TL;DR
Von Willebrand Disorder also known as Von Willebrand Disease or VWD is an inherited coagulation disorder caused by mutations in the Von Willebrand Factor (VWF). VWF is a factor VIII carrier protein and mediates platelet adhesion to the endothelium.
- Quantitative deficiency
- Type 1: Partial deficiency. Autosomal dominant, mild disorder, most common.
- Type 3: Complete deficiency. Autosomal recessive, severe disorder.
- Qualitative defects
- Type 2: Functional abnormalities.
- Excessive bleeding from cuts, injuries, surgery, dental procedures, and menstruation
- Frequent nosebleeds
- Heavy or long menstrual periods
- Heavy bleeding during labor and delivery
- Blood in the urine or stool
- Easy bruising
- Lumpy bruises
- Prothrombin Time (PT): Normal
- Platelet Count (Plt): Normal (Exception: May be ↓ in Type 2B VWD)
- Activated Partial Thromboplastin Time (aPTT): Normal or Mildly Prolonged (Often prolonged in severe VWD or when FVIII:C is very low, like in Type 3 or Type 2N)
- Decrease: vWF assay, Ristocetin-Induced Platelet Aggregation (RIPA)
- DDAVP in mild bleeding
- VWF and Factor VIII concentrates
- Fibrinolytic inhibitors
*Click ▾ for more information
What is Von Willebrand Disorder or VWD?
Von Willebrand disorder (VWD) is the most common inherited bleeding disorder, caused by a deficiency or abnormality of Von Willebrand factor (VWF) resulting from a point mutation or major deletion.

What is Von Willebrand Factor or VWF?
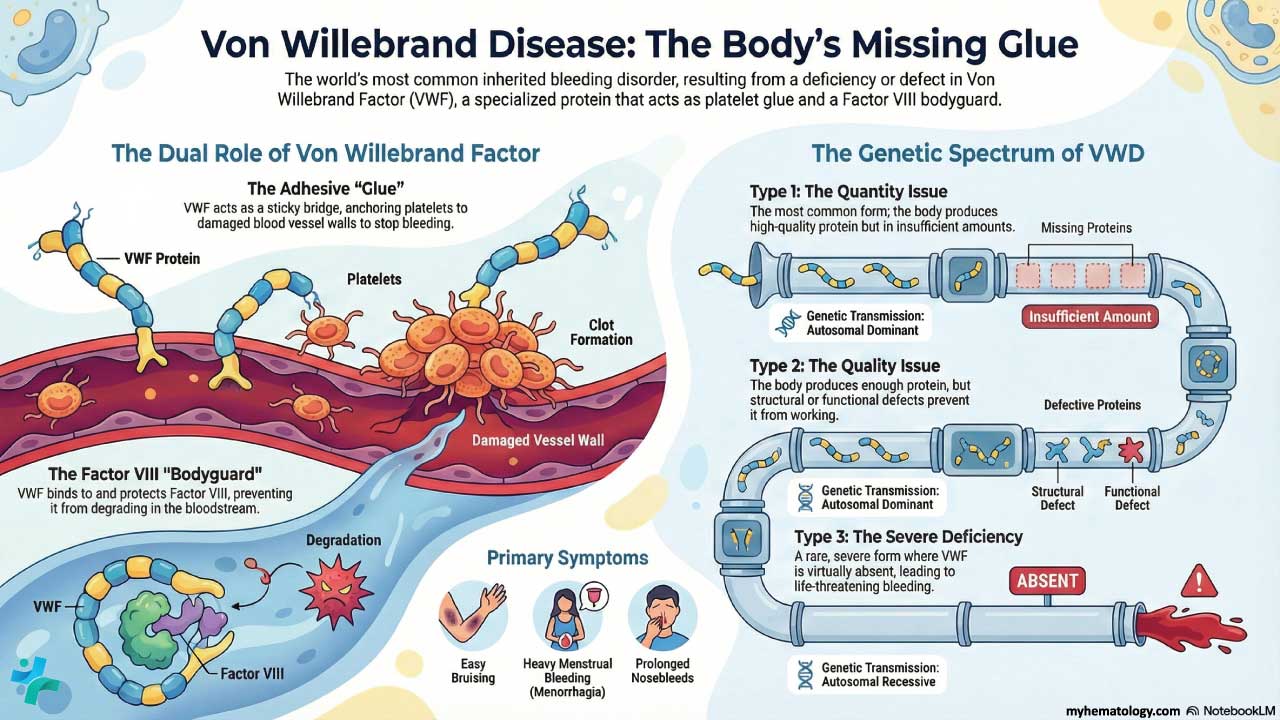
Von Willebrand factor (vWF) is a large glycoprotein that plays a vital role in hemostasis, the process by which blood clots to stop bleeding. It is encoded by the VWF gene, which is located on chromosome 12. The VWF gene is approximately 178 kilobase pairs long and contains 52 exons. VWF is synthesized as a large 300 kDa protein which then forms mutimers up to 106 in weight.
