TL;DR
Hemolytic disease of the fetus and newborn (HDFN) also known as erythroblastosis fetalis is an allo-immune hemolytic anemia in the fetus or newborn. This is due to a fetal-maternal incompatibility for one of the red cell blood group systems i.e. Rh blood group causing maternal IgG to cross the placenta and attach to fetal red cells which will be destroyed by the reticuloendothelial system.
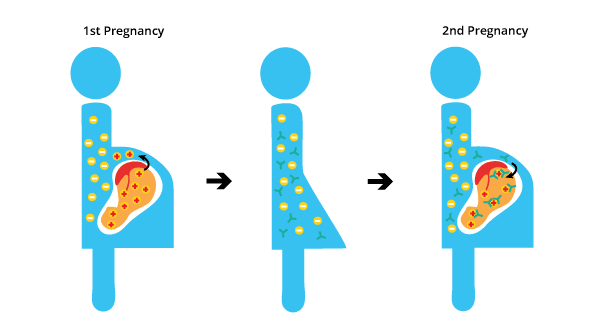
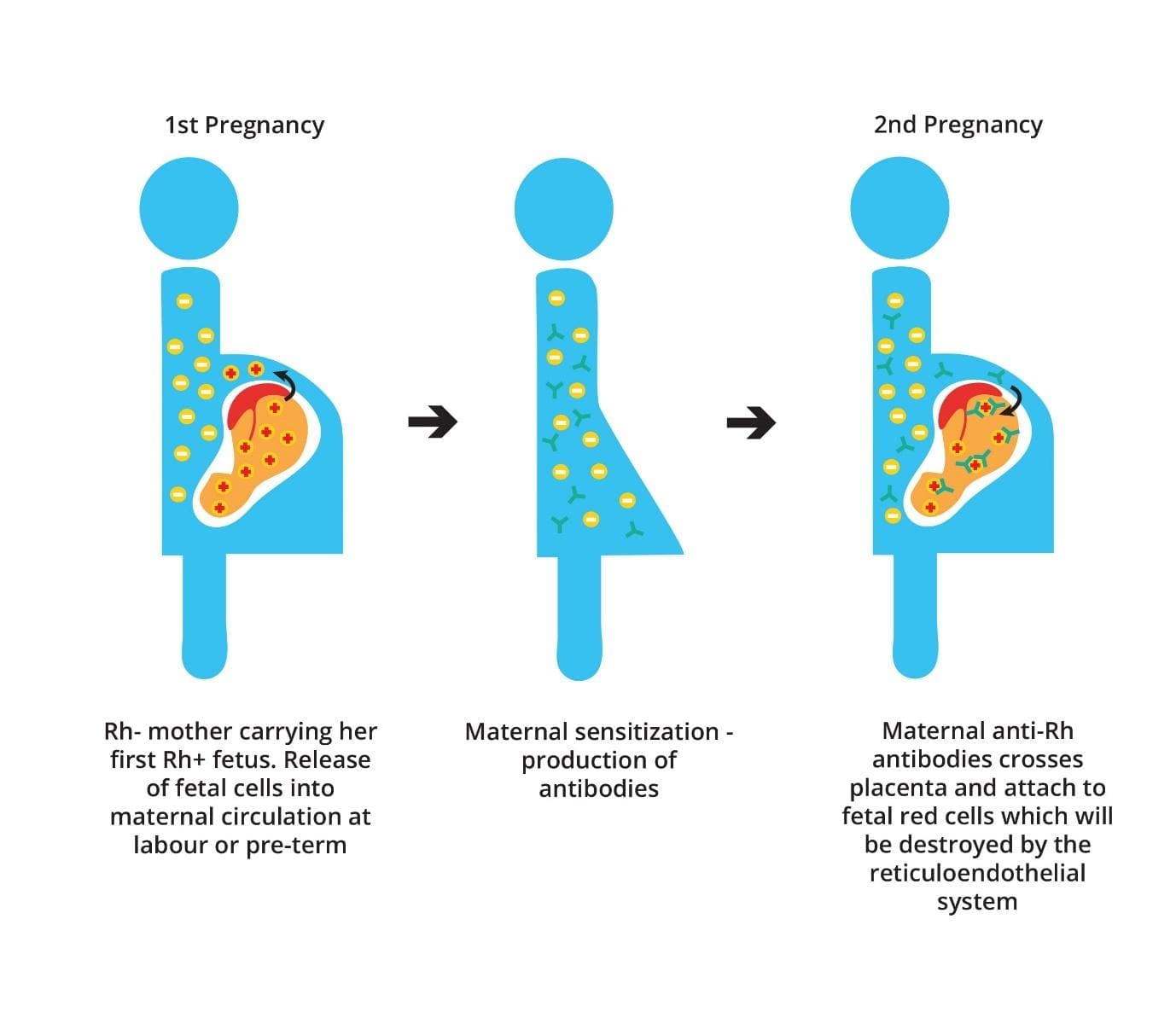
During a first pregnancy with an Rh-positive fetus, some fetal blood cells can cross the placenta into the mother’s bloodstream. This can cause the mother’s immune system to develop antibodies against the Rh-positive antigen. However, the first pregnancy is uneventful.
