to protect the body from harmful substances, such as bacteria, viruses, fungi, and toxins. It is divided into two main components: the innate immune system and the adaptive immune system.
- Innate immune system: This is the first line of defense against pathogens. It consists of physical barriers (like skin and mucous membranes), phagocytic cells (like neutrophils and macrophages), and natural killer cells. The innate immune system provides a rapid but non-specific response to threats.
- Adaptive immune system: This system is more specialized and develops over time in response to exposure to specific pathogens. It involves lymphocytes (B cells and T cells), which can recognize and target specific antigens. The adaptive immune system is slower to respond but provides a more targeted and long-lasting response.
Importance of Lymphocytes in the Immune Response
Lymphocytes are crucial components of the adaptive immune system. They play a central role in both humoral immunity and cell-mediated immunity.
- Humoral immunity: B cells are responsible for humoral immunity. They produce antibodies that bind to specific antigens on pathogens, marking them for destruction by other immune cells.
- Cell-mediated immunity: T cells are involved in cell-mediated immunity. They directly attack infected cells or abnormal cells, such as cancer cells. Helper T cells coordinate the immune response by activating other immune cells, while cytotoxic T cells directly kill infected or abnormal cells.
Lymphocytes also play a vital role in immunological memory, allowing the body to quickly recognize and respond to pathogens that it has encountered before. This is why we are often immune to diseases after having them once.
Morphology of Lymphocytes
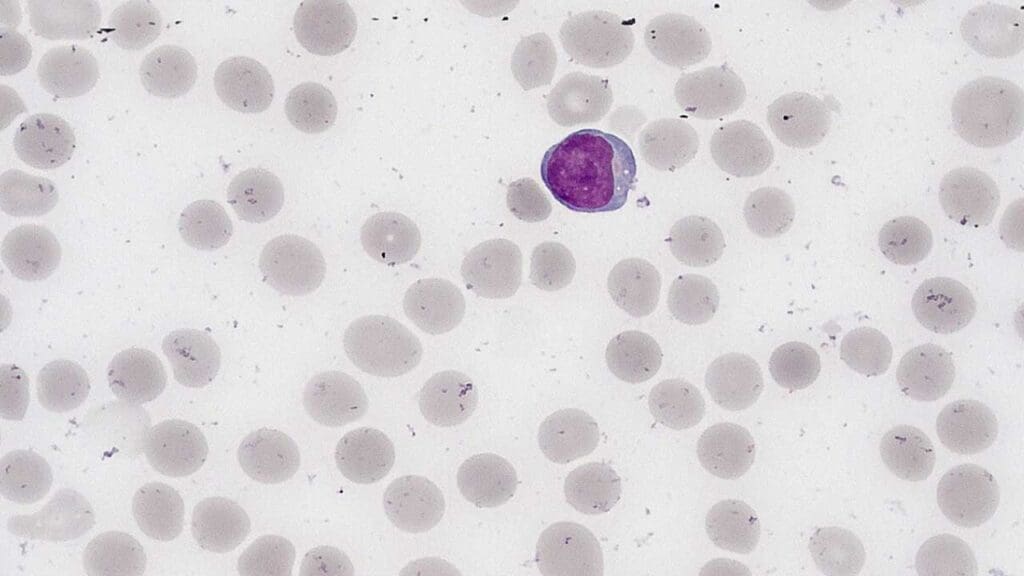
Lymphocytes are generally small, round cells with a large, round nucleus that occupies most of the cell volume. The cytoplasm is relatively thin and may contain a few azurophilic granules.
Differences in Morphology
While the basic structure of lymphocytes is similar, there are some subtle morphological differences between B cells, T cells, and NK cells.
- B cells: B cells often have a more prominent nucleolus and may contain more cytoplasm than T cells. They may also have a more irregular nuclear shape.
- T cells: T cells typically have a more condensed nucleus and less cytoplasm than B cells. Their nuclear shape is often more round or oval.
- NK cells: NK cells are similar in appearance to T cells, but they may have larger granules in their cytoplasm. They may also have a more irregular nuclear shape.