VWF is produced by endothelial cells (the cells that line the inside of blood vessels) and megakaryocytes (the cells in the bone marrow that give rise to platelets). vWF is secreted into the blood plasma, where it circulates as a multimeric protein of varying sizes. The largest VWF multimers are the most active and have the greatest ability to bind to platelets.
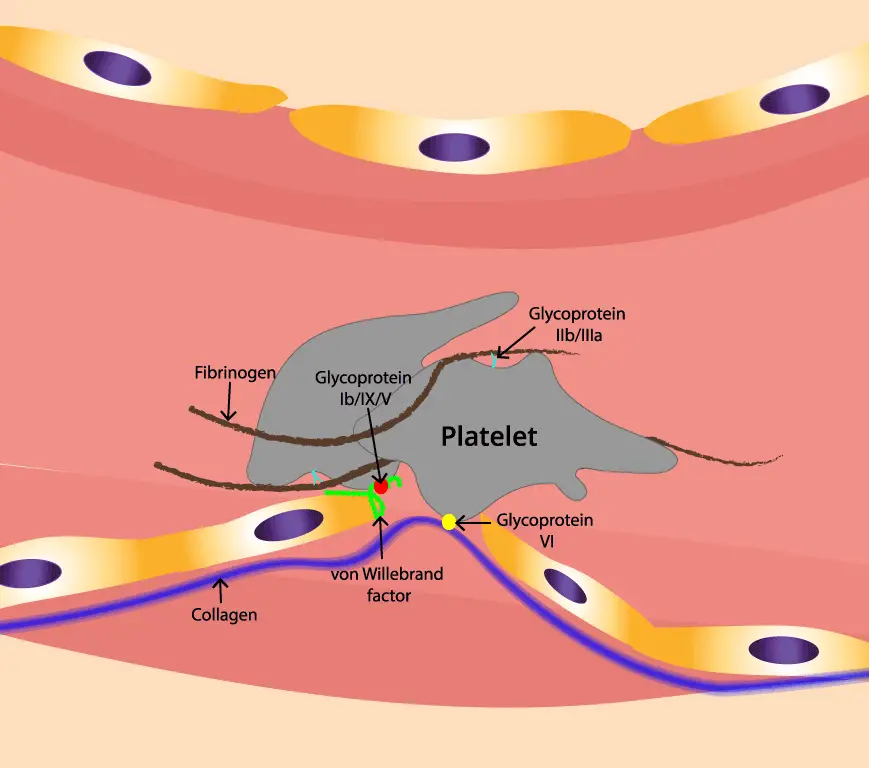
VWF has two main functions in hemostasis:
- Platelet adhesion: VWF helps platelets to adhere to each other and to the damaged blood vessel wall. This is the first step in the formation of a blood clot.
- Factor VIII stabilization: VWF binds to factor VIII, another important clotting factor, and protects it from degradation. Factor VIII is essential for the formation of thrombin, the enzyme that converts fibrinogen to fibrin, the main component of a blood clot.
Mutations in the VWF gene can cause Von Willebrand disorder (VWD), a bleeding disorder that is characterized by easy bruising, nosebleeds, and heavy menstrual periods. The severity of VWD varies depending on the type and severity of the mutation.
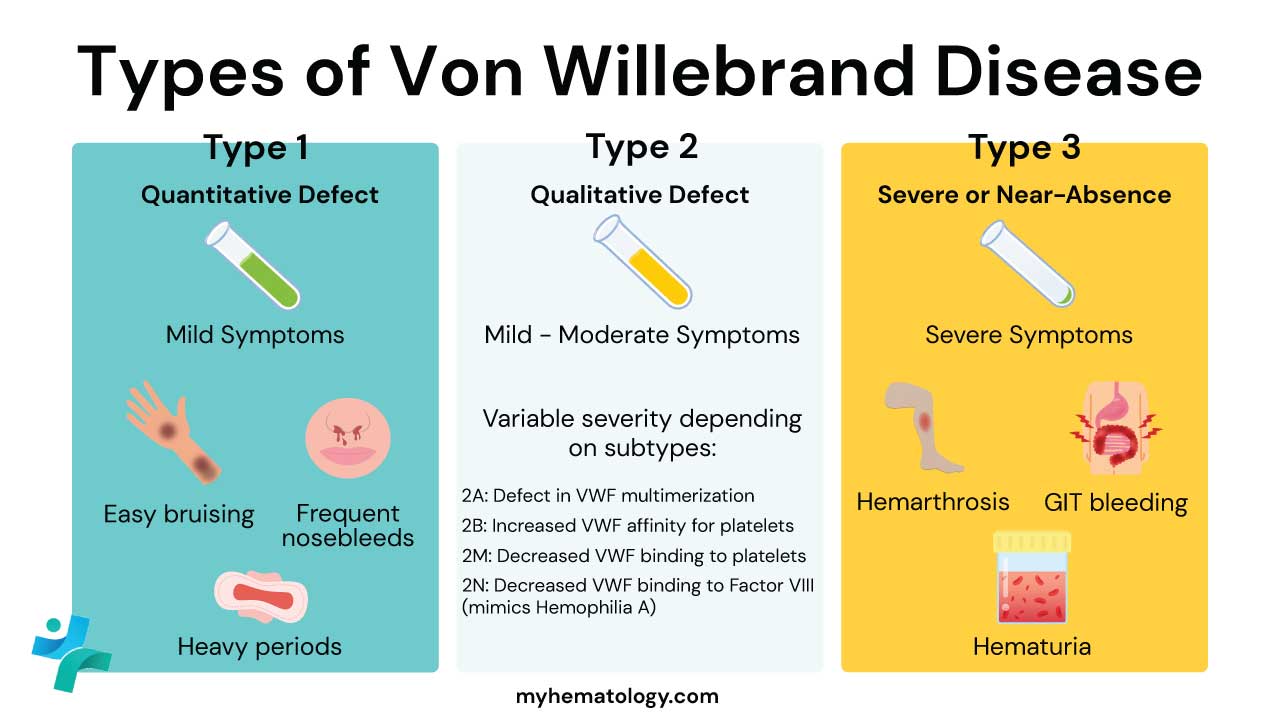
Von Willebrand Disorder (VWD) Types
Three Von Willebrand disorder (VWD) types have been described. Types 1 and 3 are caused by VWF protein deficiency, as measured by VWF antigen levels. Type 1 is by far the most common form, accounting for over 70% of affected individuals. It is inherited in an autosomal dominant manner with varying expressions of 15% – 60%. The severity of the bleeding is variable. Typically, there is mucous membrane bleeding, excessive blood loss from superficial cuts and abrasions and operative and post-traumatic hemorrhage.
In contrast to type 1 disease, levels of VWF antigen are less than 5% of normal in type 3 Von Willebrand disorder (VWD) and the inheritance pattern is autosomal recessive. Due to the fact that levels of VWF protein are so low in type 3 patients, they have much more severe bleeding and may develop hemarthrosis and other deep tissue bleeding similar to hemophilia A patients.
Type 2 disease is inherited in the same autosomal dominant manner as type 1. It accounts for about 25% of Von Willebrand disorder (VWD). In these patients, the VWF antigen and factor VIII levels are generally normal but the VWF protein is qualitatively abnormal, leading to low ristocetin cofactor activity. Type 2 disease includes several subtypes.
Most individuals with type 2A disease have defective multimerization of VWF protein thus they bleed because of failure to form large multimers which greatly impairs the ability of VWF to tether platelets to injured endothelium. In type 2B, there is also reduction in the very large multimers but due to structural mutations that increase the binding of VWF to platelet GPIb/IX. This results in clearance of large multimers from the plasma and