If the mother becomes pregnant again with another Rh-positive fetus, these antibodies can cross the placenta and attack the fetus’s red blood cells, leading to hemolytic disease of the fetus and newborn (erythroblastosis fetalis). This is known as the anamnestic response, where the immune system produces a stronger and faster antibody response in subsequent exposures to the same antigen.
Laboratory investigations of hemolytic disease of the newborn ▾
- Peripheral blood smear: Normochromic normocytic anemia with numerous nucleated red blood cells and occasional spherocytes
- DAT positive
- ↑ serum bilirubin
- ↑ LDH
- ↓ haptoglobin
- Prevention: RhIg prophylaxis
- Antenatal intervention: IUT & IVIG
- Postnatal care: Phototherapy, IVIG, exchange transfusion
*Click ▾ for more information
What is hemolytic disease of the fetus and newborn (HDFN)?
Hemolytic Disease of the Fetus and Newborn (HDFN), also known as Erythroblastosis Fetalis, is a condition where a fetus’ red blood cells are destroyed by maternal antibodies. This occurs when the mother and baby have incompatible blood types, typically involving the Rh factor or ABO blood group system.
The most common and severe form of HDN is caused by Rh (D) incompatibility, though it can also be caused by ABO incompatibility and incompatibilities with other minor blood group antigens
Blood Group System
ABO Blood Group System
The ABO blood group system is the most well-known and clinically important system for classifying human blood, after its discovery by Karl Landsteiner in the early 20th century. It’s based on the presence or absence of specific carbohydrate structures, called antigens, on the surface of red blood cells (RBCs), and corresponding antibodies in the blood plasma.
Antigens on Red Blood Cells
There are two primary antigens in the ABO system: A antigen and B antigen.
- Type A blood: Individuals have A antigens on their RBCs.
- Type B blood: Individuals have B antigens on their RBCs.
- Type AB blood: Individuals have both A and B antigens on their RBCs.
- Type O blood: Individuals have neither A nor B antigens on their RBCs (they only have a precursor antigen called the H antigen).
Antibodies in Plasma
Unlike the Rh system where antibodies are typically formed after exposure, antibodies against ABO antigens are naturally occurring in the plasma of individuals who lack the corresponding antigen. These antibodies are primarily IgM class, meaning they generally don’t cross the placenta easily.
- Type A blood: Has anti-B antibodies in their plasma.
- Type B blood: Has anti-A antibodies in their plasma.
- Type AB blood: Has neither anti-A nor anti-B antibodies in their plasma.
- Type O blood: Has both anti-A and anti-B antibodies in their plasma.
Inheritance
The ABO blood type is inherited and determined by a gene on chromosome 9 with three main alleles: A, B, and O.
- Alleles A and B are co-dominant, meaning if both are inherited (e.g., from an A parent and a B parent), both A and B antigens will be expressed (resulting in AB blood type).
- The O allele is recessive. To have Type O blood, an individual must inherit an O allele from both parents.
Clinical Significance (Transfusion)
The ABO system is critical for safe blood transfusions. If a patient receives blood with antigens they don’t have, their naturally occurring antibodies will quickly attack the transfused RBCs, leading to a severe and potentially fatal hemolytic transfusion reaction.
- Type O blood is considered the universal donor for red blood cells because its RBCs lack A and B antigens, so they won’t be attacked by anti-A or anti-B antibodies in a recipient’s plasma.
- Type AB blood is considered the universal recipient for red blood cells because individuals with AB blood have both A and B antigens, and therefore lack anti-A and anti-B antibodies, allowing them to receive blood from any ABO type.
Relevance to Hemolytic Disease of the Fetus and Newborn (Erythroblastosis Fetalis)
While less severe than Rh-hemolytic disease of the fetus and newborn (erythroblastosis fetalis), ABO incompatibility can also cause hemolytic disease of the fetus and newborn (erythroblastosis fetalis). This usually occurs when a Type O mother has a Type A or Type B fetus. The mother’s naturally occurring anti-A or anti-B antibodies (which can sometimes be IgG and thus cross the placenta, especially anti-A,B) can cause mild to moderate hemolysis in the fetus/newborn. It typically results in less severe symptoms than Rh-hemolytic disease of the newborn (erythroblastosis fetalis) and rarely leads to hydrops fetalis.
Rh Blood Group System
The Rh blood group system is a crucial classification of blood based on the presence or absence of specific proteins, called Rh antigens, on the surface of red blood cells (RBCs). It’s second only to the ABO system in clinical significance, especially in transfusion medicine and pregnancy.