Note: These differences are not always clear-cut, and it can be difficult to distinguish between B cells, T cells, and NK cells based on morphology alone. Flow cytometry and immunohistochemistry are often used to identify specific lymphocyte subsets.
Lifespan of Lymphocytes
The lifespan of lymphocytes in the blood varies widely depending on the type of lymphocyte and the body’s needs. Some lymphocytes may circulate in the blood for only a few days, while others may persist for years.
- Naive lymphocytes: These are lymphocytes that have not yet encountered an antigen. They typically have a short lifespan in the blood, ranging from a few days to a few weeks.
- Memory lymphocytes: These are lymphocytes that have been activated by an antigen and have developed the ability to recognize and respond to the same antigen in the future. Memory lymphocytes can have a very long lifespan, potentially lasting for years or even decades.
Normal Reference Range for Lymphocyte Counts
The normal reference range for lymphocyte counts varies slightly depending on the laboratory and the age of the individual. However, a typical reference range for adults is:
- Absolute lymphocyte count: 1,000 to 4,000 cells/μL
- Relative lymphocyte count: 20% to 40% of total white blood cells
Functions of Lymphocytes
B Cells
- Humoral immunity: B cells are primarily responsible for humoral immunity, which involves the production of antibodies to neutralize and eliminate pathogens. When a B cell encounters its specific antigen, it differentiates into a plasma cell, which produces antibodies. Antibodies bind to antigens on pathogens, marking them for destruction by other immune cells, such as macrophages and neutrophils.
- Immunological memory: B cells also play a crucial role in immunological memory. After encountering an antigen, some B cells become memory B cells. These cells can quickly respond to subsequent encounters with the same antigen, providing long-lasting protection against reinfection.
T Cells
- Cell-mediated immunity: T cells are involved in cell-mediated immunity, which involves the direct destruction of infected or abnormal cells.
- Helper T cells (CD4+ T cells): These cells coordinate the immune response by activating other immune cells, including B cells and cytotoxic T cells.
- Cytotoxic T cells (CD8+ T cells): These cells directly kill infected or abnormal cells by releasing toxic substances.
- Regulatory T cells: These cells help to suppress the immune response and prevent excessive inflammation.
Natural Killer (NK) Cells
- Innate immunity: NK cells are part of the innate immune system and provide a rapid response to threats.
- Cancer surveillance: NK cells play a crucial role in cancer surveillance by recognizing and destroying abnormal cells.
- Viral infections: NK cells can also help to control viral infections by killing infected cells.
Lymphopoiesis
Lymphopoiesis is the process of lymphocyte development. It occurs in the bone marrow and the thymus.
Bone Marrow
Lymphopoiesis begins with hematopoietic stem cells (HSCs) in the bone marrow. These cells have the potential to differentiate into all types of blood cells, including lymphocytes. HSCs can give rise to a common lymphoid progenitor (CLP), which is the precursor for all lymphocytes.
B cell development: In the bone marrow, CLPs can differentiate into B cells. B cell development involves a series of steps, including antigen receptor gene rearrangement and selection.
- Pro-B cell: This is the earliest stage of B-cell development. Pro-B cells express specific genes that are involved in the rearrangement of the immunoglobulin (Ig) gene loci.
- Pre-B cell: During this stage, the Ig heavy chain genes are rearranged. If successful, the cell becomes a pre-B cell.
- Immature B cell: The Ig light chain genes are then rearranged. If successful, the cell becomes an immature B cell.
- Mature B cell: Immature B cells undergo a process of negative selection to ensure that they do not react to self-antigens. If they pass this test, they become mature B cells and enter the circulation.
Thymus
T cell development: Some CLPs migrate from the bone marrow to the thymus, where they undergo T cell development. T cell development involves a process of positive and negative selection, which ensures that T cells can recognize foreign antigens but not self-antigens. The thymus is most active during childhood and adolescence. With age, the thymus gradually shrinks and becomes less active.