sometimes causes mild thrombocytopenia. Type 2M is characterised by reduced binding of VWF to GPIb while in type 2N, patients inherit 2 defective copies of VWF with mutations within the N-terminal domain that abolish VWF binding to factor VIII. As a result, the VWF factor antigen and ristocetin cofactor levels are normal but the factor VIII are very low, and the patient has a clinical phenotype very similar to that of hemophilia A.
In summary,
- Type 1 is the most common type, and it is caused by a quantitative deficiency of VWF.
- Type 2 is caused by a qualitative abnormality of VWF. There are four subtypes of type 2 VWD, each with its own characteristic defect in VWF function.
- Type 3 is the rarest and most severe type, and it is caused by a complete absence of VWF.
How does Von Willebrand disorder (VWD) cause bleeding?
The pathophysiology of Von Willebrand disorder (VWD) varies depending on the type and severity of the disorder. However, all types of Von Willebrand disorder (VWD) can lead to bleeding problems because of the deficiency or dysfunction of VWF.
In type 1, the low levels of VWF impair platelet adhesion and factor VIII stabilization. This can lead to bleeding from cuts, injuries, surgery, dental work, and childbirth.
In type 2, the qualitative defects in VWF can impair platelet adhesion and factor VIII stabilization in different ways. Some people with type 2 may have bleeding problems similar to those with type 1, while others may have more severe bleeding problems.
In type 3, the complete or nearly complete deficiency of VWF leads to severe bleeding problems from even minor cuts and injuries. People with type 3 may also experience spontaneous bleeding, such as bleeding into the joints or internal organs.
The severity of bleeding problems in Von Willebrand disorder (VWD) also depends on the level of factor VIII. People with Von Willebrand disorder (VWD) often have low levels of factor VIII because VWF helps to stabilize factor VIII in the blood. Low levels of factor VIII can make the bleeding problems in Von Willebrand disorder (VWD) worse.
In addition to the bleeding problems, Von Willebrand disorder (VWD) can also lead to other complications, such as:
- Iron deficiency anemia: This can occur from chronic blood loss.
- Arthritis: This can occur from repeated bleeding into the joints.
- Pregnancy complications: Women with Von Willebrand disorder (VWD) may have an increased risk of heavy bleeding during childbirth and miscarriage.
Signs and Symptoms of Von Willebrand Disorder (VWD)
The symptoms of Von Willebrand Disease (VWD), a common inherited bleeding disorder, primarily revolve around mucocutaneous bleeding (bleeding from the mucous membranes and skin) due to the deficiency or dysfunction of Von Willebrand factor (VWF). The severity of symptoms varies greatly depending on the type and severity of the VWF defect.