Rh Antigens
While there are over 50 identified Rh antigens, the most important and clinically significant is the D antigen. This is the antigen that determines whether a person is “Rh-positive” or “Rh-negative.” Other important Rh antigens include C, c, E, and e.
Rh-Positive vs. Rh-Negative
- Rh-positive (Rh+): If your red blood cells have the D antigen, you are Rh-positive. This is the more common status.
- Rh-negative (Rh−): If your red blood cells lack the D antigen, you are Rh-negative.
Inheritance
Inheritance of Rh status is separate from ABO blood type. The presence of the D antigen is dominant. So, if an individual inherit at least one gene for the D antigen, then he/she will be Rh-positive. To be Rh-negative, an individual must inherit two copies of the gene that results in the absence of the D antigen (one from each parent).
Clinical Significance
The Rh factor is critically important because Rh-negative individuals do not naturally produce anti-Rh antibodies. However, if an Rh-negative person is exposed to Rh-positive blood (e.g., through a blood transfusion or during pregnancy), their immune system will recognize the D antigen as foreign and produce anti-D antibodies. These antibodies are primarily of the IgG class, which means they can cross the placenta. This is the basis of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) when an Rh-negative mother is sensitized to an Rh-positive fetus.
How does hemolytic disease of the fetus and newborn (erythroblastosis fetalis) happen?
Hemolytic disease of the fetus and newborn (erythroblastosis fetalis) most commonly occurs when an Rh-negative mother has an Rh-positive baby. However, it can also occur when the mother and baby have different ABO blood types.
Naturally Occurring Antibodies vs Immune Antibodies
Naturally occurring antibodies occur in the plasma of subjects who lack the corresponding antigen and who have not been transfused or been pregnant. The most important are anti-A and anti-B. They are usually IgM and react optimally at cold temperatures also known as cold antibodies.
Immune antibodies, also known as adaptive antibodies or acquired antibodies, are highly specific proteins produced by the body’s adaptive (acquired) immune system in response to a prior exposure to a foreign substance (an antigen). These antibodies are commonly IgG, although some IgM antibodies may also develop usually in the early phase of an immune response. Immune antibodies react optimally at 37oC.
Only IgG antibodies are capable of transplacental passage from mother to foetus as IgM is too big. The most important immune antibody is the anti-D. These antibodies can cross the placenta and attack the baby’s red blood cells, causing them to break down.
Pathophysiology of hemolytic disease of the fetus and newborn (erythroblastosis fetalis)
In hemolytic disease of the fetus and newborn (erythroblastosis fetalis), the mother is Rh-negative while the father is Rh-positive.
During the first pregnancy, leakage of D+ fetal red cells across the placenta results in the mother becoming immunized against the D antigen as this antigen is not naturally occurring but is produced after sensitization as the fetus is Rh-positive. However, the amount of anti-D antibody that crosses the placenta into the fetal circulation following the initial exposure is too low to cause hemolysis.
During subsequent pregnancies, the mother mounts an anamnestic immunological response to D+ fetal red cells if the fetuses inherit the Rh-positive antigen from the father unless the father is heterozygous for the D antigen and there is a 50% probability that the fetus may be Rh-negative.
The IgG alloantibody produced by the mother is small enough to cross through the placenta into the fetal circulation and binds to fetal RBCs that are D+. The IgG-sensitized fetal RBCs are cleared from the circulation by macrophages in the fetal spleen. This leads to anemia and hyperbilirubinemia resulting in hemolytic disease of the fetus and newborn (erythroblastosis fetalis).
What are the signs and symptoms of hemolytic disease of the fetus and newborn (erythroblastosis fetalis)?
The severity of hemolytic disease of fetus and newborn (erythroblastosis fetalis) can vary from mild to severe.
Mild cases of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) may cause no symptoms, while severe cases can be life-threatening. The severity of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) depends on the number of antibodies that the mother has produced and the amount of time that the baby has been exposed to the antibodies.
Antenatal (Fetal) Presentation
During pregnancy, hemolytic disease of the newborn (erythroblastosis fetalis) symptoms are typically detected through prenatal monitoring, especially in cases of known maternal sensitization.
Fetal Anemia
This is the primary consequence of red blood cell destruction.
- Increased Middle Cerebral Artery Peak Systolic Velocity (MCA-PSV): This is the most reliable ultrasound indicator of fetal anemia. As the blood becomes thinner (less viscous) due to anemia, it flows faster through the fetal cerebral arteries.
- Cardiomegaly and Heart Failure: The fetal heart has to work harder to circulate oxygen-poor blood, leading to an enlarged heart and, in severe cases, signs of heart failure.
- Hepatosplenomegaly: The fetal liver and spleen increase in size as they try to compensate for the accelerated red blood cell destruction by producing more immature red blood cells (extramedullary hematopoiesis).
