TL;DR
The visual assessment of a peripheral blood smear allows for the identification of subtle structural and cytoplasmic changes in abnormal WBCs (white blood cells), or leukocytes, that can provide critical clues about underlying pathologies.
| Abnormality Type | Characteristics | Causes | Clinical Significance |
| Cytoplasmic Inclusions | |||
| Toxic Granulation ▾ | The presence of coarse, dark granules in the cytoplasm of neutrophils. It is part of a set of abnormal WBC morphologic changes that occur due to accelerated neutrophil production in the bone marrow. | A sign of a severe bacterial infection or inflammation. Can also be caused by certain medications. | Indicates an active immune or inflammatory response. Must be differentiated from Alder anomaly, which is a genetic condition. |
| Döhle Bodies ▾ | Small, blue-staining rods or ovals found in the periphery of a neutrophil’s cytoplasm. They are composed of agglutinated ribosomes. | A result of accelerated bone marrow neutrophil production in response to inflammation. Can also be a normal finding in small numbers in healthy individuals, especially in cats and horses. | Often a sign of “toxic change” in neutrophils, which indicates an inflammatory response. Their presence is considered a clue to an underlying inflammatory condition. |
| Vacuoles ▾ | Small, clear, unstained spaces within the cytoplasm of a cell. They can be present in neutrophils, lymphocytes, and monocytes. | In neutrophils, they can indicate phagocytosis or reflect endotoxemia leading to neutrophil autolysis. In lymphocytes, they may be a sign of rare hereditary metabolic disorders. | In neutrophils, they are a marker of cell degeneration and apoptosis and can correlate with the severity of conditions like hemorrhagic shock. In pediatric patients, vacuolated lymphocytes can be a key clue for an underlying metabolic disorder. |
| Alder Anomaly ▾ | Large, coarse, azurophilic, and basophilic granules in the cytoplasm of all types of leukocytes, including neutrophils, monocytes, and lymphocytes. The granules may be surrounded by a clear halo. | A hereditary condition strongly associated with lysosomal storage disorders like mucopolysaccharidosis (MPS) and Tay-Sachs disease. | The affected white blood cells function normally. This benign finding serves as an important non-invasive clue for a severe, underlying systemic genetic disorder. |
| Hypogranulation ▾ | A reduction or complete absence of normal cytoplasmic granules in granulocytes, especially neutrophils. Can be assessed by scoring the degree of granulation. | Can occur in benign conditions such as a severe infection or, more critically, as a hallmark feature of malignant processes like Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML). | A key cytological marker for bone marrow dysplasia, which is a failure of the bone marrow to properly produce and mature blood cells. Its diagnostic value is highest when it appears with other dysplastic features. |
| Nuclear Abnormalities | |||
| Hypersegmentation ▾ | Neutrophil nuclei with six or more lobes. | Linked to megaloblastic anemias caused by a vitamin B12 or folate deficiency. | Serves as a classic diagnostic clue for specific nutritional deficiencies. |
| Left Shift (Bandemia) ▾ | An increase in the number of immature neutrophils (band cells) in the blood. Band cells are identified by their curved, non-lobular nucleus. | Most commonly occurs in response to an acute infection or stress where the body’s demand for white blood cells outweighs its supply. Can also be a sign of a bone marrow disorder or blood cancer, such as Chronic Myeloid Leukemia (CML). | A strong indicator of an inflammatory leukogram, but the presence of a significant number of immature granulocytes can also prompt further investigation for a serious underlying condition. |
| Pelger-Huët Anomaly (PHA) ▾ | A genetic defect that results in nuclear hyposegmentation of granulocytes. Neutrophil nuclei are typically bilobed, peanut-shaped, or dumbbell-shaped. | An inherited, autosomal dominant genetic disorder. | It is a benign, incidental finding, as the affected neutrophils are fully functional. It is clinically significant because it can be mistaken for a “marked left shift” on a blood smear. |
| Neutrophil Drumsticks ▾ | A small, spherical, deeply staining mass of chromatin (~1.5 μm) connected to the main nucleus by a thin stalk. | The cytological representation of the inactivated X chromosome (Barr body) in females. They are a normal finding in females. | While not used for definitive sex determination today, they can provide a preliminary clue for sex chromosome abnormalities. They must be differentiated from non-sex-specific appendages. |
| Others | |||
| Reactive Lymphocytes ▾ | Lymphocytes with increased, distinctly basophilic cytoplasm and irregular or clefted nuclei. | Immunological or antigenic stimulation, often from a viral infection such as mononucleosis or Cytomegalovirus (CMV). Can also be caused by severe medical stress or a reaction to a new medication. | A sign that the immune system is actively fighting an invader. In rare cases, elevated levels can be a sign of certain blood cancers. |
| Dysplasia ▾ | A broad term for abnormal WBCs that occur due to a failure of proper maturation in the bone marrow. Manifests as a variety of morphological changes, including hypogranulation and nuclear abnormalities. | Underlying bone marrow disorders where the bone marrow fails to produce a sufficient number of healthy, functional blood cells. It is a hallmark feature of Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML). | The presence of dysplastic features is a key diagnostic marker for conditions like MDS and AML, and often prompts a bone marrow biopsy for a definitive diagnosis. |
*Click ▾ for more information
Introduction
This article provides an overview of key findings in abnormal WBC (white blood cell) morphology observed during a peripheral blood smear analysis. Understanding these abnormal white blood cell morphological changes can aid in diagnosing various underlying conditions.
Role of WBCs
White blood cells (WBCs), also known as leukocytes, are the body’s defense system cells, playing a critical role in the immune response. They circulate throughout the bloodstream and tissues, constantly surveying for and eliminating pathogens (disease-causing organisms) and debris. There are five main types of WBCs, each with specialized functions.
- Neutrophils: These are the most abundant WBCs, acting as the first responders to infections by engulfing and destroying bacteria through a process called phagocytosis. They are also the first to migrate to sites of inflammation.
- Lymphocytes: These are further divided into B and T cells. B cells produce antibodies, which are proteins that specifically target and neutralize pathogens. T cells come in various subtypes, some of which directly attack infected cells or regulate the immune response by helping other immune cells function.
- Monocytes: They mature into macrophages in tissues. Macrophages are like bigger, stronger phagocytes that engulf debris, pathogens, and even cancer cells. They also process and present antigens (foreign molecules) to lymphocytes, helping them recognize and target the invaders.
- Eosinophils: They are involved in allergic reactions and defense against parasitic infections. Eosinophils release chemicals that damage parasites and contribute to inflammatory responses.
- Basophils: These are the least common WBCs, releasing histamine and other chemicals during allergic reactions and inflammation. Histamine causes vasodilation (widening of blood vessels) and increased permeability, leading to allergy symptoms like runny nose and itchy eyes.
WBC Morphology
WBC morphology plays a crucial role in blood smear analysis for several reasons:
1. Detailed Information Beyond Cell Count: Some complete blood count (CBC) for example a 3-part full blood count analyzer provides the total WBC count, but it doesn’t differentiate between the types of WBCs. Examining the morphology of WBCs in a blood smear allows for a differential white blood cell count (WBC diff). This diff reveals the percentages of each WBC type, which can be highly informative. For example, an elevated eosinophil count on the diff might suggest an allergic reaction or parasitic infection, even with a normal total WBC count.
2. Identification of Abnormal WBC: The morphology of healthy WBCs has specific characteristics. Deviations from these characteristics (abnormal WBC morphology), like the presence of toxic granulation or Dohle bodies, can indicate underlying conditions like infections or myeloproliferative disorders. Early detection of these abnormal white blood cells can prompt further investigation and lead to a faster diagnosis.
