What is beta-thalassemia?
Thalassemia is defined as the quantitative reduction of globin chains production leading to anemia. Thalassemia can be loosely classified into alpha thalassemia and beta thalassemia depending on the affected globin gene. Beta thalassemia is a genetic blood disorder that reduces the production of hemoglobin, the protein in red blood cells that carries oxygen throughout the body. Beta-thalassemia occurs due to a mutation found in the beta globin gene or the promoter region which leads to a quantitative reduction or absence of the beta globin chain production depending on the severity that the mutation has caused. Even though the production of the beta globin chain has decreased, the production of alpha globin chains is not affected and produces normally. These will lead to a lack of Hb A formation as well as an excess of free alpha globin chains. Free alpha globin chains precipitate as monomers and cause membrane damage leading to anemia as well as an increase in oxidative reactive oxygen species and thus oxidative stress in the cell.
What is the function of the beta-globin gene in hemoglobin?
The beta-globin gene is a gene that provides instructions for making a protein called beta-globin. Beta-globin is a component (subunit) of a larger protein called hemoglobin, which is located inside red blood cells. Hemoglobin is responsible for carrying oxygen from the lungs to the body’s tissues and carbon dioxide from the tissues back to the lungs.
The beta-globin gene is located on chromosome 11. Humans have two copies of the beta-globin gene, one on each chromosome 11. In adults, hemoglobin consists of four protein subunits: usually two subunits of beta-globin and two subunits of a protein called alpha-globin, which is produced from another gene called HBA.
The beta-globin gene is responsible for producing the beta-globin subunit of hemoglobin. Beta-globin is a globular protein, meaning that it folds into a three-dimensional structure. The beta-globin subunit has a pocket that can bind to an iron-containing molecule called heme. Hemoglobin can bind up to four oxygen molecules, one to each heme molecule.
When red blood cells reach the lungs, oxygen binds to the beta-globin subunits of hemoglobin. The red blood cells then travel throughout the body, delivering oxygen to the tissues. When the red blood cells reach the tissues, oxygen is released from the beta-globin subunits of hemoglobin and diffuses into the tissues.
Carbon dioxide is a waste product of metabolism. It is produced in the tissues and diffuses into the red blood cells. In the red blood cells, carbon dioxide binds to the beta-globin subunits of hemoglobin. The red blood cells then travel back to the lungs, where the carbon dioxide is released and exhaled.
The beta-globin gene is essential for life. Without the beta-globin gene, humans would not be able to produce hemoglobin and would not be able to carry oxygen to the tissues. Mutations in the beta-globin gene can lead to a variety of blood disorders, including beta thalassemia and sickle cell anemia.
How does beta-thalassemia occur?
Beta thalassemia occurs when there is a mutation in the beta-globin gene. The beta-globin gene is responsible for producing the beta-globin protein, which is one of the two proteins that make up hemoglobin, the protein in red blood cells that carries oxygen.
Mutations in the beta-globin gene can reduce or eliminate the production of beta-globin. This can lead to a deficiency of hemoglobin and anemia. The severity of beta thalassemia depends on the severity of the mutation in the beta-globin gene.
Beta thalassemia is an inherited disorder, meaning that it is passed down from parents to children. It is caused by a mutation in the beta-globin gene. This mutation can be inherited from one parent (carrier) or from both parents (carriers).
If one parent is a carrier of the beta-globin gene mutation, there is a 50% chance that each child will inherit the mutation. If both parents are carriers of the beta-globin gene mutation, there is a 25% chance that each child will have beta thalassemia major, a 50% chance that each child will have beta thalassemia minor, and a 25% chance that each child will not have beta thalassemia at all.
Beta thalassemia is most common in people of Mediterranean, Middle Eastern, and Southeast Asian descent. However, it can occur in people of any ethnicity.
Molecular classifications of beta thalassemia
The molecular classifications of beta thalassemia can be loosely classified into
- Beta thalassemia trait: Usually represents the inheritance of a single beta-thalassemia allele and the hemoglobin reduction is mild.
- Beta-thalassemia intermedia: Could involve homozygosity of compound heterozygosity for mild beta-thalassemia mutations or homozygosity or compound heterozygosity for severe mutations with the coinheritance of alpha thalassemia or heterocellular hereditary persistence of foetal hemoglobin (HPFH).