Hydrops Fetalis
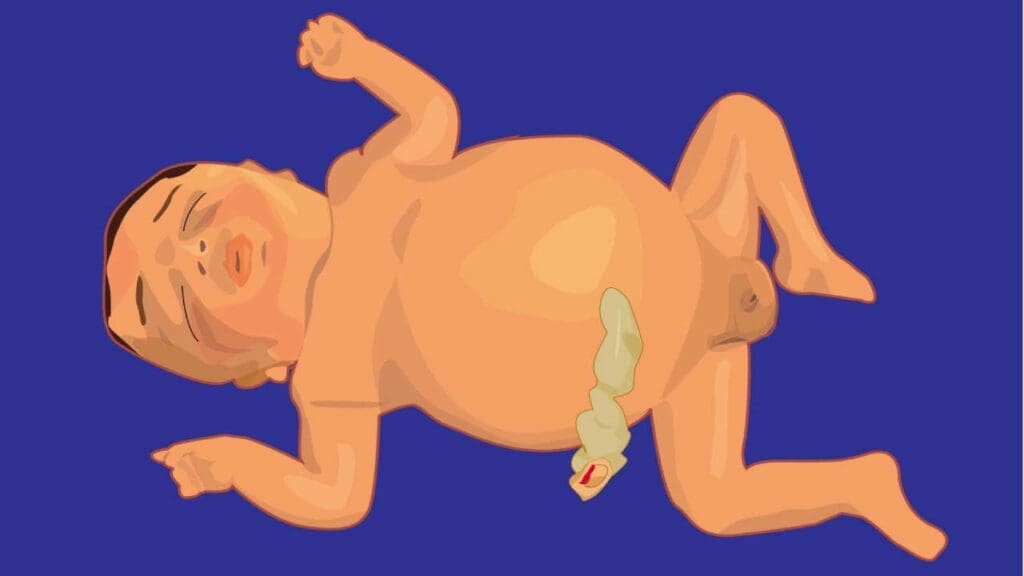
This is the most severe and life-threatening manifestation of fetal hemolytic disease of the fetus and newborn (erythroblastosis fetalis), occurring when the fetal body can no longer compensate for severe anemia and heart failure. It involves widespread fluid accumulation in at least two different fetal compartments.
- Ascites: Fluid buildup in the abdomen.
- Pleural Effusions: Fluid around the lungs.
- Pericardial Effusions: Fluid around the heart.
- Skin Edema: Generalized swelling, often visible as thickened skin.
- Polyhydramnios: Excessive amniotic fluid, often associated with hydrops.
- Thickened Placenta: The placenta may also appear enlarged and edematous on ultrasound.
- Decreased Fetal Movement: In severe cases, the fetus may become less active due to profound anemia and distress.
Postnatal (Neonatal) Presentation
After birth, the signs and symptoms of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) become more apparent as the newborn’s immature liver struggles to clear the large amount of bilirubin produced from the rapid breakdown of red blood cells.
- Jaundice: This is the most common and often the earliest postnatal sign of hemolytic disease of the fetus and newborn (erythroblastosis fetalis).
- Early Onset: Jaundice typically appears within the first 24 hours of life, which is a key indicator of a pathological cause (as opposed to physiological jaundice, which usually appears after 24-48 hours).
- Rapid Progression: The yellowing of the skin and whites of the eyes (sclera) progresses quickly and can be severe.
- Orange-Yellow Color: The jaundice in hemolytic disease of the fetus and newborn (erythroblastosis fetalis) often has a more pronounced orange-yellow hue due to the high levels of unconjugated bilirubin.
- Pallor (Anemia): The newborn may appear pale due to the ongoing destruction of red blood cells and insufficient compensation by the bone marrow. The severity of pallor correlates with the degree of anemia.
- Hepatosplenomegaly: The liver and spleen may be enlarged upon physical examination in hemolytic disease of the fetus and newborn (erythroblastosis fetalis), reflecting the increased erythropoiesis (red blood cell production) and red blood cell sequestration occurring in these organs.
- Edema: In severe cases of hemolytic disease of the fetus and newborn (erythroblastosis fetalis), particularly if hydrops fetalis was present prenatally, the newborn may still exhibit generalized swelling or edema.
- Signs of Respiratory Distress: If lung development was compromised by hydrops (pleural effusions), or if the infant is severely anemic, they may present with tachypnea, grunting, or retractions.
- Signs of Kernicterus (Bilirubin Encephalopathy): This is the most dreaded complication of severe, untreated hyperbilirubinemia in hemolytic disease of the fetus and newborn (erythroblastosis fetalis), where unconjugated bilirubin crosses the blood-brain barrier and deposits in brain tissue, causing damage. Early signs of kernicterus can be subtle and progress to more severe neurological damage:
- Initial/Early Signs
- Lethargy, extreme sleepiness, or difficulty waking.
- Poor feeding or sucking.
- Irritability.
- High-pitched cry.
- Decreased or absent startle reflex.
- Hypotonia (floppy muscle tone).