3. Supporting Diagnosis: Abnormal WBC morphology findings can support diagnoses suggested by other clinical findings or laboratory tests. For instance, hypersegmented neutrophils are abnormal WBCs and might point towards vitamin B12 deficiency, while May-Hegglin anomaly strengthens the suspicion of May-Hegglin syndrome when giant platelets are also observed.
4. Guiding Treatment Decisions: Identifying the specific type of WBC involved in an elevated count can guide treatment decisions. For example, an elevated neutrophil (neutrophilia) count suggests a bacterial infection, prompting antibiotic therapy, whereas an elevated eosinophil count (eosinophilia) might point towards an allergy, requiring a different treatment approach.
5. Monitoring Treatment Response: WBC morphology can be used to monitor the effectiveness of treatment. For example, a decrease in abnormal WBCs following treatment for an infection indicates a positive response.
Cytoplasmic Inclusions Abnormal WBC
Toxic Granulation in Neutrophils
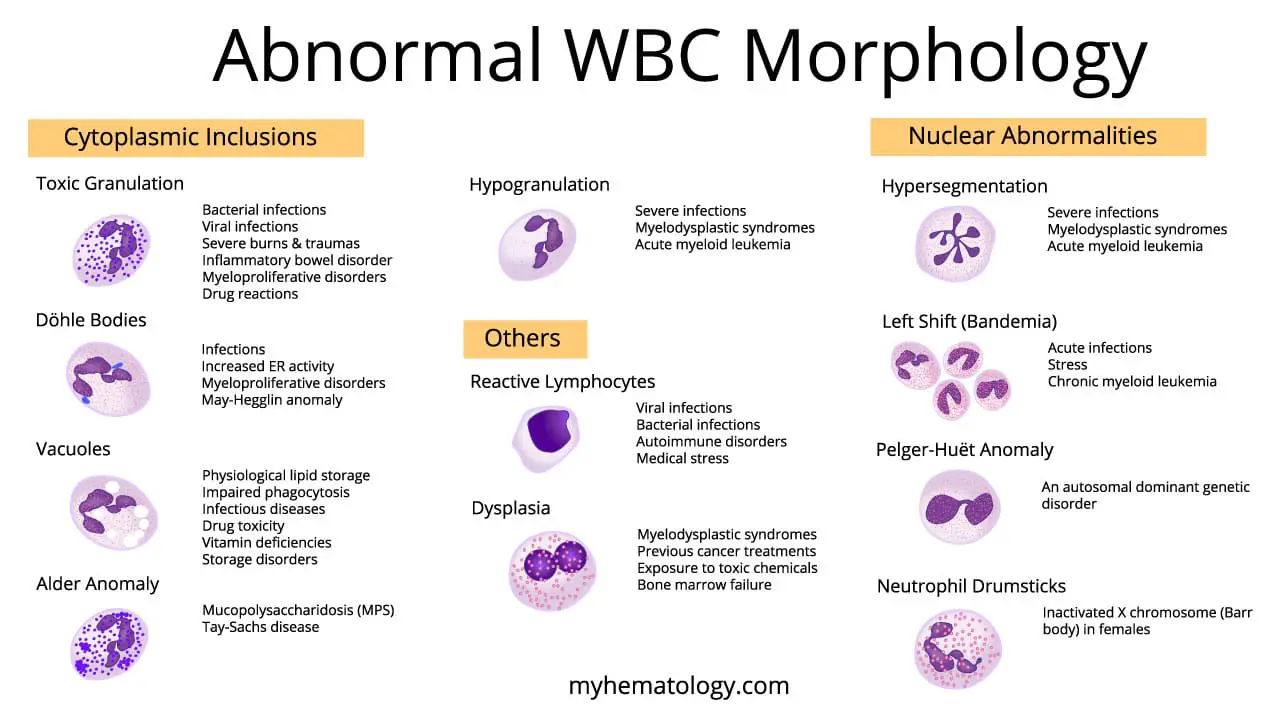
Toxic granulation refers to the presence of abnormal granules within neutrophils observed during a blood smear analysis. These abnormal WBC granules have a distinct appearance compared to normal neutrophil granules.
Appearance on Abnormal WBC in Blood Smear
- Size and Color: These abnormal WBC granules are larger and more coarse compared to the fine, evenly distributed pink or lilac granules in mature neutrophils.
- Staining: These abnormal WBC granules stain a darker blue or purple with traditional blood stains (e.g., Leishman stain) due to a higher concentration of enzymes.
Causes of Toxic Granulation in Neutrophils
While toxic granulation is a strong indicator of an inflammatory response, it can be caused by various conditions.
- Bacterial Infections: This is the most common cause for this abnormal WBC morphology. The presence of bacteria triggers the release of cytokines, which stimulate neutrophils to mature more rapidly particularly in severe bacterial infections like sepsis, pneumonia, or meningitis. This rapid maturation leads to incomplete packaging of enzymes within the granules, resulting in their coarse and hyper-staining appearance.
- Viral Infections: Some viral infections, particularly severe ones, can also induce toxic granulation in neutrophils.
- Severe Burns and Trauma: Extensive tissue damage triggers an inflammatory response, leading to the release of cytokines and potential toxic granulation.
- Inflammatory Bowel Disease (IBD): Active phases of IBD involve significant inflammation, which can be reflected in the blood smear by the presence of toxic granulation as abnormal WBC morphology.
- Myeloproliferative Disorders: These are a group of bone marrow diseases characterized by abnormal growth of myeloid cells. Some types of myeloproliferative disorders can cause toxic granulation for example chronic neutrophilic leukemia.
- Drug Reactions: Certain medications can cause toxic granulation as a side effect for example chemotherapy or granulocyte colony-stimulating factor (G-CSF), can stimulate neutrophil production and lead to toxic granulation.
Clinical Significance
It is a clear sign of an active immune response, usually indicating a bacterial infection or a severe inflammatory condition.
Döhle Bodies in Neutrophils
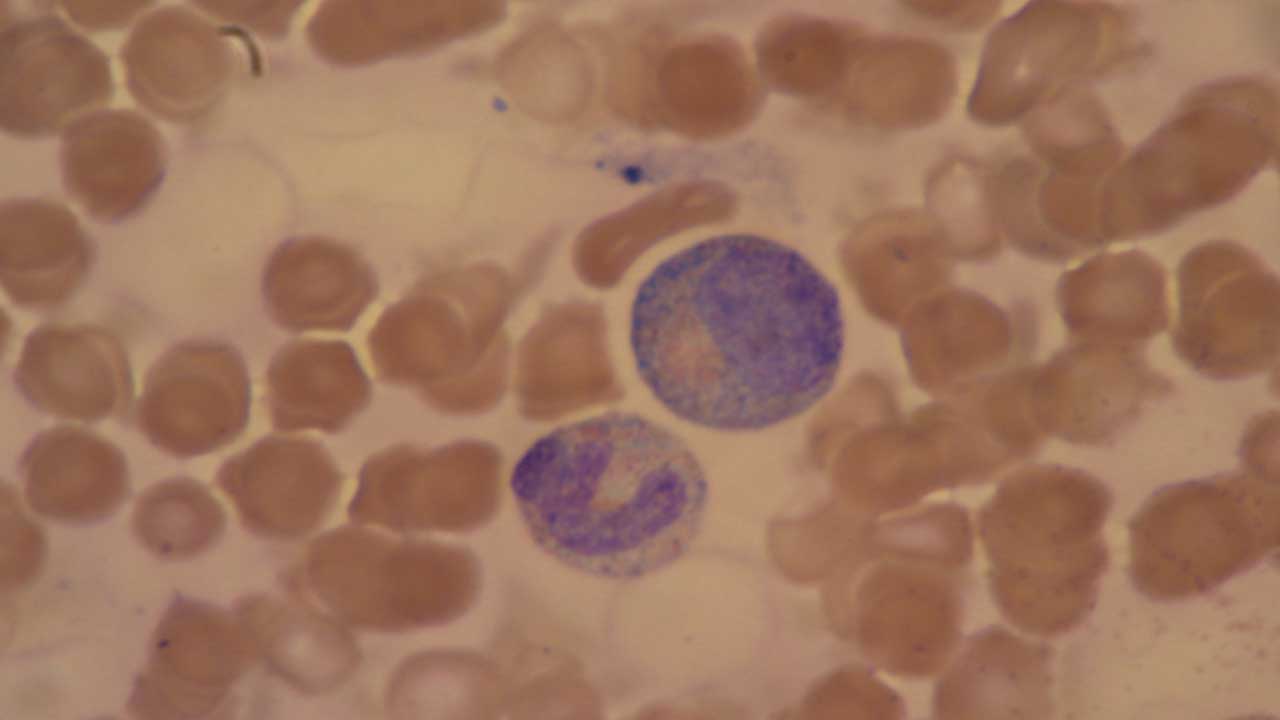
Döhle bodies are another type of abnormal WBC morphology. They are pale blue-gray, oval-shaped inclusions found within the cytoplasm of neutrophils, typically near the cell membrane.