- Beta-thalassemia major: Usually represents the inheritance of homozygosity or compound heterozygosity for severe mutations.
Clinical features of beta-thalassemia
The clinical features of beta thalassemia vary depending on the severity of the condition.
Thalassaemia trait carriers are typically asymptomatic with or without mild anemia. Clinically beta thalassemia trait individuals are asymptomatic and are only picked up through a routine blood test. The alpha/beta chain ratio is not affected much thus there are minimal haematologic abnormalities.
Phenotypically, beta-thalassemia intermedia falls in between relatively severe anemia to a clinically asymptomatic phenotype. Patients with a more severe phenotype usually manifest symptoms from 2 years to 6 years of age compared to those with thalassemia major who present within the first year. These patients are able to survive without regular blood transfusions or blood transfusions may be required at longer intervals although growth and development may be retarded. Clinical phenotypes of beta-thalassaemia intermedia are caused by the ineffective erythropoiesis, chronic anemia and iron overload.
Homozygosity or compound heterozygosity for severe mutations usually presents a thalassaemia major phenotype, however, coinheritance of alpha thalassemia or HPFH ameliorates the severity to an intermediate phenotype. Beta thalassemia intermedia patients are pale and have a slightly enlarged spleen as they grow older and moderate anemia presents around 1 – 2 years of age. A lower beta globin chain production will lead to excess unpaired alpha globin chains which precipitate to cause membrane damage in the erythroblasts. This leads to ineffective erythropoiesis and moderate anemia. Signs and symptoms of beta-thalassemia are highly variable depending on the various genetic modifiers involved.
Thalassemia major refers to the severe end of the clinical spectrum and these patients are severely anemic and dependent on regular blood transfusion for survival. Beta thalassemia major patients usually present with lethargy and anaemia around 6 months of age. Children fail to thrive with intercurrent infections, hepatosplenomegaly, frontal bossing, thal facies i.e. chipmunk face and features of iron overload if not treated quickly. Without treatment, children usually die within the first decade of life. With the absence of beta globin chain production, the excess alpha globin chains will cause ineffective erythropoiesis and extravascular hemolysis in the reticuloendothelial system. Severe anemia will lead to increased medullary and extramedullary hematopoiesis.
Complications related to beta-thalassemia
The hallmark of beta-thalassemia is ineffective erythropoiesis where 10 -15% of the erythroblasts dies in the bone marrow prematurely. This leads to anemia, bone marrow expansion and extramedullary erythropoiesis.
Complications related to ineffective erythropoiesis include bone pain and fractures and hepatosplenomegaly. The high red cell turnover rate predisposes to gallstone formation. Iron overload due to increased GIT iron absorption for erythropoiesis and iron from transfused blood leads to iron deposition in visceral organs which causes heart failure, growth retardation and even delayed sexual maturity.
Clinically, the anemia caused by the premature destruction of the erythroid cells due to alpha globin chain excess induces a hyperproliferation of erythropoiesis leading to an expansion of the bone marrow. This contributes to serious deformities of the skull and long bones if untreated. A classic ‘hair-on end’ appearance on skull radiography is apparent due to marked erythroid hyperplasia. Splenomegaly results from a constant influx and destruction of abnormal red blood cells by the spleen.
Complications of severe beta-thalassemia intermedia and major include bone expansion, hypopituitarism, hypothyroidism and hypoparathyroidism, cardiomyopathy, pulmonary hypertension and embolism, hemosiderosis, liver cirrhosis and extramedullary hematopoiesis, splenomegaly, diabetes mellitus, delayed puberty and secondary sexual characteristics, osteoporosis and short stature.
Laboratory investigations for beta-thalassemia
Laboratory investigations play an important role in the diagnosis and management of beta thalassemia. The following tests are commonly used to investigate beta thalassemia:
Full blood count (FBC)
A FBC is a test that measures the number of different types of blood cells in the blood. A low red blood cell count and a low hemoglobin level are suggestive of anemia. Beta thalassemia is characterised by low hemoglobin level, low MCV and MCH and increased reticulocyte count.
Thalassemia trait has hypochromic microcytic red blood cells with or without a mild anemia, increased levels of Hb A2 and variable increases in Hb F levels.