Common and Mild Symptoms (Often Seen in Type 1)
Most people with VWD have Type 1, which is often mild, and they may only notice bleeding problems after surgery, injury, or dental work. Common symptoms include:
- Easy or frequent bruising (often large or lumpy bruises).
- Frequent or prolonged nosebleeds (≥5 per year, or lasting >10 minutes).
- Bleeding from the gums (e.g., after brushing).
- Prolonged or excessive bleeding from minor cuts, injuries, or after surgical procedures (including dental work).
Symptoms Affecting Women
Women often experience more noticeable symptoms due to menstruation and childbirth:
- Heavy or prolonged menstrual periods (menorrhagia):
- Soaking through a pad or tampon every 1–2 hours on the heaviest days.
- Periods lasting longer than 7 days.
- Passing blood clots larger than a quarter/grape size.
- Leading to iron deficiency anemia (low red blood cell count), causing fatigue and shortness of breath.
- Heavy bleeding after childbirth or miscarriage.
Severe Symptoms (Most Common in Type 3)
The rarest and most severe form, Type 3 VWD, involves almost no VWF and can cause bleeding similar to severe hemophilia, including:
- Spontaneous bleeding episodes (bleeding for no apparent reason).
- Bleeding into joints and muscles (hemarthrosis), causing swelling, pain, and stiffness.
- Gastrointestinal (GI) bleeding, leading to blood in the stool.
- Blood in the urine (hematuria).
How is Von Willebrand disorder (VWD) diagnosed?
Von Willebrand disorder (VWD) can be difficult to diagnose because the bleeding problems can vary in severity and can be similar to other bleeding disorders. However, there are a number of tests that can be used to diagnose Von Willebrand disorder (VWD).
The first step in diagnosing Von Willebrand disorder (VWD) is to take a medical history and perform a physical examination. Medical history must include symptoms, when they started, how often they occur, how severe they are and patient’s family history of bleeding disorders. Physical examination include signs of bleeding, such as bruises, nosebleeds, and heavy menstrual periods .
The laboratory diagnosis of Von Willebrand Disease (VWD) follows a standardized two-tiered approach to accurately determine the amount and function of the Von Willebrand Factor (VWF) and distinguish between the various subtypes.
Routine Screening Test Results (Usually Normal)
These initial coagulation tests are often normal, especially in mild VWD, but are typically included in the workup:
- Complete blood count (CBC): Platelet count is normal in this disease except for type 2B where it is low.
- Prothrombin time (PT): PT is normal in vWD (Exception: May be ↓ in Type 2B VWD).
- Activated partial thromboplastin time (aPTT): Normal or Mildly Prolonged (Often prolonged in severe VWD or when FVIII:C is very low, like in Type 3 or Type 2N)
Tier 1: Screening and Initial Evaluation
The first tier of testing is performed on all patients suspected of having VWD and measures the quantity and basic function of VWF, along with related clotting factors.
| Test Name | Abbreviation | What It Measures | Clinical Significance in VWD |
| Von Willebrand Factor Antigen | VWF:Ag | The total quantity or concentration of the VWF protein in the plasma. | Low levels indicate a quantitative deficiency (Type 1 and Type 3). |
| Von Willebrand Factor Activity* | VWF:Act | The function of the VWF protein—its ability to bind to platelets and initiate clot formation. | Low activity despite normal or near-normal VWF:Ag suggests a qualitative defect (Type 2). |
| Factor VIII Coagulant Activity | FVIII:C | The activity of clotting factor VIII, which VWF protects from degradation. | Often low, especially in Type 3 and Type 2N VWD, where the phenotype can mimic Hemophilia A. |
*VWF Activity Assays: Historically, the primary assay was VWF:RCo (Ristocetin Cofactor). However, newer, more precise assays are often used today, such as:
- VWF:GPIbM (Glycoprotein Ib binding capacity with a modified VWF)
- VWF:GPIbR (Glycoprotein Ib binding capacity with a recombinant VWF)
Tier 2: Subtyping and Confirmatory Testing
If Tier 1 results are abnormal, Tier 2 tests are performed to determine the specific VWD subtype, which is essential for guiding appropriate treatment (e.g., the contraindication for Desmopressin in Type 2B).
VWF Multimer Analysis
This is a critical test for diagnosing Type 2 VWD. It uses gel electrophoresis to visualize the different sizes (multimers) of the VWF protein.
Normal plasma contains a full range of large, medium, and small multimers. The absence of the high molecular weight (HMW) multimers is the hallmark finding in several Type 2 subtypes (e.g., Type 2A and Type 2B), indicating defective VWF assembly or increased clearance.
Ristocetin-Induced Platelet Aggregation (RIPA)
This functional test specifically examines the interaction between a patient’s platelets and VWF in the presence of the antibiotic Ristocetin.
It is the key test to differentiate Type 2B VWD. In Type 2B, the VWF has an increased affinity for platelets, resulting in enhanced aggregation at low concentrations of Ristocetin, a characteristic finding that dictates specific treatment avoidance.