Appearance of Abnormal WBC on Blood Smear
- Size: Döhle bodies measure 1-3 micrometers in diameter, which is relatively small compared to a neutrophil.
- Color: They appear pale blue or gray due to their composition of rough endoplasmic reticulum (RER) remnants.
- Shape: Döhle bodies are usually oval or round, but they can also be crescent-shaped or elongated.
- Location: These inclusions are most commonly seen close to the neutrophil’s cell membrane.
Causes of Döhle Bodies in Neutrophils
The presence of Döhle bodies can be due to various factors:
- Infections: Döhle bodies are often associated with viral infections, particularly severe ones like cytomegalovirus (CMV) or influenza. They can also be seen in some bacterial infections.
- Increased Endoplasmic Reticulum Activity: Conditions that stimulate the endoplasmic reticulum (ER) in neutrophils, like drug reactions or deficiencies (vitamin B12, folate), can lead to Döhle body formation.
- Myeloproliferative Disorders: These are bone marrow disorders characterized by abnormal overproduction of blood cells. Some myeloproliferative disorders, like chronic myeloid leukemia (CML), may be associated with Döhle bodies.
- May-Hegglin Anomaly: This is a rare inherited condition where Döhle bodies as abnormal WBC morphology, are consistently present along with giant platelets in the blood smear.
Clinical Significance
Döhle bodies are a common sign of “toxic change” in neutrophils, which indicates an inflammatory leukogram. While often associated with toxic conditions, a small number of Döhle bodies may also be a normal finding in healthy individuals, particularly in cats and horses.
The presence of Döhle bodies alone is not diagnostic of any specific condition. It should be evaluated in conjunction with other clinical findings and laboratory tests. The number and prominence of Döhle bodies may offer some clues about the underlying cause. For example, a larger number of Döhle bodies might suggest a more severe condition.
Döhle bodies are sometimes confused with toxic granulation, but this abnormal WBC morphology differ significantly in appearance and cause. Toxic granules are coarse, basophilic, and associated with inflammation, while Döhle bodies are pale, composed of RER remnants, and linked to viral infections or increased ER activity.
Differences between Toxic Granulation and Döhle Bodies
| Feature | Toxic Granulation | Döhle Bodies |
| Description | The presence of coarse, dark granules in the cytoplasm of neutrophils. It is considered a set of morphologic changes that occur due to accelerated neutrophil production in the bone marrow. | Intra-cytoplasmic structures composed of agglutinated ribosomes. They appear as small, blue-staining rods or ovals in the periphery of the cytoplasm. |
| Causes | Often a sign of a severe bacterial infection, inflammation, or certain medications. It reflects “defects” in cell production and maturation during active granulocytopoiesis. | A result of accelerated bone marrow neutrophil production in response to inflammation. |
| Diagnostic Significance | A clear sign of an active immune or inflammatory response. The presence of toxic granules, along with other changes like cytoplasmic basophilia, is a component of “toxic change” in neutrophils. | While often associated with toxic change, a small number can be a normal finding in healthy individuals. They are also normally more abundant in healthy cats and horses. |
Vacuolation in Abnormal WBCs
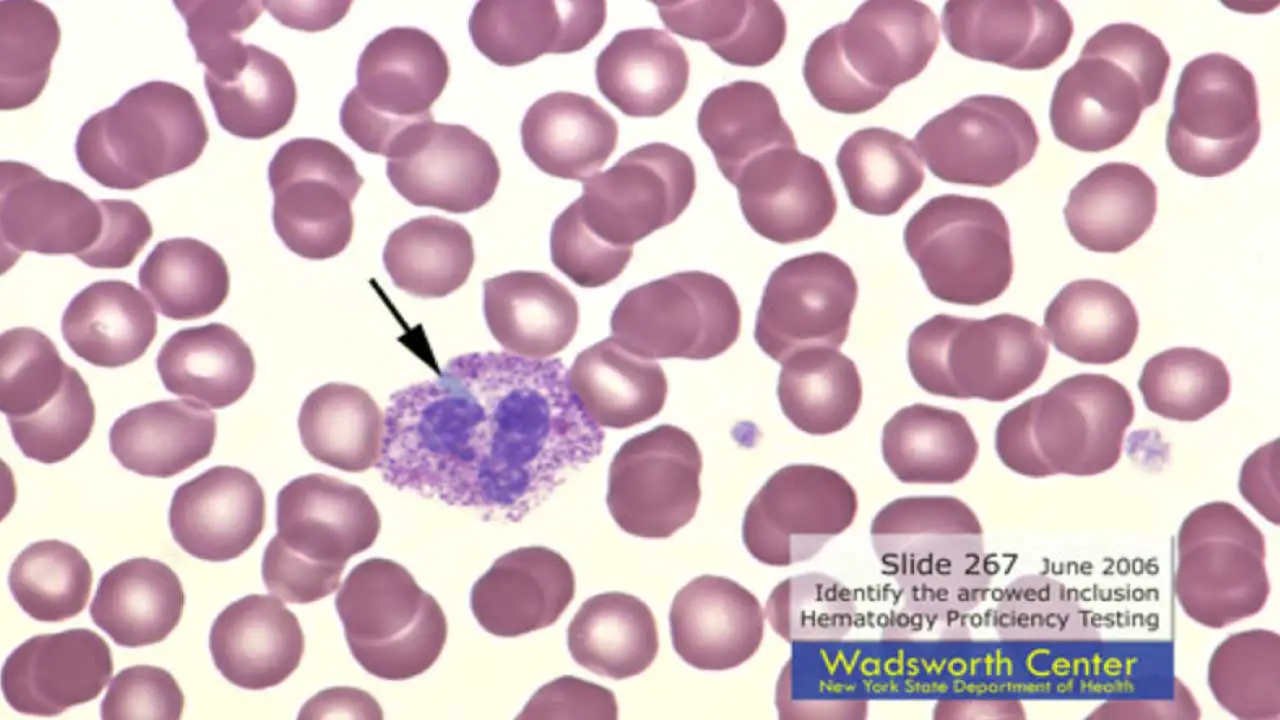
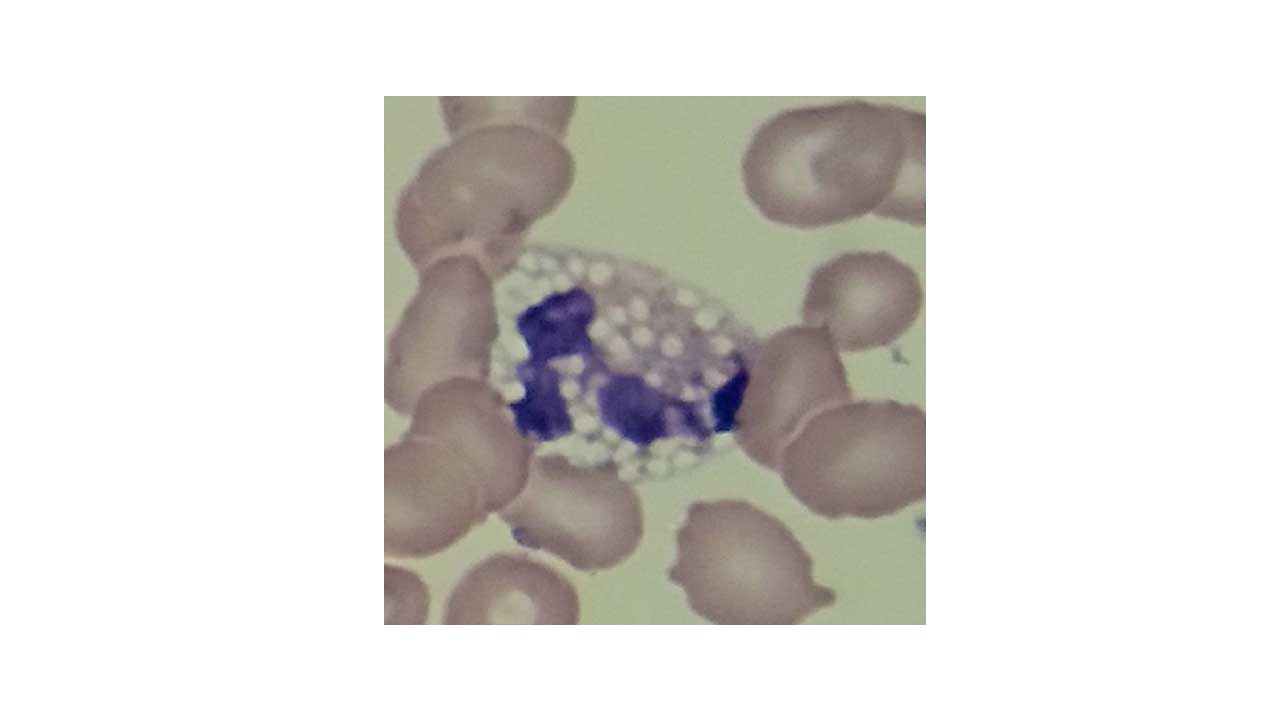
Vacuoles in abnormal WBCs appear as clear, round, or oval spaces within the cytoplasm. They lack the staining properties of normal cellular components and typically appear empty or light blue due to the presence of fluid in the abnormal WBCs.
Appearance on Blood Smear