- Progressive/Late Signs
- Fever.
- Hypertonia (increased muscle tone), leading to arching of the back and neck (opisthotonus).
- Seizures.
- Abnormal eye movements, particularly an upward gaze (setting sun sign).
- Apnea (pauses in breathing).
- Long-term Sequelae of Kernicterus: If brain damage occurs, long-term complications can include:
- Cerebral palsy (especially athetoid cerebral palsy, characterized by involuntary, uncontrolled movements).
- Hearing loss or auditory neuropathy.
- Intellectual disabilities.
- Dental enamel hypoplasia.
- Gaze palsies.
- Initial/Early Signs
How is hemolytic disease of the newborn (erythroblastosis fetalis) tested?
Laboratory investigations for hemolytic disease of the fetus and newborn (erythroblastosis fetalis) are crucial for diagnosis, assessment of severity, monitoring, and guiding treatment decisions. These investigations are performed at different stages: antenatally (on the mother and fetus) and postnatally (on the newborn).
Antenatal Investigations (Maternal and Fetal)
The primary goal of antenatal labs is to identify the risk of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) and monitor the fetus for signs of severe anemia.
Maternal Screening (Initial Risk Assessment)
- ABO and RhD Blood Typing: Performed early in every pregnancy to identify RhD-negative women at risk of alloimmunization.
- Red Cell Antibody Screen (Indirect Antiglobulin Test or IAT): Checks the maternal plasma for the presence of alloantibodies (e.g., anti-D, anti-Kell, anti-c) that can cross the placenta and attack fetal red blood cells.
- Antibody Titration/Quantification: If a clinically significant antibody is detected, its concentration (titer) is measured periodically. Reaching a critical threshold (e.g., typically ≥16 for non-Kell antibodies) signals the point at which the risk of severe hemolytic disease of the fetus and newborn (erythroblastosis fetalis) is high enough to warrant close fetal monitoring (usually with MCA-PSV).
Non-Invasive Fetal Genotyping (NIFG)
This investigation represents the most significant recent advancement in hemolytic disease of the fetus and newborn (erythroblastosis fetalis) diagnostics.
A maternal blood sample is analyzed for cell-free fetal DNA (cfDNA), which is derived from the placenta and circulates in the mother’s plasma. This test is to reliably and non-invasively determine the fetal blood group antigen status (e.g., whether the fetus is RhD-positive or RhD-negative).
- Targeted Monitoring: If the mother has a dangerous antibody (e.g., anti-D) but NIFG shows the fetus is negative for that antigen (e.g., RhD-negative), further invasive monitoring or intervention is completely unnecessary, reducing patient anxiety and cost.
- Targeted Prophylaxis: In non-sensitized RhD-negative women, NIFG can determine if the fetus is RhD-positive, allowing for targeted RhIg prophylaxis only for those who need it, conserving a limited blood product.
Fetal Surveillance (Monitoring Severity)
Middle Cerebral Artery Peak Systolic Velocity (MCA-PSV) Doppler Ultrasound
This is the current gold standard for non-invasive monitoring of fetal anemia. As the fetus becomes anemic, the blood becomes less viscous. To maintain oxygen delivery, the heart pumps the thinner blood faster, especially to the brain.
A velocity measurement exceeding 1.5 Multiples of the Median (MoM) for gestational age is highly predictive of moderate to severe fetal anemia, often triggering the need for an intrauterine transfusion (IUT).
Invasive Tests (Now less frequent, primarily for confirmation or intervention)
- Cordocentesis (Percutaneous Umbilical Blood Sampling – PUBS): Used to directly sample fetal blood. This is the most definitive test as it directly measures fetal hemoglobin, hematocrit, and bilirubin levels, and is performed immediately before an IUT.
- Amniocentesis for ΔOD450: Measures the change in optical density at 450 nm in the amniotic fluid, which correlates with the concentration of bilirubin. This method has been largely supplanted by MCA-PSV due to the invasive risk.
Postnatal Investigations (Neonatal)
The goal of postnatal labs in hemolytic disease of the fetus and newborn (erythroblastosis fetalis) is to confirm the diagnosis, assess the degree of red blood cell destruction, and monitor for the risk of kernicterus.
Diagnostic Confirmation
- ABO and RhD Blood Typing (Newborn): Determines the baby’s blood group.
- Direct Antiglobulin Test (DAT) / Direct Coombs Test: This is the hallmark diagnostic test for immune-mediated hemolysis in the newborn. The test detects maternal IgG antibodies that have already coated the surface of the newborn’s red blood cells. A positive DAT confirms that the mother’s antibodies are causing red cell destruction.
Severity Assessment
- Complete Blood Count (CBC) with Reticulocyte Count:
- Hemoglobin/Hematocrit: Measures the degree of anemia caused by red cell destruction.
- Reticulocyte Count: A high count indicates the marrow is actively trying to compensate for the rapid hemolysis.