T-cell development occurs in the thymus. It involves a series of stages, including:
- Double-negative thymocyte: This is the earliest stage of T-cell development. Double-negative thymocytes lack expression of the CD4 and CD8 co-receptors.
- Double-positive thymocyte: During this stage, the T-cell receptor (TCR) genes are rearranged. If successful, the cell becomes a double-positive thymocyte, expressing both CD4 and CD8.
- Single-positive thymocyte: Double-positive thymocytes undergo a process of positive and negative selection to ensure that they can recognize self-major histocompatibility complex (MHC) molecules but not self-antigens. Successful T cells become either CD4+ helper T cells or CD8+ cytotoxic T cells.
Positive Selection
- MHC recognition: Positive selection ensures that T cells can recognize self-MHC molecules. T cells that can interact with either MHC class I or MHC class II molecules are selected for survival.
- Thymic epithelial cells: Thymic epithelial cells present self-MHC molecules to T cells. T cells that can bind to self-MHC molecules receive survival signals.
Negative Selection
- Self-antigen recognition: Negative selection ensures that T cells do not recognize self-antigens. T cells that bind too strongly to self-antigens are eliminated.
- Medullary thymic epithelial cells: Medullary thymic epithelial cells present self-antigens to T cells. T cells that react strongly to self-antigens are deleted.
After passing positive and negative selection, T cells exit the thymus as mature single-positive T cells. CD4+ helper T cells can assist in both humoral and cell-mediated immunity, while CD8+ cytotoxic T cells are primarily involved in cell-mediated immunity.
Causes of High Lymphocytes (Lymphocytosis)
Lymphocytosis (high lymphocytes) is a condition characterized by an abnormally high number of lymphocytes in the blood. It can be caused by a variety of factors, including:
Infections
- Viral infections: Many viral infections, such as infectious mononucleosis, hepatitis, and HIV, can cause lymphocytosis (high lymphocytes).
- Bacterial infections: Some bacterial infections, such as pertussis and tuberculosis, can also lead to increased lymphocyte counts.
- Parasitic infections: Certain parasitic infections, such as malaria and toxoplasmosis, can cause lymphocytosis (high lymphocytes).
Autoimmune Diseases
- Systemic lupus erythematosus (SLE): This autoimmune disease can cause lymphocytosis (high lymphocytes), particularly when there is active inflammation.
- Rheumatoid arthritis: Lymphocyte counts may be elevated in patients with rheumatoid arthritis.
- Hyperthyroidism: Overactive thyroid function can lead to lymphocytosis (high lymphocytes).
Leukemia
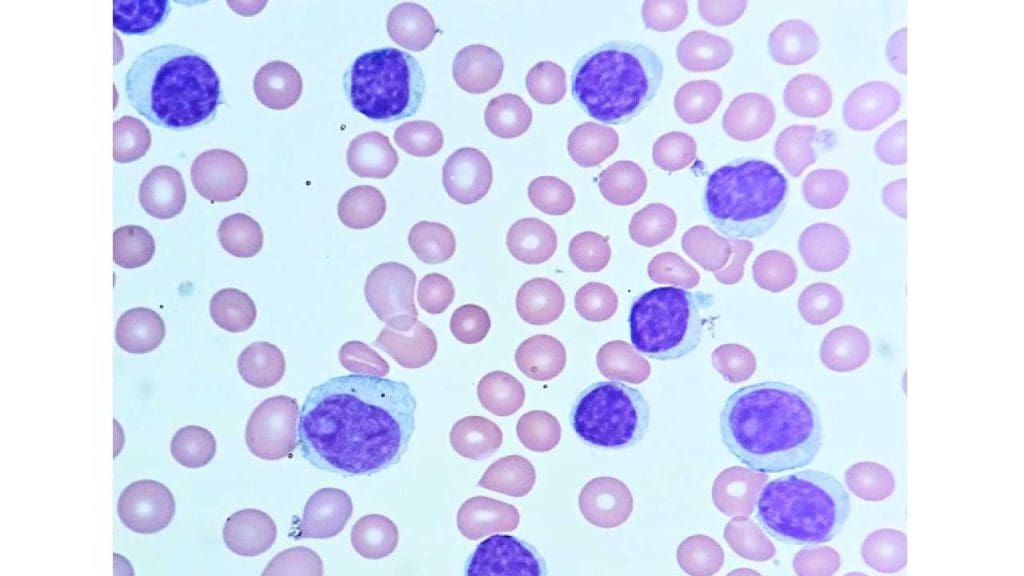
- Chronic lymphocytic leukemia (CLL): This is the most common type of leukemia in adults and is characterized by the accumulation of abnormal B cells.