- Size: The size of vacuoles in abnormal WBCs can vary depending on the underlying cause. They can range from small and inconspicuous to large and prominent, occupying a significant portion of the cytoplasm.
- Number: The number of vacuoles in abnormal WBCs can also be variable. Some abnormal WBCs might have a single, isolated vacuole, while others might exhibit multiple vacuoles scattered throughout the cytoplasm.
Causes of Vacuolation in Abnormal WBCs
The presence of vacuoles in abnormal WBCs necessitates considering several potential causes.
| Cause | Description | Characteristics |
| Physiological Lipid Storage (Monocytes) | In some cases, particularly in monocytes, small vacuoles might represent the storage of excess lipids within the cell. This is a normal physiological process and doesn’t necessarily indicate pathology. | The vacuoles in abnormal WBCs are typically small and may not be a prominent feature. |
| Impaired Phagocytosis | When WBCs, particularly neutrophils, engulf foreign material (bacteria, debris) through phagocytosis, they normally break it down within lysosomes. If lysosomal function is impaired (e.g., Chediak-Higashi syndrome), undigested material can accumulate, forming vacuoles. | The vacuoles in abnormal WBCs may be larger and more prominent compared to physiological lipid storage. |
| Infectious Diseases | Certain viral or parasitic infections can trigger the formation of vacuoles within WBCs. The mechanism can vary depending on the specific pathogen. | The type and size of vacuoles might offer clues about the causative agent. Additional tests would be needed for definitive diagnosis. |
| Drug Toxicity | Some medications, particularly chemotherapy drugs, can have a toxic effect on WBCs, leading to vacuolization as a side effect. | The presence of vacuoles along with other abnormal WBC morphological changes and a history of recent medication use can be suggestive of drug toxicity. |
| Vitamin Deficiencies | Deficiencies in vitamins like B12 or folate can disrupt the normal maturation and function of WBCs, potentially leading to vacuolization. | Vacuoles might be accompanied by other abnormal WBC morphologies suggestive of a deficiency state. |
| Storage Disorders | Inherited metabolic disorders that affect how cells store specific molecules can cause vacuole formation within WBCs due to the accumulation of unprocessed materials. Examples include Gaucher disease and Niemann-Pick disease. | The specific type of storage disorder can be identified through further investigations based on clinical presentation and additional laboratory tests. |
Clinical Significance
The presence of vacuoles in neutrophils has been shown to correlate directly with the severity of conditions like hemorrhagic shock and systemic inflammatory response syndrome (SIRS). In pediatric patients, the identification of vacuolated lymphocytes can be a key clue to an underlying metabolic disorder, prompting further specific testing.
Alder Anomaly
Alder anomaly is a rare benign finding in blood smear analysis characterized by the presence of abnormal WBC morphology such as coarse, basophilic granules within the cytoplasm of lymphocytes and, less commonly, neutrophils.
Appearance of Abnormal WBC on Blood Smear
- Granules: These granules are:
- Basophilic: They stain dark blue with basophilic dyes used in blood smears.
- Coarse: They are larger and more prominent compared to the fine, azurophilic granules typically seen in lymphocytes.
- Variable number: The number of basophilic granules can vary within lymphocytes, with some cells having just a few and others exhibiting a more prominent display.
- Lymphocyte Morphology: While lymphocytes with Alder anomaly have abnormal granules, their overall size and nuclear morphology typically remain within the normal range.