- Serum Bilirubin Levels (Total and Fractionated): Measures the amount of bilirubin produced from the breakdown of red blood cells. High levels of unconjugated bilirubin pose a risk of neurotoxicity (kernicterus). Bilirubin levels are monitored frequently and plotted on hour-specific nomograms to determine the need for interventions like phototherapy or exchange transfusion.
Differential Diagnosis of Hemolytic Disease of the Fetus and Newborn (Erythroblastosis Fetalis)
| Category | Condition | Key Pathophysiology | Typical Newborn Presentation | Key Diagnostic Test Result |
| Alloimmune (HDFN) | Rh, ABO, or Minor Antigen Incompatibility | Maternal IgG antibodies cross the placenta and destroy fetal RBCs. | Anemia, Jaundice (early onset, severe), Hepatosplenomegaly, Hydrops fetalis (severe cases). | Positive Direct Antiglobulin Test (DAT) (Newborn’s RBCs are coated with maternal IgG). |
| Intrinsic RBC Defects | RBC Enzyme Deficiencies (e.g., G6PD or Pyruvate Kinase Deficiency) | Defect in RBC metabolism makes the cells vulnerable to oxidative stress/destruction. | Jaundice (may be severe, often later onset or triggered by an external agent), Anemia. | Negative DAT, specific enzyme assay (e.g., G6PD assay), Bite cells on blood smear (G6PD). |
| RBC Membrane Defects (e.g., Hereditary Spherocytosis) | Defect in structural proteins causes RBCs to be rigid, spherical, and easily destroyed in the spleen. | Anemia, Jaundice, Splenomegaly (due to RBC destruction), Microspherocytes on blood smear. | Negative DAT, Osmotic Fragility Test or Eosin-5-Maleimide (EMA) binding test. | |
| Other Hemolytic Causes | Sepsis (Systemic Infection) | Bacterial or viral toxins/inflammation cause RBC damage (non-immune hemolysis) or associated DIC. | Jaundice, Anemia, Fever/Hypothermia, Lethargy, signs of multiorgan dysfunction. | Negative DAT, Positive Blood Culture, signs of inflammation (CRP, WBC derangements). |
| Concealed Hemorrhage (e.g., Cephalohematoma, Internal) | RBCs are sequestered outside the circulation, where they break down, leading to bilirubin load and anemia. | Anemia (often immediate), Jaundice (delayed onset as the hematoma breaks down). | Negative DAT, Anemia, localized swelling/bruising visible on imaging/exam. | |
| Non-Hemolytic Causes | Physiologic Jaundice | Normal increased RBC turnover combined with an immature liver enzyme (UGT1A1) system. | Jaundice appearing after the first 24 hours of life, peaking around day 3-5. | Negative DAT, Normal CBC (no significant anemia/hemolysis). |
| Non-Immune Hydrops Fetalis (e.g., Parvovirus B19 infection, severe Alpha-Thalassemia) | Various underlying conditions cause heart failure or low protein, leading to severe fluid leakage (hydrops) and anemia. | Hydrops (before birth/at delivery), severe anemia. | Negative DAT (rules out alloimmune cause), specific viral or genetic testing. |
How is hemolytic disease of the newborn (erythroblastosis fetalis) treated?
The treatment and management of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) are complex, ranging from prevention during the mother’s pregnancy to intensive care for the newborn. The primary goals of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) are to prevent sensitization, correct fetal anemia, and prevent neonatal neurotoxicity (kernicterus).
Prevention (Antenatal)
The most effective management strategy for hemolytic disease of the fetus and newborn (erythroblastosis fetalis) is preventing the maternal immune system from ever producing the alloantibodies.
- RhD Immune Globulin (RhIg / RhoGAM): RhIg is a concentrated preparation of anti-D antibodies. When administered to an RhD-negative mother, it binds to any RhD-positive fetal red blood cells that may have entered the maternal circulation. This action prevents the mother’s own immune system from recognizing the fetal RBCs and initiating a primary immune response (sensitization). Routinely given to RhD-negative women at ~28 weeks gestation and again within 72 hours postpartum if the newborn is RhD-positive. It is also given after any potential feto-maternal hemorrhage (e.g., trauma, amniocentesis, miscarriage).
- Targeted Prophylaxis: Advances in Non-Invasive Fetal Genotyping (NIFG) now allow clinicians in some regions to determine if the fetus is RhD-negative early in pregnancy, enabling them to withhold RhIg and avoid giving a blood product unnecessarily.
Antenatal Management (Fetal)
Once the mother is sensitized (antibodies are present), management of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) focuses on monitoring the fetus and intervening if anemia is severe.
- Monitoring via MCA-PSV Doppler: This non-invasive ultrasound technique measures the peak velocity of blood flow in the fetal middle cerebral artery. An increased velocity indicates a lower blood viscosity, which is highly predictive of moderate-to-severe fetal anemia. If the velocity exceeds a critical threshold (1.5 Multiples of the Median), it triggers the need for immediate intervention.