VWF Binding Assays
These specialized tests focus on specific functions that may be defective in Type 2.
- VWF:CBA (Collagen-Binding Assay): Measures VWF’s ability to bind to collagen, often defective in Type 2A.
- VWF:FVIIIB (Factor VIII Binding Assay): Measures VWF’s ability to bind and protect Factor VIII. If this is low, it confirms Type 2N VWD (Normandy), which is important for distinguishing it from mild Hemophilia A.
Genetic Testing
Gene sequencing of the VWF gene is not a routine first-line test but is essential for:
- Confirmation of Type 2N and Type 3 VWD.
- Resolving ambiguous phenotypes.
- Genetic counseling for family planning.
Expected Laboratory Results in Von Willebrand Disease
| VWD Type | VWF Antigen (VWF:Ag) (Quantity) | VWF Activity (VWF:Act or VWF:RCo) (Function) | FVIII Coagulant Activity (FVIII:C) | VWF Activity/Antigen Ratio (VWF:Act/Ag)* | VWF Multimer Analysis |
| Normal | N (typically 50−200 IU/dL) | N | N | ≈1.0 | N (Full range of multimers) |
| Type 1 (Partial Quantitative Defect) | ↓ (Proportionally) | ↓ (Proportionally) | ↓ or N | ≥0.7 | N |
| Type 2A (Qualitative Defect – Multimerization) | N or ↓ | ↓↓ | N or ↓ | <0.7 | Absence of HMW Multimers |
| Type 2B (Qualitative Defect – Gain of Function) | N or ↓ | ↓↓ | N or ↓ | <0.7 | Absence of HMW Multimers |
| Type 2M (Qualitative Defect – Binding) | N or ↓ | ↓↓ | N or ↓ | <0.7 | N (HMW Multimers are present) |
| Type 2N (Qualitative Defect – FVIII Binding) | N or ↓ | N or ↓ | ↓↓ | ≥0.7 | N |
| Type 3 (Complete Quantitative Defect) | Undetectable/Absent | Undetectable/Absent | ↓↓ (Severe) | N/A | Absent |
Key:
- ↓: Decreased or Low
- ↓↓: Markedly Decreased or Very Low
- N: Normal
- N/A: Not Applicable / Undetectable
Key Tier 2 Confirmatory Test Results
| VWD Subtype | Confirmatory Test Finding | Rationale |
| Type 2B | Low-Dose RIPA (Ristocetin-Induced Platelet Aggregation) is Increased | Due to the VWF gaining a function that causes it to bind platelets more aggressively (hypersensitivity to Ristocetin). |
| Type 2N | VWF:FVIIIB (Factor VIII Binding) is Markedly Decreased | Confirms the specific defect in VWF’s ability to protect Factor VIII. |
| Type 1C (High Clearance) | VWFpp/VWF:Ag Ratio is Elevated (>3.0) | Indicates a normal amount of newly synthesized VWF propeptide (VWFpp) but rapid loss of mature VWF from circulation. |
Differential Diagnosis of VWD
| Condition | Primary Mechanism | Inheritance | Bleeding Pattern | Activated Partial Thromboplastin Time (aPTT) | Factor VIII Activity (FVIII:C) | VWF Activity (VWF:RCo) / Antigen (VWF:Ag) | Platelet Count |
| Von Willebrand Disease (VWD) | Deficiency or dysfunction of von Willebrand factor (VWF). | Autosomal Dominant (Types 1, 2) or Recessive (Type 3). | Primarily mucocutaneous (nosebleeds, easy bruising, heavy periods, GI bleeding). Joint/muscle bleeding in severe (Type 3) cases. | Normal (Type 1, 2) to Prolonged (Type 3, Type 2N) | Decreased (due to VWF clearance/binding FVIII) | Decreased VWF:Ag and/or VWF:RCo. VWF:RCo/VWF:Ag ratio < 0.7 in Type 2. | Usually Normal (Thrombocytopenia can occur in Type 2B). |
| Hemophilia A (Classic Hemophilia) | Deficiency of Clotting Factor VIII (FVIII). | X-linked Recessive (almost exclusively affects males). | Primarily deep tissue bleeding (hemarthrosis, muscle hematomas), less mucocutaneous bleeding. | Prolonged (proportional to FVIII deficiency). | Markedly Decreased | Normal | Normal |
| Platelet Function Disorders (e.g., Glanzmann’s thrombasthenia) | Defect in platelet adhesion or aggregation (platelets don’t stick properly). | Autosomal Recessive | Severe mucocutaneous bleeding (similar to VWD, often more severe). | Normal | Normal | Normal | Normal or Normal (but function is abnormal) |
| Thrombocytopenia | Low number of Platelets. | Variable (e.g., ITP, TTP, drug-induced). | Petechiae, purpura, immediate bleeding from superficial cuts/trauma. | Normal | Normal | Normal | Low |
| Type 2N VWD | Qualitative defect where VWF cannot bind Factor VIII effectively. | Autosomal Recessive | Mucocutaneous bleeding, but may have joint/muscle bleeding due to very low FVIII. | Prolonged | Markedly Decreased (mimics mild Hemophilia A) | VWF:Ag/RCo are usually Normal. Distinguishing test: VWF:FVIIIB (VWF-Factor VIII binding) is decreased. | Normal |
How is Von Willebrand disorder (VWD) treated?