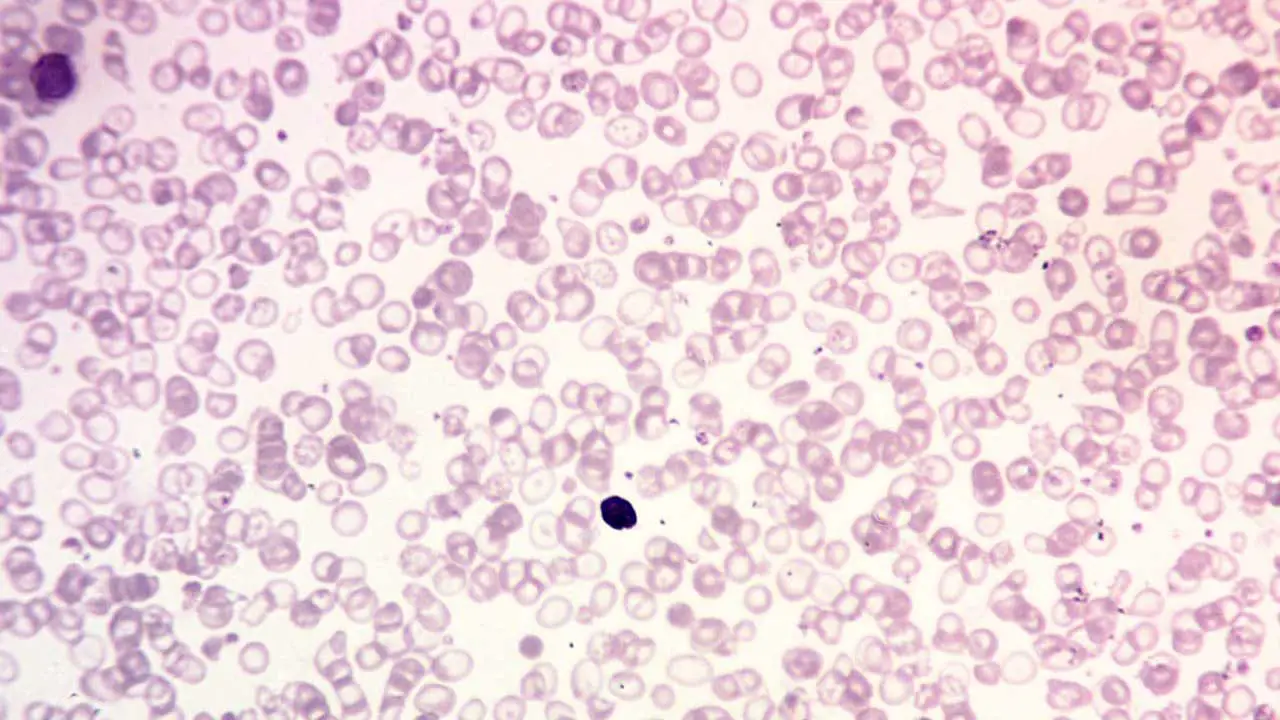
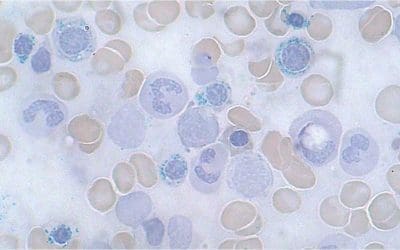
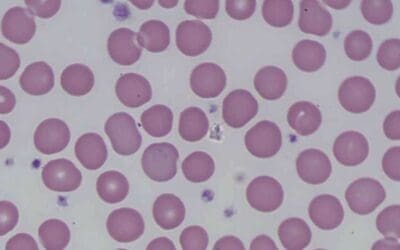
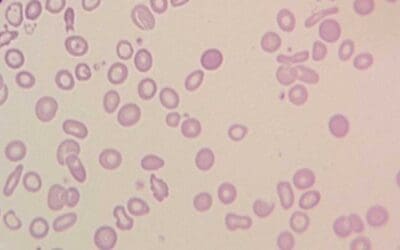
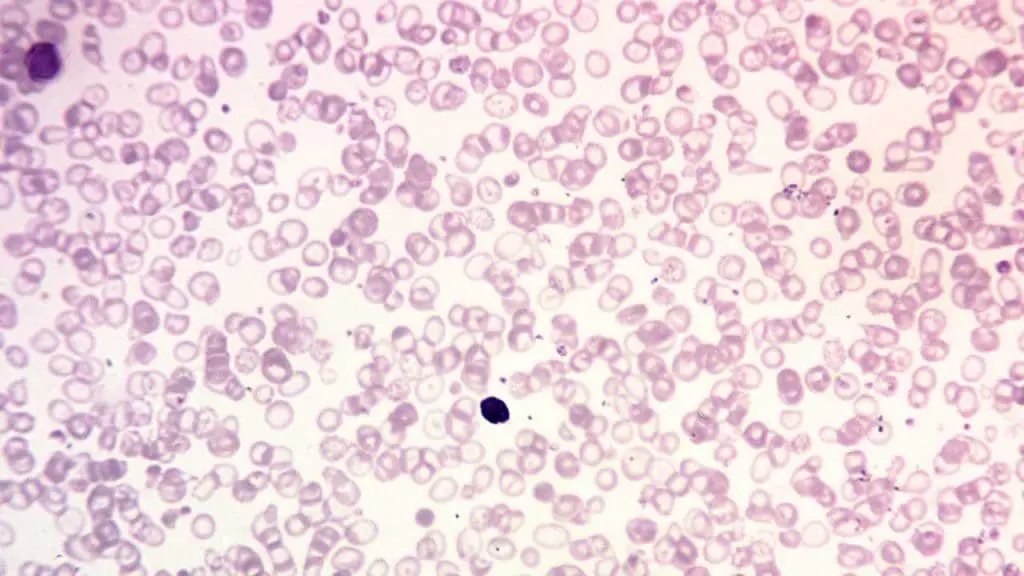
Peripheral blood smear
A peripheral blood smear is a microscopic examination of blood cells. It can be used to look for signs of anemia, such as small, pale red blood cells.
- In thalassemia trait, the peripheral blood smear shows hypochromic microcytic red cells with presence of target cells.