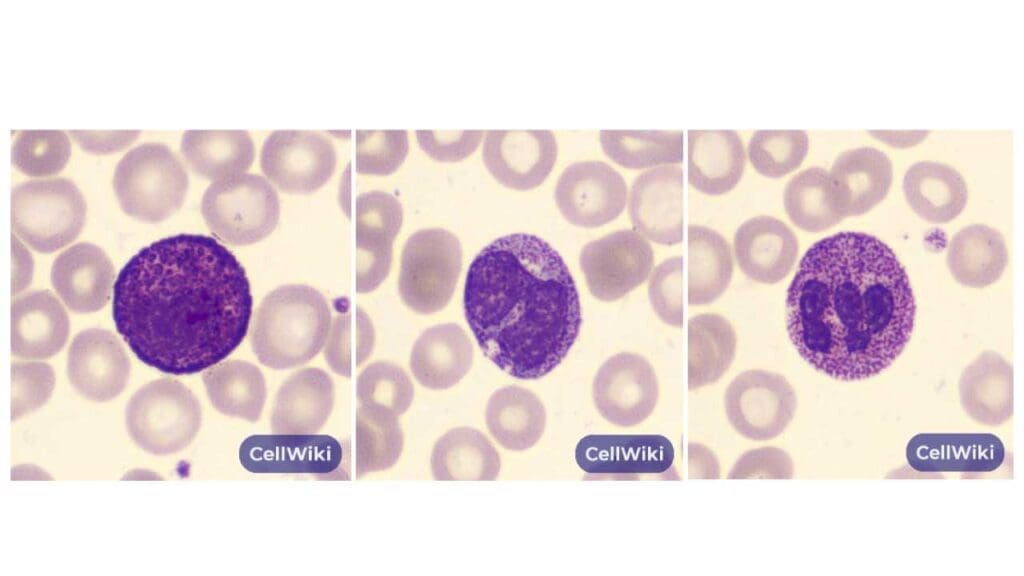
Cause of Alder Anomaly
The exact cause of Alder anomaly remains unclear. This anomaly is strongly associated with a group of genetic conditions known as lysosomal storage disorders, most notably mucopolysaccharidosis (MPS) and, less commonly, Tay-Sachs disease. The underlying pathology is a genetic defect that impairs the function of lysosomal enzymes responsible for the degradation of complex carbohydrates, such as glycosaminoglycans. This defect leads to the accumulation of undegraded metabolic products, which form the characteristic dense, clustered granules visible within the cytoplasm of the white blood cells on a peripheral blood smear.
Alder anomaly is a benign finding and doesn’t necessarily indicate any underlying medical condition. It’s more frequently observed in individuals with a history of myeloproliferative disorders, but it can also occur in healthy individuals without any associated disease.
Differential Diagnosis
It is important to differentiate Alder anomaly from other conditions that might exhibit basophilic granules.
- Toxic Granulation: This abnormal WBCs morphology is more commonly associated with neutrophils and presents with coarse, basophilic granules, but they are typically more numerous and evenly distributed throughout the cytoplasm compared to the more localized and variable distribution seen in Alder anomaly.
- Chronic Myeloproliferative Neoplasms (CMPN): These are bone marrow disorders that can cause abnormal basophilic granules in precursors of various blood cells, including lymphocytes. However, CMPN typically presents with other abnormal WBC morphologies in blood smear analysis and additional clinical features that differentiate it from Alder anomaly.
- Mast Cell Activation Syndrome (MCAS): This condition can sometimes lead to the release of basophilic granules from mast cells into the bloodstream, which might be mistaken for abnormal basophilia in lymphocytes on a blood smear. However, the presence of mast cell fragments or atypical cells can help differentiate MCAS from Alder anomaly.
Hypogranularity in WBCs
Hypogranularity refers to a decrease in the number and/or staining intensity of granules within the cytoplasm of white blood cells (WBCs). This abnormal WBC morphology can be observed in various types of WBCs, including neutrophils, eosinophils, and basophils.
Appearance of Abnormal WBC on Blood Smear
- Reduced Granules: Abnormal WBCs exhibit a noticeable decrease in the number of visible granules compared to healthy cells. The cytoplasm appears relatively clear or pale due to the reduced granularity.
- Fainter Staining: In some cases, the remaining granules might also stain less intensely with specific dyes used in blood smears, further contributing to the pale appearance in the abnormal white blood cells.
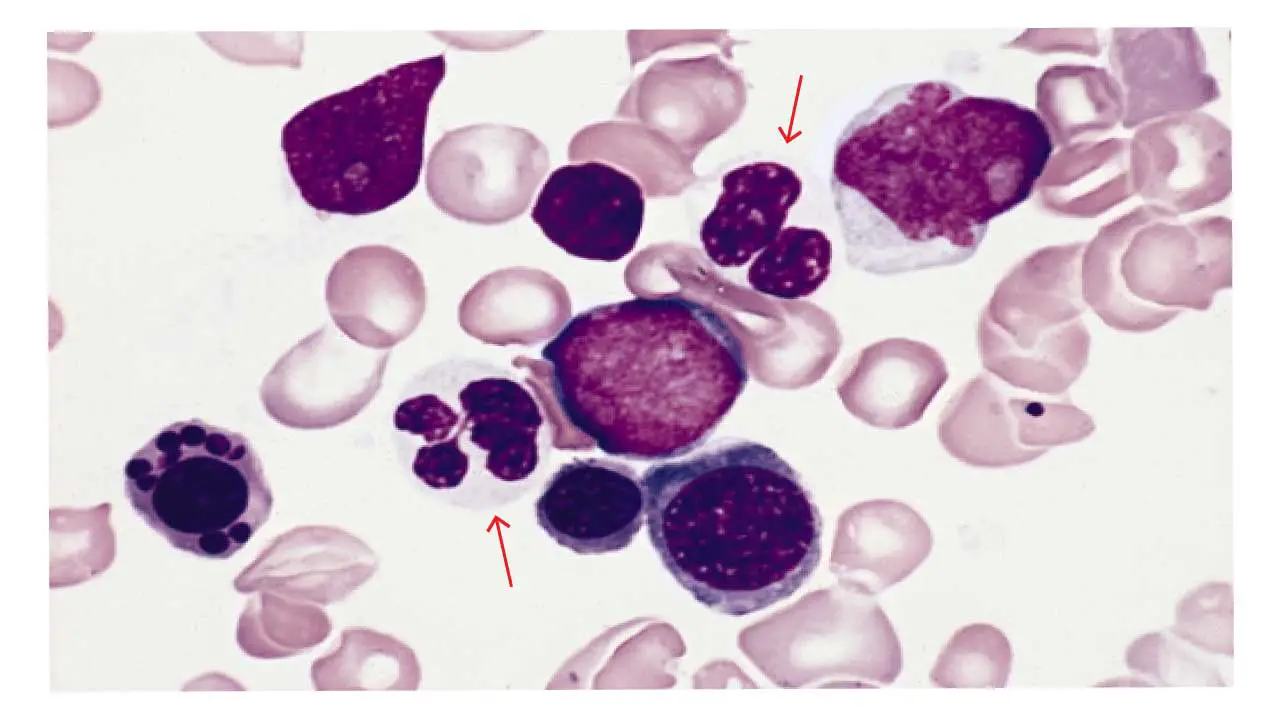
Causes of Hypogranularity in Abnormal WBCs
The presence of hypogranularity in abnormal WBCs necessitates considering several potential causes:
- Immature Cells: During maturation in the bone marrow, WBCs develop and acquire their characteristic granules. The release of immature WBCs into the bloodstream due to conditions like infections or bone marrow stress can lead to hypogranularity, as these precursors have fewer or less developed granules.
- Vitamin B12 or Folate Deficiency: Deficiencies in vitamin B12 or folate are essential for healthy DNA synthesis and cell division. These deficiencies can disrupt the proper maturation of WBCs, leading to the release of hypogranular forms with abnormal WBC morphology into the bloodstream.
- Myelodysplastic Syndromes (MDS): These are a group of bone marrow disorders characterized by abnormal blood cell production. In some cases of MDS, hypogranularity in neutrophils or other WBCs might be observed due to disrupted maturation processes.
- Pelger-Huët Anomaly: This is a benign, inherited condition primarily affecting neutrophils. While the hallmark feature in the abnormal WBC is bilobed nuclei, some individuals with Pelger-Huët anomaly might also exhibit a slight decrease in the number and staining intensity of their neutrophil granules.
Clinical Significance
The primary diagnostic value of hypogranulation lies in its role as a cytological marker of myelodysplasia, signifying a fundamental disruption in the normal maturation process within the bone marrow.