The treatment and management of Von Willebrand Disease (VWD) is highly individualized, depending on the type and severity of the disease, and the specific clinical situation (e.g., managing a bleed, preventing bleeding during surgery, or treating heavy menstrual bleeding).
The goal of treatment is to increase or replace the deficient/defective von Willebrand Factor (VWF) and/or Factor VIII (FVIII).
Primary Treatment Agents
Desmopressin (DDAVP)
Desmopressin is a synthetic hormone that acts by stimulating the release of stored, endogenous VWF and FVIII from the endothelial cells lining the blood vessels. This provides a temporary, natural boost to the patient’s clotting factors. Typically given intravenously (IV) or subcutaneously (SC). A nasal spray formulation is also available for some patients.
It is the first-line therapy for most patients with Type 1 VWD (mild/moderate deficiency). It may also be effective for some patients with Type 2A or 2M VWD, but its efficacy must be confirmed by a trial dose.
It is generally contraindicated in Type 2B VWD because the VWF in this subtype has an enhanced affinity for platelets. Releasing more of this abnormal VWF can trigger widespread platelet binding, leading to a temporary drop in platelet count (thrombocytopenia) and potentially worsening the bleeding or causing thrombosis.
VWF and Factor VIII Concentrates
These are products infused directly into a vein to replace the missing or dysfunctional VWF and FVIII.
- Plasma-Derived Concentrates (pd-VWF/FVIII): Derived from human plasma, these products contain both VWF and FVIII. They are virally inactivated to minimize infection risk.
- Recombinant VWF (rVWF): A newer, genetically engineered product that contains VWF but no plasma. This eliminates the risk of transmitting human blood-borne pathogens and is especially useful as it allows for independent dosing of VWF.
- Indication:
- Essential for managing and preventing bleeds in patients with Type 3 VWD (severe deficiency).
- The treatment of choice for Type 2B VWD (where DDAVP is contraindicated).
- Used in Type 1 VWD patients who do not respond adequately to DDAVP.
- Used for major surgery or significant trauma across all types when DDAVP is insufficient.
Adjunctive/Supportive Therapies
These medications help stabilize the clot once it has formed and are often used in combination with DDAVP or concentrates.
| Drug Class | Examples | Action | Common Use |
| Antifibrinolytics | Tranexamic acid (TXA), Aminocaproic acid (Amicar) | Prevent the natural breakdown of blood clots (fibrinolysis), thereby stabilizing the clot at the wound site. | Treating or preventing bleeding in high-fibrinolytic areas, such as dental procedures, nosebleeds (epistaxis), and heavy menstrual bleeding. |
| Hormonal Therapy | Oral contraceptives (Birth Control Pills) | The estrogen in these pills can increase endogenous VWF and FVIII levels. | Management of heavy menstrual bleeding (menorrhagia) in women with VWD. |
| Topical Agents | Fibrin Sealant, Thrombin Spray | Applied directly to a wound or site of bleeding (e.g., in the mouth or nose) to promote local clotting. | Minor mucosal bleeds, or during surgical procedures. |
General Management Strategies
- Avoid Antiplatelet Medications: Patients should avoid aspirin, ibuprofen (Advil/Motrin), and naproxen (Aleve) unless specifically recommended by their hematologist, as these drugs inhibit normal platelet function and can worsen bleeding. Acetaminophen (Tylenol) is generally a safe alternative for pain and fever.
- Elective Procedures: All patients should have a full treatment plan, including dosing and factor level targets, devised by their hematologist before any surgery or invasive procedure (including dental work).
- Specialized Care: VWD patients are often best managed at a Hemophilia Treatment Center (HTC), which offers comprehensive care from a team of hematologists, nurses, social workers, and physical therapists.
- Medical Alert: Wearing a medical identification bracelet or carrying a medical alert card is strongly recommended to ensure appropriate treatment in an emergency.
With proper management, most people with VWD can live long and healthy lives.
Frequently Asked Questions (FAQs)
What is the life expectancy of someone with Von Willebrand Disease?
People with Von Willebrand disorder (VWD) generally have a normal life expectancy, especially those with the most common and mild form, type 1. This is because Von Willebrand disorder (VWD) is a manageable bleeding disorder, and with proper treatment, people with Von Willebrand disorder (VWD) can live long and healthy lives.
However, there are different types of Von Willebrand disorder (VWD), and the severity of symptoms can vary. In rare cases, people with severe VWD may experience life-threatening bleeding episodes.
Is Von Willebrand disorder (VWD) painful?
Von Willebrand disorder (VWD) itself doesn’t directly cause pain, but it can lead to symptoms that can be painful, such as:
- Bleeding into joints and muscles: This is a less common symptom but can occur in some people with von Willebrand disorder (vWD), especially those with the more severe type 3. This bleeding can cause swelling, stiffness, and pain in the affected joint or muscle.