- Thalassemia intermedia will have moderate microcytic hypochromic anemia with reticulocytosis.
- Thalassemia major will show marked anisopoikilocytosis with numerous target cells and nucleated red cells may be seen.
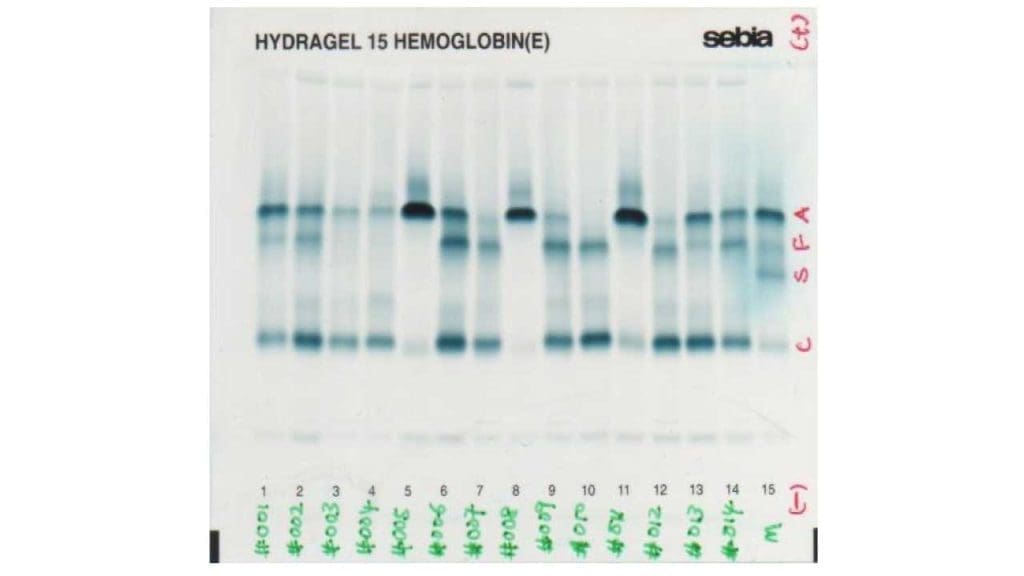
Hemoglobin electrophoresis
Hemoglobin electrophoresis is a test that is used to identify the different types of hemoglobin in the blood. In beta thalassemia, hemoglobin electrophoresis may show a decrease in the amount of normal hemoglobin (HbA) and an increase in the amount of abnormal hemoglobin (such as HbS, HbE, or HbF).