- Intrauterine Fetal Transfusion (IUT): This is the definitive treatment for severe fetal anemia. It prevents hydrops fetalis and fetal death. Compatible donor blood (usually O-negative, irradiated, and CMV-safe) is transfused directly into the fetal umbilical vein under ultrasound guidance. This procedure replaces the hemolyzing fetal RBCs and can be repeated multiple times throughout the pregnancy.
- Maternal IVIG (High-Risk Cases): This is used in a select group of very high-risk pregnancies (e.g., previous fetal loss or IUT before 24 weeks). The goal is to bind to immune receptors and prevent a high concentration of maternal antibodies from crossing the placenta, thereby attempting to delay the onset or severity of fetal anemia.
- Early Delivery: If MCA-PSV monitoring shows worsening anemia near term (~35−37 weeks) and the risk of continued hemolysis in the womb is greater than the risks associated with mild prematurity, delivery is often induced so the newborn can be managed directly.
Postnatal Management (Newborn)
After birth, management of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) shifts entirely to preventing brain damage (kernicterus) from hyperbilirubinemia and managing anemia.
Phototherapy for Hyperbilirubinemia
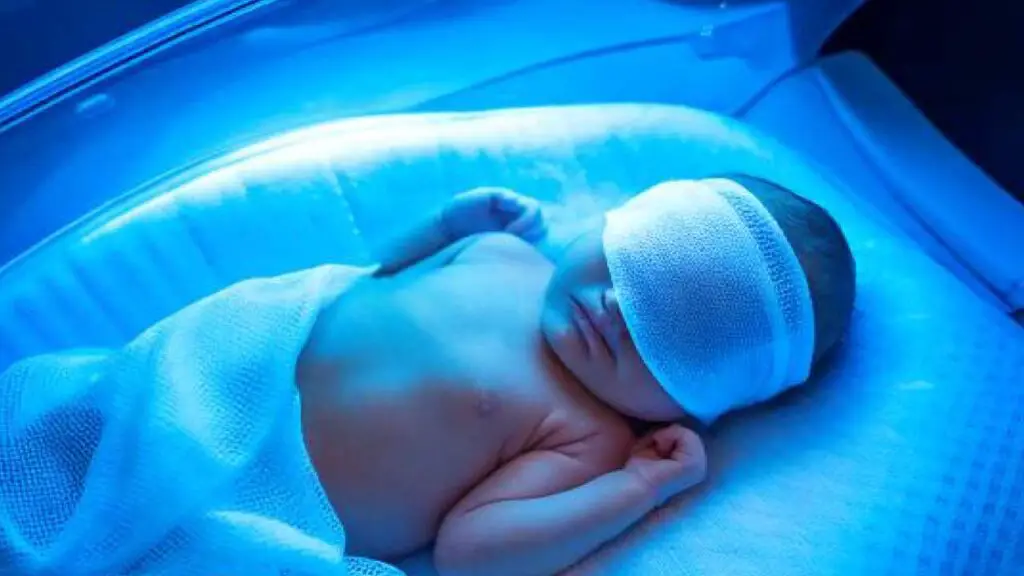
Phototherapy uses specific wavelengths of blue-green light (420-490 nm). This light is absorbed by bilirubin in the skin and converts it into water-soluble photoisomers and oxidation products (lumirubin) that can be excreted in bile and urine without requiring liver conjugation. This prevents the accumulation of toxic unconjugated bilirubin.
Treatment is initiated based on the newborn’s total serum bilirubin (TSB) levels plotted on hour-specific nomograms, considering gestational age, postnatal age, and risk factors (like positive DAT, rapid rise in bilirubin, prematurity). Intensive phototherapy is used for higher bilirubin levels.
Exchange Transfusion
This is a more invasive procedure reserved for severe hyperbilirubinemia in hemolytic disease of the fetus and newborn (erythroblastosis fetalis) when phototherapy is insufficient or for significant anemia at birth. It is indicated if:
- TSB levels reach a critical threshold, despite intensive phototherapy, and are approaching levels associated with kernicterus.
- Signs of acute bilirubin encephalopathy (kernicterus) are present.
- Severe anemia is present at birth (Hb < 10-12 g/dL, depending on the clinical scenario).
A “double volume” exchange transfusion is typically performed, where small aliquots of the newborn’s blood are incrementally removed and replaced with an equal amount of compatible donor blood (usually O-negative packed red blood cells suspended in AB plasma). This process aims to remove sensitized red blood cells, circulating maternal antibodies, and excess bilirubin, while correcting anemia.
While effective, it carries risks including electrolyte imbalances (hypocalcemia, hyperkalemia, hypoglycemia), acid-base disturbances, infection, vascular complications (e.g., thrombosis, air embolism), cardiac arrhythmias, necrotizing enterocolitis, and even death.