Nuclear Abnormalities
Hypersegmented Neutrophils
Hypersegmented neutrophils are mature neutrophils with an abnormally high number of nuclear lobes. Typically, a healthy neutrophil has 2-5 nuclear lobes connected by thin strands of chromatin, however, hypersegmented neutrophils are abnormal WBCs with 6 or more nuclear lobes.
Appearance of Abnormal WBC on Blood Smear
- Number of Lobes: Has six or more distinct lobes as the abnormal WBC morphology.
- Shape: The individual lobes can be round, oval, or even horseshoe-shaped.
- Chromatin Connections: The lobes may be connected by thin chromatin strands, but sometimes appear more separated.

Causes of Hypersegmented Neutrophils
Hypersegmented neutrophils as abnormal WBCs can be a sign of several underlying conditions.
- Megaloblastic Anemia: This is a type of anemia caused by vitamin B12 or folate deficiency. These deficiencies disrupt DNA synthesis, leading to the formation of mature neutrophils with more nuclear divisions, resulting in hypersegmentation.
- Myelodysplastic Syndromes (MDS): These are a group of bone marrow disorders characterized by abnormal blood cell production. In some cases of MDS, hypersegmented neutrophils as abnormal WBCs might be observed due to altered nuclear maturation.
- Liver Disease: Severe liver disease can sometimes lead to hypersegmentation of neutrophils.
- Hydroxyurea Therapy: This medication is used to treat certain cancers like chronic myeloid leukemia (CML). As a side effect, it can cause hypersegmentation of neutrophils as abnormal WBCs.
Clinical Significance
Hypersegmentation serves as a classic diagnostic clue for a vitamin B12 or folate deficiency. Hypersegmented neutrophils are often accompanied by macrocytosis (larger than normal red blood cells) in megaloblastic anemia due to the same underlying cause of DNA synthesis disruption. Recognizing hypersegmented neutrophils can be a valuable clue for identifying potential vitamin B12 or folate deficiencies, prompting further investigation for early intervention.
Left Shift (Bandemia)
Bandemia, or a “left shift,” refers to the presence of an increased number of immature neutrophils, known as band cells, in the blood. These cells are precursors to mature neutrophils.
Appearance of Abnormal WBC on Blood Smear
Band cells are identified by their curved, non-lobular nucleus, which gives them a characteristic banded or C-shaped appearance, as opposed to the segmented nucleus of a mature neutrophil.
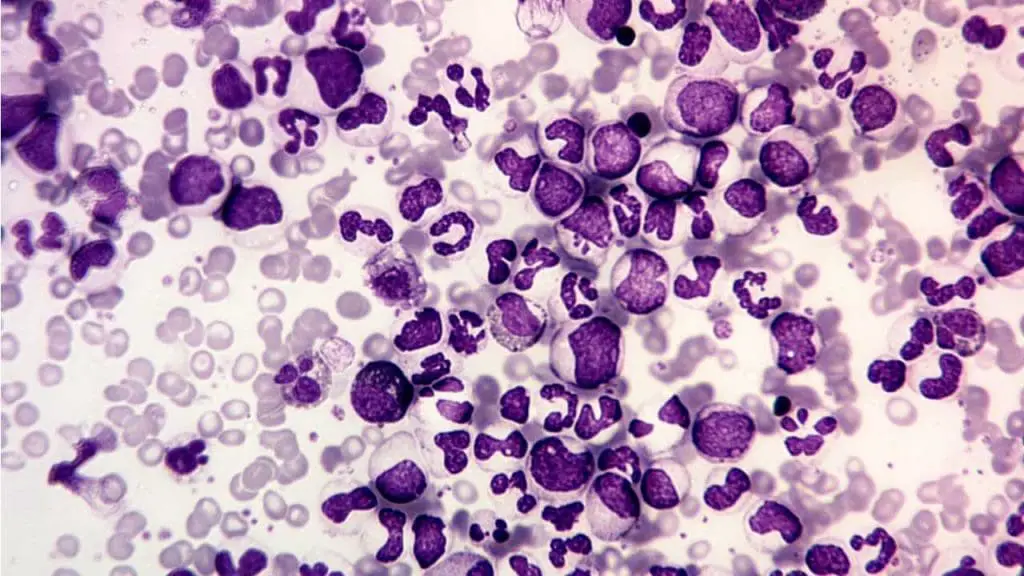
Causes of Left Shift (Bandemia)
- Acute Infection or Inflammation: This is the most frequent cause of a left shift. During an acute infection, the body’s demand for white blood cells to fight the invading pathogen increases rapidly, causing the bone marrow to release neutrophils prematurely before they have fully matured. Conditions like severe bacterial infections and inflammation are primary examples.
- Physical Injury or Stress: Severe physical stress or tissue trauma can also prompt an acute inflammatory response that leads to a left shift.
- Bone Marrow Disorders and Leukemias: While an inflammatory response is the most common reason, a left shift can also be a sign of a more serious underlying condition. Bone marrow disorders or blood cancers, such as Chronic Myeloid Leukemia (CML), can cause the bone marrow to release a significant number of immature granulocytes into the bloodstream.
Clinical Significance
While a left shift is a strong indicator of an inflammatory leukogram, signaling that the body is actively responding to an infectious or inflammatory process, it is also a key marker that can prompt further investigation into the bone marrow. The presence of a significant number of immature granulocytes can be a sign of a blood cancer or other bone marrow-related diseases.
Pelger-Huët Anomaly
Pelger-Huët anomaly (PHA) is a benign, inherited condition affecting the morphology of neutrophils and sometimes eosinophils giving rise to abnormal WBCs.
Appearance of Abnormal WBC on Blood Smear
- Nuclear Shape: Neutrophils exhibit bilobed nuclei instead of the usual segmented (3-5 lobes) appearance. These bilobed nuclei can be:
- Dumbbell-shaped: Resembling two connected spheres, resembling a dumbbell.
- Peanut-shaped: Oval with a central constriction, similar to a peanut.
- Chromatin Condensation: The abnormal WBC’s nuclear chromatin may appear condensed and coarse compared to a healthy neutrophil.
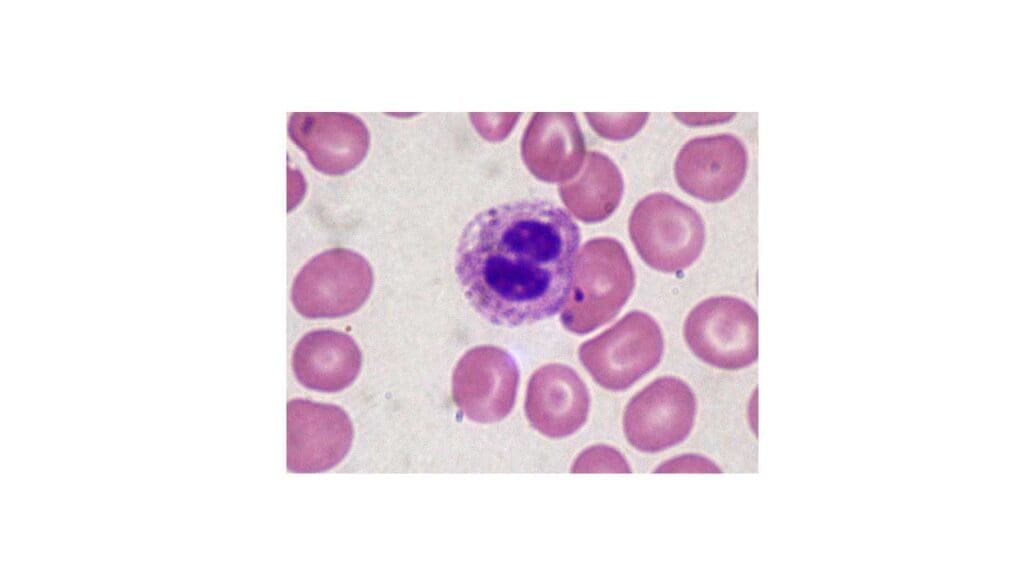
Causes of Pelger-Huët anomaly (PHA)
PHA is caused by mutations in the lamin B receptor (LBR) gene, which plays a role in nuclear envelope formation during neutrophil maturation. It’s inherited in an autosomal dominant pattern, meaning only one copy of the mutated gene is needed for the condition to manifest.