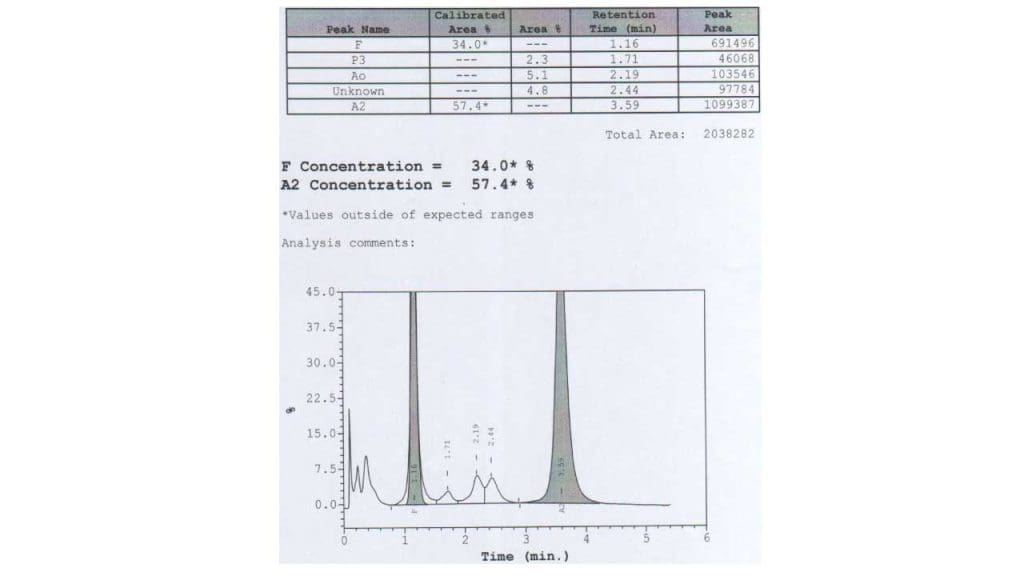
High performance liquid chromatography (HPLC)
Similar to hemoglobin electrophoresis, there will be a quantitative reduction or absence of Hb A with an increase of Hb A2 and Hb F.
DNA testing
DNA testing is the most definitive test for the diagnosis of beta thalassemia. It can be used to identify the specific mutation in the beta-globin gene that is causing beta thalassemia. DNA testing for example PCR can also be used to identify carriers of beta thalassemia, which is important for genetic counseling and prenatal diagnosis.
Other laboratory tests
Other laboratory tests that may be used to investigate beta thalassemia include:
- Iron studies: Iron studies are used to measure the amount of iron in the blood and body tissues. People with beta thalassemia are at risk for iron overload due to the regular blood transfusions that they require. There will be a marked increase in serum iron, serum ferritin and transferrin saturation level while low TIBC.
- Liver function tests: Liver function tests are used to assess the function of the liver. People with beta thalassemia are at risk for liver damage due to iron overload.
- Cardiac function tests: Cardiac function tests are used to assess the function of the heart. People with beta thalassemia are at risk for heart problems due to iron overload, anemia, and other complications of the disorder.
- Other investigations will show increased bilirubin and urobilinogen level with decreased serum haptoglobin.
How is beta-thalassemia treated?
There is no treatment necessary for beta-thalassemia trait.
As for beta-thalassemia intermedia, intermittent transfusions can be given to patients as and when needed. Iron chelation therapy can be used to prevent iron overload when the patient is older.
The first line treatment of beta-thalassaemia major is packed red cell transfusion every 2 – 4 weeks and splenectomy is indicated if there is significant splenomegaly and excessive consumption of the red cells. However, splenectomy has a higher risk of thrombotic complications and post-splenectomy sepsis thus splenectomy is recommended only in children older than 6 years old or extremely necessary.
Apart from iron accumulation due to transfusion, iron loading and overloading are common complications in thalassaemia individuals due to excess intestinal iron absorption and accumulation in the tissues rising from ineffective erythropoiesis even without transfusion. Therefore, iron chelation therapy is recommended to prevent the deleterious effects of iron overload.
As thalassaemia intermedia and major can be a debilitating disorder, the only current cure is bone marrow transplantation and as scientists and medical doctors look for better treatment and cure for this disease, gene therapy has made remarkable progress in this area.
Many countries with high frequency of beta-thalassemia run a prevention screening program while offering genetic counselling to those affected.