- Acute lymphoblastic leukemia (ALL): This is a type of leukemia that affects primarily children and young adults. It is characterized by the overproduction of immature lymphocytes.
Other Causes
- Stress: Emotional or physical stress can temporarily increase lymphocyte counts.
- Medications: Certain medications, such as corticosteroids and anticonvulsants, can affect lymphocyte counts.
- Genetic disorders: Some genetic disorders, such as Wiskott-Aldrich syndrome and X-linked agammaglobulinemia, can lead to lymphocytosis (high lymphocytes).
Absolute High Lymphocytes vs. Relative Lymphocytosis
Lymphocytosis (high lymphocytes) can be classified as either absolute or relative.
- Absolute high lymphocytes (lymphocytosis): This occurs when the absolute number of lymphocytes in the blood is increased. It is typically indicated by an absolute lymphocyte count above the normal reference range.
- Relative lymphocytosis (high lymphocytes): This occurs when the percentage of lymphocytes among total white blood cells is increased, even though the absolute number of lymphocytes may be within the normal range. This can happen when there is a decrease in other types of white blood cells, such as neutrophils.
To accurately diagnose the cause of lymphocytosis (high lymphocytes), it is important to consider the patient’s clinical history, physical examination, and laboratory tests, including a complete blood count (CBC) with differential and possibly additional tests such as flow cytometry or bone marrow biopsy.
Causes of Low Lymphocytes (Lymphopenia)
Lymphopenia (low lymphocytes) is a condition characterized by an abnormally low number of lymphocytes in the blood.
Infections
- HIV infection: HIV infection is a major cause of lymphopenia (low lymphocytes). The virus directly targets and destroys CD4+ T cells, which are essential for immune function.
- Viral infections: Other viral infections, such as measles, influenza, and Epstein-Barr virus infection, can temporarily decrease lymphocyte counts.
- Bacterial infections: Some bacterial infections, such as sepsis, can lead to lymphopenia (low lymphocytes).
Autoimmune Diseases
- Systemic lupus erythematosus (SLE): In some cases, SLE can cause lymphopenia (low lymphocytes) due to autoimmune destruction of lymphocytes.
- Rheumatoid arthritis: Lymphocyte counts may be decreased in patients with rheumatoid arthritis, especially when there is active inflammation.
Immunosuppressive Drugs
- Chemotherapy: Chemotherapy drugs used to treat cancer can significantly reduce lymphocyte counts.
- Corticosteroids: High doses of corticosteroids can suppress the immune system and lead to lymphopenia (low lymphocytes).
- Immunosuppressant drugs: Medications used to prevent organ rejection in transplant patients can also cause lymphopenia (low lymphocytes).
Bone Marrow Disorders
- Aplastic anemia: This is a rare condition characterized by the failure of bone marrow to produce blood cells, including lymphocytes.
- Bone marrow infiltration: Certain diseases, such as leukemia and lymphoma, can infiltrate the bone marrow and interfere with lymphocyte production.
Other Causes
- Nutritional deficiencies: Deficiencies of certain nutrients, such as zinc and selenium, can affect lymphocyte function.
- Stress: Severe stress can temporarily decrease lymphocyte counts.
- Aging: Lymphocyte counts tend to decrease with age.
It is important to note that lymphopenia (low lymphocytes) can be a serious condition, as it can increase the risk of infections and other complications.