PHA is a benign condition and does not affect the function or lifespan of neutrophils. While PHA itself doesn’t require treatment. Recognizing it can prevent unnecessary investigations prompted by the abnormal WBC morphology.
PHA typically affects all granulocytes (neutrophils, eosinophils, basophils) to some degree, but the most prominent visual changes are usually seen in neutrophils.
Differences of PHA from Other Abnormal WBCs with Similar Morphology
| Feature | Pelger-Huët Anomaly (PHA) | Left Shift (Bandemia) | Hypersegmentation |
| Description | A genetic defect resulting in nuclear hyposegmentation of granulocytes. Neutrophil nuclei are typically bilobed, peanut-shaped, or dumbbell-shaped. | The presence of an increased number of immature neutrophils, known as band cells, in the blood. These cells are identified by their curved, non-lobular nucleus. | A condition where neutrophil nuclei have six or more lobes. |
| Cause | An inherited, autosomal dominant genetic disorder. Affected individuals are clinically normal. | An acute infection or stress response that triggers the accelerated release of immature neutrophils from the bone marrow. | Typically linked to megaloblastic anemias caused by vitamin B12 or folate deficiency. |
| Diagnostic Significance | It is typically an incidental finding and does not cause symptoms. PHA can be mistaken for a marked left shift on a blood smear. | A strong indicator of an active inflammatory leukogram or severe infection. The body is responding to an increased demand for white blood cells. | A key diagnostic clue for specific nutritional deficiencies. |
Drumsticks in Neutrophils
Drumsticks are a specific type of nuclear appendage observed in neutrophils during blood smear analysis. They are named for their characteristic drumstick-like shape and are considered a marker of sex chromatin in females.This is because the drumstick represents the condensed, inactive X chromosome (Barr body) in females (inactivation of one X chromosome).
Appearance on Blood Smear
- Shape: They resemble a small drumstick with a:
- Head: A rounded or oval-shaped terminal portion.
- Stalk: A thin, thread-like connection to the main body of the neutrophil nucleus.
- Size: Drumsticks are typically smaller than the main nucleus but can vary slightly in size.
- Location: They can be attached to any lobe of the neutrophil nucleus but are most commonly seen near the periphery.
- Frequency: In healthy females, the drumstick appearance is usually seen in only a small percentage (1-2%) of circulating neutrophils.
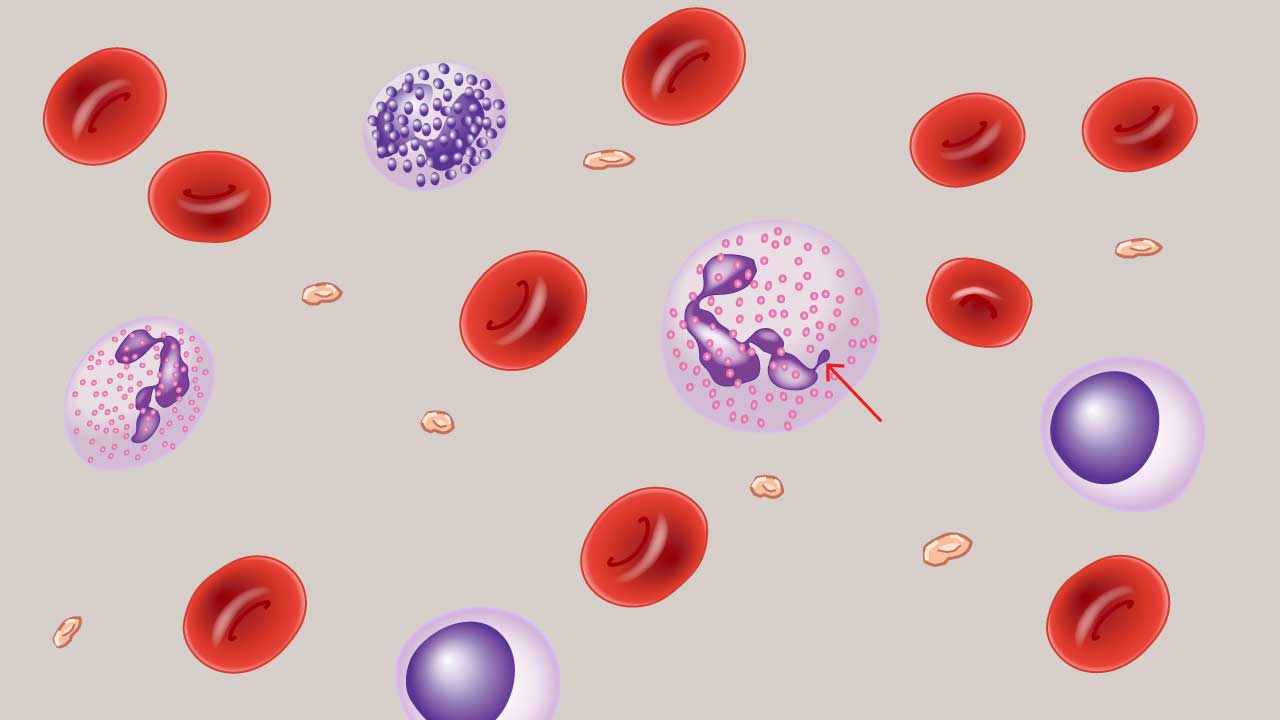
Causes of Drumsticks in Neutrophils
While drumsticks are primarily associated with sex chromatin in females, there are a few other considerations.
- Males: The presence of drumsticks in males is very rare (less than 0.1%) and might be associated with Klinefelter syndrome (XXY karyotype) or other chromosomal abnormalities.
- Non-Specific Nuclear Appendages: Occasionally, neutrophils can exhibit other nuclear appendages besides drumsticks as abnormal white blood cell morphology. These appendages can be:
- Sessile nodules: Small, round protuberances attached to the nucleus.
- Racquet forms: Resemble a drumstick but with a wider, flattened head. These non-specific appendages are usually smaller and lack the distinct drumstick shape. However, in rare cases, they might require further evaluation to rule out underlying conditions.
Differential Diagnosis
Identifying neutrophil drumsticks is important to differentiate them from other morphologically similar but non-sex-specific nuclear appendages. These non-specific appendages, which are not clinically significant, include “racquet forms,” “sessile nodules,” “small clubs,” and “minor lobes”. These structures are distinguished from true drumsticks by their smaller size (often less than 1 μm), their less intense staining, and a more variable shape and attachment.
While some investigators have suggested that sessile nodules are also sex-specific, they are more difficult to recognize and can be easily confused with non-specific appendages, which increases the potential for misinterpretation. Therefore, only the true drumstick is considered a reliable marker for sex diagnosis in clinical practice.
Others
Reactive Lymphocytes
Reactive lymphocytes are a type of white blood cell that has undergone morphological changes due to immunological stimulation.
Appearance of Abnormal WBC on Blood Smear
They are larger than normal lymphocytes, with increased, distinctly basophilic (dark blue) cytoplasm. Their nuclei may also have an irregular or clefted shape.