Key points of beta-thalassemia
Definition
A hereditary hemoglobin disorder due to reduced or absence of beta-globin chain production leading to ineffective erythropoiesis and anemia. It is predominantly an autosomal recessive gene disorder.
Epidemiology
Approximately 1.5% of the global population are β-thalassemia carriers. High prevalence in the Mediterranean, Southeast Asia, Africa, Middle East, India, Pakistan and Southern China.
Genetics of beta-thalassemia
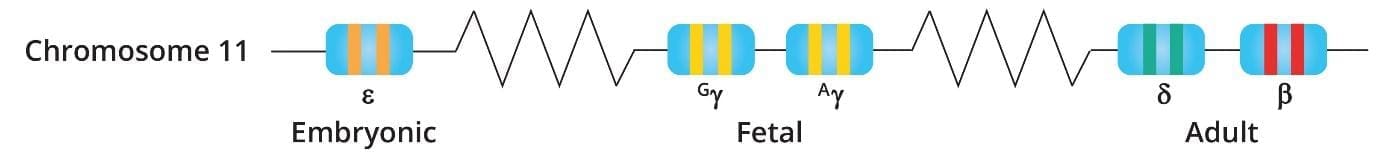
More than 400 β-thalassemia mutations have been recorded and they are mainly single nucleotide polymorphisms (SNPs), insertions or small deletions. Mutations cause impairments in transcription, RNA splicing and modifications, translations due to frame shift mutations and nonsense codons among others.
Classifications
| β-Thalassemia minor | β-Thalassemia intermedia | β-Thalassemia major | |
| Pathogenesis | E.g.: single β++ or β+ or β0 mutation | E.g.: β+/ β+ or β+/ β0 | E.g.: β0/ β0 |
| Signs and Symptoms | Mainly asymptomatic | Pallor, splenomegaly, moderate anemia presenting after the age of 1 – 2 years old | Noticeable anemia around 3 – 6 months of age, failure to thrive, intercurrent infections, mild jaundice, hepatosplenomegaly, frontal bossing, thal facies, features of iron overload |
| Pathophysiology | α/β chain ratio is almost balanced thus minimal hematologic abnormalities | Low β chain production → excess unpaired α chains → membrane damage in erythroblasts → ineffective erythropoiesis → moderate anemia and extramedullary hemopoiesis. Signs and symptoms depends on impact of various genetic modifiers involved | No β chain production → excess unpaired α chains → membrane damage in erythroblasts→ ineffective erythropoiesis and extravascular hemolysis in RE → severe anemia → medullary and extramedullary hemopoiesis |