Laboratory Tests for Lymphocyte Evaluation
To assess lymphocyte function and identify potential abnormalities, several laboratory tests can be performed.
Complete Blood Count (CBC) with Differential
- Absolute lymphocyte count: This provides the total number of lymphocytes in the blood.
- Relative lymphocyte count: This indicates the percentage of lymphocytes among total white blood cells.
Flow Cytometry
- Lymphocyte subset analysis: Flow cytometry can be used to quantify specific lymphocyte subsets, such as CD4+ T cells, CD8+ T cells, B cells, and NK cells.
- Immunophenotyping: This technique can identify abnormal lymphocyte populations, such as those seen in leukemia or lymphoma.
Immunoglobulin Levels
- Serum immunoglobulin levels: Measuring the levels of different immunoglobulin classes (IgG, IgA, IgM, IgE) can help assess B cell function and identify immunodeficiency disorders.
T Cell Function Tests
- Delayed-type hypersensitivity (DTH) skin test: This test assesses the ability of T cells to respond to antigens.
- In vitro T cell proliferation assay: This test measures the ability of T cells to proliferate in response to stimulation.
NK Cell Function Tests
- NK cell cytotoxicity assay: This test measures the ability of NK cells to kill target cells.
Bone Marrow Biopsy
- Lymphoid cell morphology: A bone marrow biopsy can help evaluate the morphology and maturation of lymphocytes.
- Lymphoid cell markers: Immunohistochemical staining can be used to identify abnormal lymphocyte populations.
Other Tests
- Genetic testing: In some cases like immunodeficiency disorders, genetic testing may be used to identify inherited disorders that affect lymphocyte function.
- Infectious disease testing: If lymphocytosis or lymphopenia is suspected to be due to an infection, specific tests may be needed to identify the causative agent.
Note: The choice of laboratory tests will depend on the specific clinical context and suspected diagnosis.
Therapeutic Applications of Lymphocytes: Immunotherapy
Immunotherapy is a rapidly evolving field of medicine that utilizes the immune system to treat diseases, particularly cancer. Lymphocytes, as key players in the immune response, are central to many immunotherapy approaches.
Types of Lymphocyte-Based Immunotherapy
Adoptive Cell Therapy (ACT)
- T cell therapy: This involves harvesting T cells from a patient, genetically engineering them to recognize and target cancer cells, and then re-infusing them into the patient.
- Chimeric antigen receptor (CAR) T cell therapy: CAR T cells are engineered with a synthetic receptor that allows them to specifically target and kill cancer cells. This approach has shown remarkable success in treating certain types of blood cancers.
- Natural killer (NK) cell therapy: NK cells can also be used in ACT to target and kill cancer cells.
Checkpoint Inhibitors
- Antibodies: These drugs block checkpoints in the immune system that prevent T cells from attacking cancer cells. By releasing these brakes, checkpoint inhibitors can help the immune system recognize and eliminate tumor cells.
- Examples: PD-1 inhibitors (e.g., nivolumab, pembrolizumab) and CTLA-4 inhibitors (e.g., ipilimumab).
Cancer Vaccines
- Therapeutic vaccines: These vaccines aim to stimulate the immune system to recognize and attack cancer cells. They can be made from tumor cells or tumor-derived antigens.
- Prophylactic vaccines: These vaccines aim to prevent cancer by stimulating the immune system to recognize and eliminate pre-cancerous cells.
Lymphocyte-Directed Therapies
These therapies aim to modulate lymphocyte function to enhance their ability to fight disease. For example, certain drugs can stimulate the production of lymphocytes or enhance their effector functions.
Applications of Lymphocyte-Based Immunotherapy
- Cancer Treatment: Lymphocyte-based immunotherapies have shown promising results in treating various cancers, including hematological malignancies (e.g., leukemia, lymphoma) and solid tumors (e.g., melanoma, lung cancer).
- Autoimmune Diseases: While immunotherapy is primarily used for cancer treatment, it is also being explored for autoimmune diseases. By targeting specific lymphocyte subsets or pathways, researchers hope to modulate the immune response and reduce inflammation.