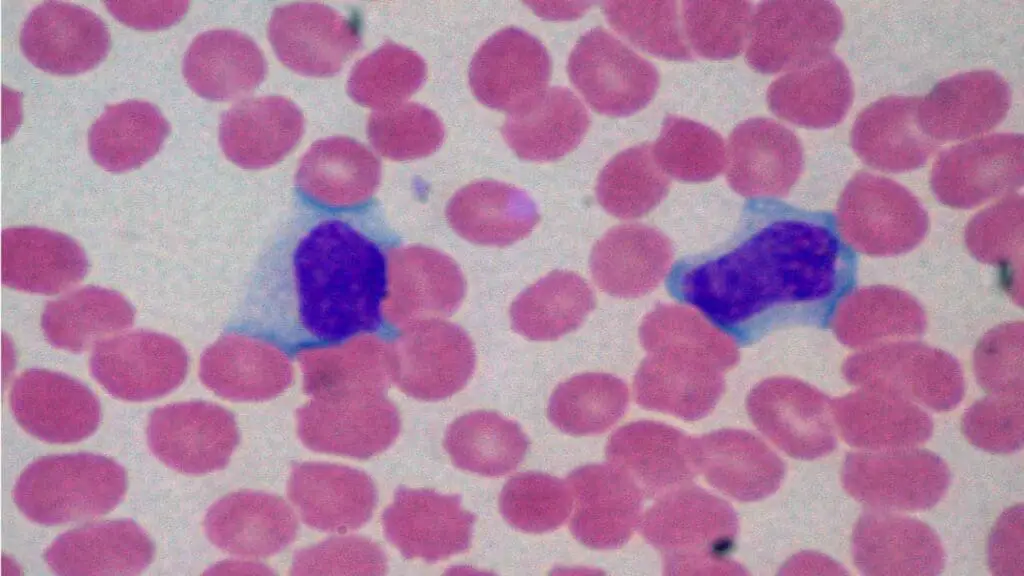
Causes of Reactive Lymphocytes
Common causes of reactive lymphocytes include:
- Viral Infections: This is the most common cause. Examples include infectious mononucleosis (caused by the Epstein-Barr virus), Cytomegalovirus (CMV), influenza, and hepatitis.
- Bacterial Infections: While less common than viral causes, certain bacterial infections can also trigger a reactive lymphocyte response, such as whooping cough, brucellosis, or cat-scratch disease.
- Autoimmune Diseases: Conditions that cause long-lasting inflammation, such as lupus and rheumatoid arthritis, can lead to elevated lymphocyte levels.
- Medical Stress: Severe physical stress, such as from trauma, can cause a spike in lymphocyte counts.
- Allergic Reactions: Reactions to a new medication or other allergens can cause a temporary increase in lymphocytes.
Clinical Significance
While they often signify a benign response to a recent infection, in some cases, elevated levels of reactive lymphocytes can be a sign of a more serious condition, such as a blood cancer like leukemia or lymphoma.
Dysplasia
Dysplasia refers to the abnormal features and development of white blood cells caused by abnormal maturation in the bone marrow.
Appearance of Abnormal WBC on Blood Smear
It can manifest as a variety of morphological changes, including granulocytic hypogranulation, nuclear abnormalities (like the Pelger-Huët-like anomaly), or the presence of immature myeloid cells in the blood. The cells that are produced are often abnormal in shape and unable to function properly.
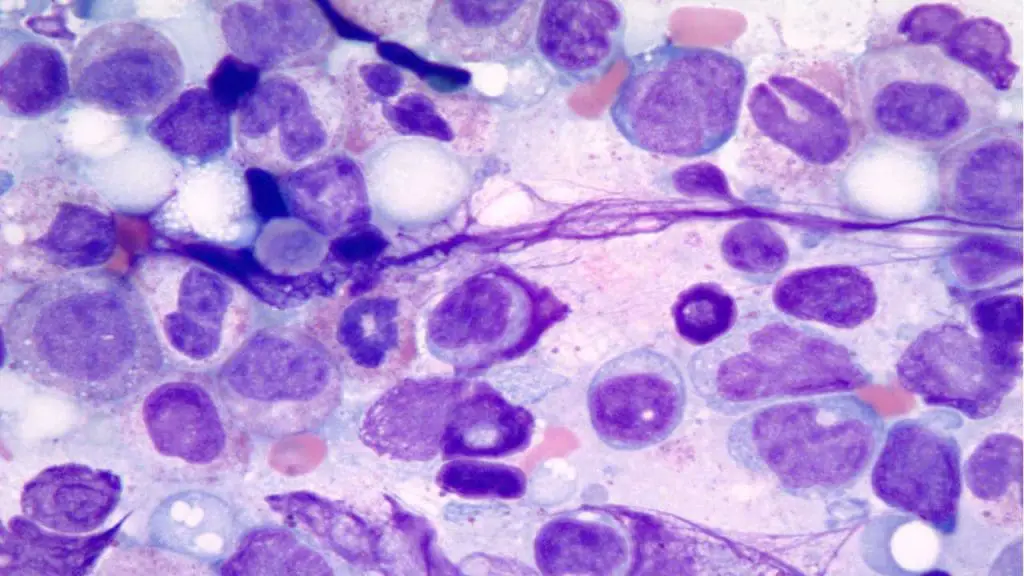
Causes of Dysplasia
The common causes of dysplasia include:
- Primary (Idiopathic) Dysplasia: In many cases, the cause of myelodysplastic syndrome (MDS)—a group of disorders characterized by dysplasia—is unknown. This is referred to as idiopathic MDS.
- Previous Cancer Treatments: Exposure to chemotherapy or radiation therapy, which are commonly used to treat cancer, can disrupt the normal function of the bone marrow and lead to secondary MDS.
- Exposure to Toxic Chemicals: Exposure to certain chemicals, particularly benzene, has been linked to the development of MDS.
- Underlying Bone Marrow Failure: The core of dysplasia is the bone marrow’s failure to produce a sufficient number of healthy, functional blood cells. This can lead to a range of symptoms, including fatigue, frequent infections, and easy bruising.
Clinical Significance
The presence of dysplastic features is a key diagnostic marker for conditions like MDS and Acute Myeloid Leukemia (AML). The symptoms experienced by a patient with MDS, such as fatigue, frequent infections, and easy bruising, are a direct result of the bone marrow’s failure to produce healthy cells. A definitive diagnosis often requires a bone marrow biopsy.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Blumenreich MS. The White Blood Cell and Differential Count. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 153.
- Farrelly, S. J., & O’Connor, K. A. (2017). Hypersegmented neutrophils and oval macrocytes in the setting of B12 deficiency and pancytopaenia. BMJ case reports, 2017, bcr2016218508. https://doi.org/10.1136/bcr-2016-218508
- Shah, S. S., Parikh, R. S., Vaswani, L. P., & Divkar, R. (2016). Familial Pelger-Huet Anomaly. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion, 32(Suppl 1), 347–350. https://doi.org/10.1007/s12288-015-0508-3
- Akyay, A., Demir Şahin, F., & Şen, A. (2020). Vacuolated Leukocytes in the Peripheral Blood Smear of a Child with Chanarin-Dorfman Syndrome. Turkish journal of haematology : official journal of Turkish Society of Haematology, 37(4), 299–300. https://doi.org/10.4274/tjh.galenos.2020.2020.0242
- Miknienė, Z. and Ivanauskaitė, R. (2018) Sex Chromatin in Peripheral Blood Neutrophils and Sex Determination. Case Reports in Clinical Medicine, 7, 55-62. doi: 10.4236/crcm.2018.71005.
- Méhes K. (1966). Nuclear projections in neutrophils. Blood, 28(4), 598–601.
- https://www.cellwiki.net/en/aberrations