- Heavy menstrual periods: This can be a significant issue for some women with Von Willebrand disorder (VWD) and can cause pain and discomfort.
Is Von Willebrand disorder (VWD) life threatening?
In most cases, Von Willebrand disorder (VWD) is not life-threatening. The most common type, type 1 Von Willebrand disorder (VWD), is usually mild and doesn’t cause life-threatening bleeding. Even with other types, proper management through treatment significantly reduces the risk.
However,
- Uncontrolled bleeding can be life-threatening: While rare, severe bleeding episodes in any person, regardless of Von Willebrand disorder (VWD), can become life-threatening if not addressed promptly.
- Severity of Von Willebrand disorder (VWD) matters: People with severe forms of Von Willebrand disorder (VWD) (types 2 and 3) are more prone to experiencing heavy, prolonged, or internal bleeding that can be life-threatening if left untreated.
- Certain situations increase risk: Situations like surgery, childbirth, or major injuries can increase the risk of life-threatening bleeding in individuals with Von Willebrand disorder (VWD), even those with mild forms.
Can people with Von Willebrand disorder (VWD) have babies?
Yes, women with Von Willebrand disorder (VWD) can have healthy pregnancies and births. However, due to the potential for bleeding complications, pre-conceptional counseling and close monitoring throughout pregnancy are essential.
- Von Willebrand disorder (VWD) Types and Pregnancy Risk
- Type 1: Most common and mildest form. VWF levels often increase during pregnancy, minimizing bleeding risk.
- Type 2: More severe bleeding symptoms. VWF levels may not rise significantly, requiring closer monitoring and potential treatment adjustments.
- Type 3: Rarest and most severe. VWF levels remain low, and factor replacement therapy is likely necessary throughout pregnancy.
- Increased Bleeding Risks During Pregnancy
- Vaginal bleeding: More common in the third trimester, especially with type 3 Von Willebrand disorder (VWD).
- Postpartum hemorrhage (PPH): Increased risk for all Von Willebrand disorder (VWD) types due to physiological changes after delivery.
- Management Strategies
- Pre-pregnancy counseling: Discussing Von Willebrand disorder (VWD) severity, potential risks, and individualized management plans with a hematologist familiar with Von Willebrand disorder (VWD) and obstetrics.
- vWF level monitoring: Regular monitoring of VWF activity throughout pregnancy to assess potential need for factor replacement therapy.
- Delivery planning: Minimizing invasive procedures (forceps, vacuum) and considering prophylactic factor replacement therapy around delivery, especially for types 2 & 3.
- Multidisciplinary team approach: Collaboration between hematologist, obstetrician, and potentially anesthesiologist for optimal care.
- Fetal Considerations
- Risk of inheriting Von Willebrand disorder (VWD): Offspring have a 50% chance of inheriting the condition, but genetic counseling can provide further information.
Can Von Willebrand disorder (VWD) affect the baby?
Yes, Von Willebrand disorder (VWD) can potentially affect a baby in a few ways, but the risk depends on the severity of the mother’s Von Willebrand disorder (VWD) and the specific type.
Risk of inheriting Von Willebrand disorder (VWD)
- Babies born to a parent with Von Willebrand disorder (VWD) have a 50% chance of inheriting the condition. However, the severity of the inherited Von Willebrand disorder (VWD) can vary.
Bleeding complications during pregnancy
- Mothers with Von Willebrand disorder (VWD), particularly those with severe types (2 & 3), have a higher risk of excessive bleeding during pregnancy, delivery, and postpartum. This can potentially lead to complications like:
- Anemia: If blood loss is significant, the mother might develop iron deficiency anemia, which can affect the baby’s growth and development.
- Premature birth or low birth weight: In severe cases, excessive blood loss can compromise blood flow to the baby, potentially leading to these complications.
What not to do with Von Willebrand disorder (VWD)?
Medications
- Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen and aspirin: These medications can further inhibit platelet function and worsen bleeding tendencies in VWD patients. Consider alternative pain relievers like acetaminophen.
- Antiplatelet medications: Drugs like clopidogrel (Plavix) and ticagrelor (Brilinta) are typically contraindicated due to their additional inhibition of platelet function, which can exacerbate bleeding in VWD.
Procedures
- Unnecessary invasive procedures: Practices like unnecessary biopsies, venipunctures, or intramuscular injections can increase bleeding risk. Explore alternative non-invasive options whenever possible.
- Uncontrolled blood pressure: Elevated blood pressure can put additional stress on already weakened blood vessels in VWD patients, potentially increasing bleeding risk. Ensure proper blood pressure management.
Activities
- High-contact sports and activities: Activities with a high risk of falls or injuries, like contact sports (rugby, football), should be approached with caution and discussed individually with the patient. Alternative lower-risk activities might be encouraged.
Other
- Smoking and alcohol: These habits can negatively impact overall health and potentially worsen bleeding tendencies. Encourage smoking cessation and moderate alcohol consumption.