Intravenous Immunoglobulin (IVIG)
IVIG may be used in moderate to severe cases of ABO or Rh-hemolytic disease of the fetus and newborn (erythroblastosis fetalis) (often in conjunction with phototherapy) to reduce the need for exchange transfusion. IVIG is a preparation of pooled antibodies from human plasma. It is thought to work by saturating the Fc receptors on the newborn’s macrophages and reticuloendothelial cells, thereby blocking the destruction of antibody-coated red blood cells and reducing the rate of hemolysis.
Supportive Care
- Correction of Anemia: Besides exchange transfusion, some newborns with less severe anemia may require simple top-up blood transfusions during the first few weeks or months of life as maternal antibodies persist and continue to cause hemolysis.
- Fluid Management: Adequate hydration is important, especially during phototherapy, to facilitate bilirubin excretion.
- Respiratory Support: For newborns with hydrops fetalis or severe anemia leading to heart failure, respiratory support (oxygen, mechanical ventilation, surfactant) may be necessary.
Long-term Follow-up
Newborns treated for hemolytic disease of the newborn (erythroblastosis fetalis) require careful follow-up due to potential long-term complications.
- Anemia: May persist for several weeks or months due to ongoing hemolysis and suppression of erythropoiesis, requiring repeat CBCs and potentially further transfusions.
- Hearing Assessment: Due to the risk of bilirubin neurotoxicity, especially to the auditory pathway, all infants with severe hyperbilirubinemia should have auditory brainstem response (ABR) testing to screen for hearing loss.
- Neurological Development: Follow-up for neurological development is crucial to detect any signs of kernicterus sequelae (e.g., cerebral palsy, developmental delays).
- Liver Function: Monitoring for any persistent effects on liver function.
Frequently Asked Questions (FAQs)
What happens to a mother in erythroblastosis fetalis?
Erythroblastosis fetalis or hemolytic disease of fetus and newborn primarily affects the fetus, but it can also have implications for the mother. While the mother’s immune system is responsible for producing the anti-D antibodies that cause the condition, she typically doesn’t experience severe symptoms.
However, the mother’s body may respond to the excessive breakdown of fetal red blood cells by:
- Anemia: In severe cases of hemolytic disease of fetus and newborn (erythroblastosis fetalis), the mother’s blood may become anemic due to the loss of red blood cells from the fetus.
- Jaundice: If the mother’s liver cannot process the breakdown products of red blood cells efficiently, she may develop jaundice, characterized by yellowing of the skin and eyes.
- Other complications: In rare cases of hemolytic disease of the fetus and newborn (erythroblastosis fetalis), severe hemolysis (the breakdown of red blood cells) can lead to complications like kidney failure or liver damage.
These complications of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) are uncommon and typically occur only in severe cases of hemolytic disease of the fetus and newborn (erythroblastosis fetalis). Modern prenatal care and preventive measures like Rh immunoglobulin (RhIg) have significantly reduced the risk of severe maternal complications.
What happens if mother is Rh positive and baby is Rh negative?
If a mother is Rh-positive and her baby is Rh-negative, there is no risk of Rh incompatibility.
Rh incompatibility only occurs when the mother is Rh-negative and the baby is Rh-positive. In this case, the mother’s immune system may produce antibodies against the Rh-positive blood cells, which can cross the placenta and harm the baby.
If both mother and baby are Rh-positive or both are Rh-negative, there is no risk of Rh incompatibility.
How long does hemolytic disease of the fetus and newborn last?
The duration of hemolytic disease of the fetus and newborn (erythroblastosis fetalis) can vary depending on its severity.
In mild cases, hemolytic disease of the fetus and newborn (erythroblastosis fetalis) may resolve within a few days or weeks with supportive care, such as phototherapy to reduce jaundice. However, in more severe cases, hemolytic disease of the fetus and newborn (erythroblastosis fetalis) can last for several weeks or even months.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Hall V, Vadakekut ES, Avulakunta ID. Hemolytic Disease of the Fetus and Newborn. [Updated 2025 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557423/
- Delaney, M., & Matthews, D. C. (2015). Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematology. American Society of Hematology. Education Program, 2015, 146–151. https://doi.org/10.1182/asheducation-2015.1.146
- De Winter, D. P., Hulzebos, C., Van ‘t Oever, R. M., De Haas, M., Verweij, E. J., & Lopriore, E. (2023). History and current standard of postnatal management in hemolytic disease of the fetus and newborn. European journal of pediatrics, 182(2), 489–500. https://doi.org/10.1007/s00431-022-04724-0
- Yu, D., Ling, L. E., Krumme, A. A., Tjoa, M. L., & Moise, K. J., Jr (2023). Live birth prevalence of hemolytic disease of the fetus and newborn in the United States from 1996 to 2010. AJOG global reports, 3(2), 100203. https://doi.org/10.1016/j.xagr.2023.100203