- Infectious Diseases: Lymphocyte-based therapies may have potential applications in treating certain infectious diseases, particularly those that are difficult to treat with traditional antibiotics.
Impact of Aging on Lymphocyte Function
Aging is associated with a decline in immune function, including a reduction in lymphocyte activity. This phenomenon, known as immunosenescence, contributes to increased susceptibility to infections, autoimmune diseases, and cancer in older adults.
Key Changes in Lymphocyte Function with Aging
Decreased Lymphocyte Production
- Bone marrow function: With age, the bone marrow becomes less efficient at producing lymphocytes, leading to a decrease in their absolute numbers.
- Thymic involution: The thymus, a primary site of T cell development, undergoes involution with age. This results in a reduced capacity to generate new T cells.
Impaired Lymphocyte Function
- Reduced antigen recognition: Aging lymphocytes may become less efficient at recognizing and responding to foreign antigens.
- Decreased proliferation: Lymphocytes from older individuals may have a reduced ability to divide and proliferate in response to stimuli.
- Altered cytokine production: The balance of cytokines produced by lymphocytes may shift with age, leading to a less effective immune response.
- Increased susceptibility to infections: The decline in lymphocyte function contributes to increased susceptibility to infections in older adults.
Immunosenescence
The overall age-related decline in immune function is referred to as immunosenescence. It is characterized by:
- Decreased T cell function: A reduction in the number and function of T cells, particularly CD4+ helper T cells and CD8+ cytotoxic T cells.
- Increased B cell dysfunction: B cells from older individuals may produce fewer antibodies and have impaired responses to antigens.
- Altered NK cell activity: NK cell function may also decline with age, leading to increased susceptibility to infections and cancer.
Consequences of Immunosenescence
- Increased susceptibility to infections: Older adults are more prone to infections due to their weakened immune systems.
- Autoimmune diseases: The decline in immune function can lead to an increased risk of autoimmune diseases, where the immune system mistakenly attacks healthy tissues.
- Cancer: Older individuals are at a higher risk of developing cancer, in part due to their weakened immune system’s inability to effectively detect and eliminate tumor cells.
- Reduced response to vaccines: Older adults may have a reduced response to vaccines, such as influenza and pneumococcal vaccines.
Strategies for Mitigating Immunosenescence
- Healthy lifestyle: A healthy lifestyle, including a balanced diet, regular exercise, and adequate sleep, can help to maintain immune function in older adults.
- Vaccinations: Staying up-to-date on vaccinations is crucial for protecting older adults from infectious diseases.
- Immunizations: Some immunizations, such as pneumococcal and influenza vaccines, are specifically recommended for older adults to boost their immune response.
- Nutritional supplements: Certain nutritional supplements, such as vitamin D and zinc, may help to support immune function in older adults.
- Immunotherapy: While still under development, immunotherapy strategies may be able to help restore immune function in older adults.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Generation of lymphocytes in bone marrow and thymus. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27123/
- Cano RLE, Lopera HDE. Introduction to T and B lymphocytes. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459471/
- Linton, P., Dorshkind, K. Age-related changes in lymphocyte development and function. Nat Immunol 5, 133–139 (2004). https://doi.org/10.1038/ni1033
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014 Oct 16;371(16):1507-17. doi: 10.1056/NEJMoa1407222. Erratum in: N Engl J Med. 2016 Mar 10;374(10):998. doi: 10.1056/NEJMx160005. PMID: 25317870; PMCID: PMC4267531.
- Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016 Aug 23;16(9):566-81. doi: 10.1038/nrc.2016.97. PMID: 27550819; PMCID: PMC5543811.
- Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017 Feb 9;168(4):724-740. doi: 10.1016/j.cell.2017.01.016. PMID: 28187291; PMCID: PMC5553442.
- Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189-225. doi: 10.1146/annurev-immunol-032713-120136. Epub 2014 Jan 9. PMID: 24423116; PMCID: PMC4533835.