- Ignoring warning signs: Educate patients to recognize and report any unusual bleeding symptoms promptly, such as prolonged nosebleeds, excessive menstrual bleeding, or bleeding into joints or muscles.
Can I donate blood if I have Von Willebrand disorder (VWD)?
Generally, people with Von Willebrand disorder (VWD) are not recommended to donate blood. This is due to a few key reasons:
- Risk of bleeding complications: Individuals with Von Willebrand disorder (VWD) already have a tendency to bleed due to a deficiency or malfunction of the Von Willebrand Factor, a protein crucial for blood clot formation. Donating blood further reduces their blood volume and potentially exacerbates this bleeding risk.
- Donor safety: The blood donation process itself involves needle punctures, which can increase the risk of bleeding at the puncture site for individuals with Von Willebrand disorder (VWD).
- Blood product quality: Because of the potential presence of low levels of Von Willebrand Factor (VWF) in the donated blood, it might not be suitable for all transfusion recipients, especially those who also have bleeding disorders.
Does Von Willebrand disorder (VWD) worsen with age?
Von Willebrand disorder (VWD) itself doesn’t necessarily worsen with age. VWF levels may increase with age in some, particularly those with type 1, but this doesn’t always translate to reduced bleeding risk. Individuals with severe forms (types 2 & 3) are unlikely to experience significant improvement in symptoms with age.
Does Von Willebrand disorder (VWD) make you tired?
Von Willebrand disorder (VWD) itself doesn’t directly cause fatigue. However, it can lead to situations that can contribute to fatigue in some individuals, such as:
- Iron deficiency anemia: This is a common complication of heavy menstrual bleeding, especially in women with Von Willebrand disorder (VWD). Iron deficiency can lead to symptoms like fatigue, weakness, shortness of breath, and pale skin.
- Frequent bleeding episodes: Even if not severe, repeated bleeding episodes can lead to iron loss and contribute to fatigue, especially if iron stores are not adequately replenished.
- Pain: Von Willebrand disorder (VWD) can sometimes cause painful symptoms like bleeding into joints and muscles, which can disrupt sleep and contribute to fatigue.
- Psychological stress: The burden of managing a chronic condition like Von Willebrand disorder (VWD), including potential anxieties about bleeding episodes or limitations in activities, can contribute to psychological stress and potentially lead to fatigue.
At what age is Von Willebrand disorder (VWD) diagnosed?
- Men: Studies suggest an average diagnosis age of around 30-40 years.
- Women: Due to menstrual bleeding being a common symptom, women are diagnosed earlier, on average between 12 and 50 years old.
- Children: While less common, Von Willebrand disorder (VWD) can be diagnosed in children as well, especially if they experience frequent nosebleeds, prolonged bleeding from cuts, or excessive bleeding after procedures like tooth extraction.
Overall, there’s no single definitive age for VWD diagnosis.
Is it harder to get pregnant with Von Willebrand disorder (VWD)?
No, Von Willebrand disorder (VWD) itself doesn’t necessarily make it harder to get pregnant. In fact, many women with Von Willebrand disorder (VWD) have successful pregnancies. However, there are some factors to consider:
- Von Willebrand disorder (VWD) type:
- Type 1 (most common): Fertility is usually not affected. VWF levels may even increase during pregnancy, further reducing bleeding risk.
- Type 2 & 3 (more severe): While conception is possible, there might be a higher risk of miscarriage or bleeding complications due to lower VWF levels.
- Hormonal therapy: Medications used to regulate heavy menstrual bleeding, a common symptom in some women with Von Willebrand disorder (VWD), can interfere with ovulation and make conception more challenging.
Can you have Von Willebrand disorder (VWD) if your parents don’t?
While Von Willebrand disorder (VWD) is typically inherited from parents, it’s possible to have it even if your parents don’t, due to a phenomenon called sporadic mutations. In rare cases, Von Willebrand disorder (VWD) can occur due to a spontaneous genetic mutation in the egg or sperm cell, not inherited from either parent. This is known as a de novo mutation. These mutations can happen during cell division and are not present in the parents’ genes. Such sporadic mutations account for approximately 10-30% of Von Willebrand disorder (VWD) cases.
Is Von Willebrand disorder (VWD) curable?
No, there is currently no cure for Von Willebrand disorder (VWD). However, it is a treatable and manageable condition.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Kaushansky K, Levi M. Williams Hematology Hemostasis and Thrombosis (McGraw-Hill). 2017.
- Yawn BP, Nichols WL, Rick ME. Diagnosis and Management of Von Willebrand Disease: Guidelines for Primary Care. Am Fam Physician. 2009;80(11):1261-1268.
- Sabih A, Babiker HM. Von Willebrand Disease. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459222/
- Seidizadeh, O., Eikenboom, J.C.J., Denis, C.V. et al. von Willebrand disease. Nat Rev Dis Primers 10, 51 (2024). https://doi.org/10.1038/s41572-024-00536-8
- Castaman, G., Goodeve, A., Eikenboom, J., & European Group on von Willebrand Disease (2013). Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica, 98(5), 667–674. https://doi.org/10.3324/haematol.2012.077263