| Laboratory investigations | FBC: Slight reduction or normal Hb level with reduced MCV and MCH. Hb A2 3.5 – 7.0%. Hb F 1.0 – 5.0%PBF: Hypochromic microcytic red cells. Presence of some target cells. | FBC: Moderate anemia (Hb 7 – 9 g/dL), variable reticulocytes count (5 – 10%).PBF: Moderate microcytic hypochromic RBCS with marked poikilocytosis including target cells.Bone marrow: erythroid hyperplasia.Splenomegaly | FBC: Severe anemia (Hb 3 – 7 g/dL), low MCV and MCH. PBF: Severe microcytic hypochromic anemia with numerous nucleated RBCs. Bone marrow: marked erythroid hyperplasia. Splenomegaly. Growth retardation, bony abnormalities if untreated. DNA mutation analysis: positive HPLC and Hb electrophoresis: No Hb A and predominantly Hb F (30 – 90%) Other investigations: ↑ bilirubin, ↑ urine urobilinogen, ↓ serum haptoglobin, markedly ↑ serum iron, serum ferritin and transferrin saturation, ↓TIBC |
| Treatment | None required | Intermittent transfusions dependent on severity | Transfusion dependent. Iron chelation therapy. Splenectomy if recommended due to splenomegaly, trapping of transfused red cells and increased transfusion requirements. Oral penicillin therapy for life following splenectomy. Cure: HLA-matched hemopoietic stem cell transplantation. |
| Management | Genetic counselling and prenatal diagnosis recommended | ||
Complications of β-thalassemia major
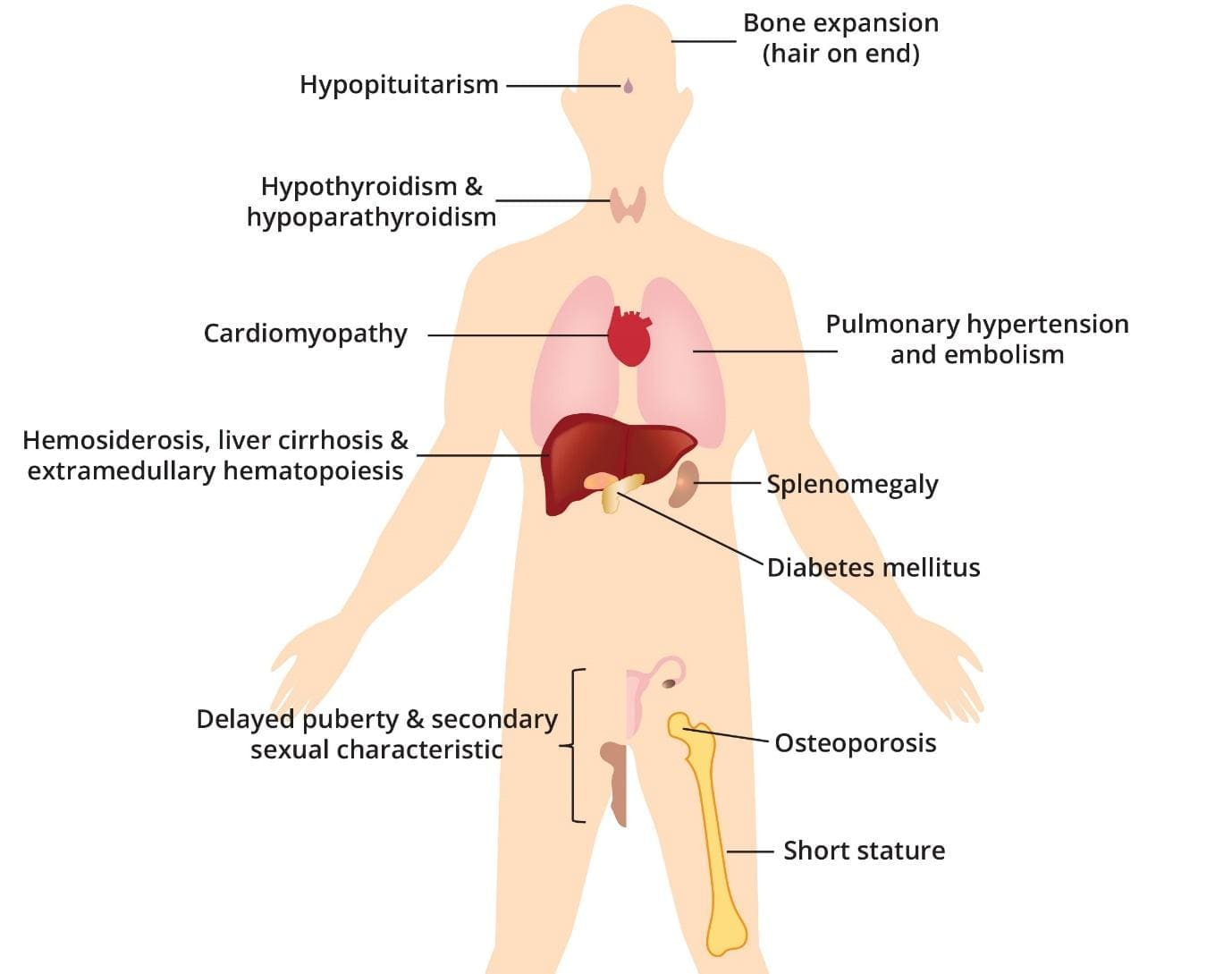
Beta-thalassemia major, a severe form of genetic anemia, results in the abnormal production of hemoglobin, leading to chronic anemia. The repeated blood transfusions required to manage anemia introduce excess iron into the body, gradually accumulating in various organs.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Weatherall D. 2003 William Allan Award address. The Thalassemias: the role of molecular genetics in an evolving global health problem. Am J Hum Genet. 2004 Mar;74(3):385-92. doi: 10.1086/381402. PMID: 15053011; PMCID: PMC1182250.
- Steinberg MH, Forget BG, Higgs DR, Weatherall DJ. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (Cambridge Medicine) 2nd Edition. 2009.
- Weatherall D. Thalassaemia: The Biography (Biographies of Disease)(OUP Oxford). 2010.