TL;DR
Multiple myeloma is a cancer of plasma cells, a type of white blood cell that produces antibodies. In this condition, abnormal plasma cells multiply uncontrollably and accumulate in the bone marrow. It is slightly more common in males than females and most common between 50 – 80 years old.
- Pathophysiology ▾: Multiple Myeloma is characterized by the clonal proliferation of malignant plasma cells in the bone marrow, leading to the overproduction of a single, non-functional M-protein.
- Multiple myeloma symptoms ▾: Clinical presentation is defined by the CRAB criteria (HyperCalcemia, Renal failure, Anemia, Bone lesions), which signify disease-related end-organ damage.
- Laboratory investigations ▾: Diagnosis relies on finding the M-protein (via SPEP/SFLC), confirming ≥ 10% clonal plasma cells in the bone marrow, and identifying a Myeloma Defining Event (MDE) through lab work or imaging.
- Treatment and management ▾: Treatment combines novel agents (PIs, IMiDs, and MoAbs) in multidrug regimens, often followed by consolidation with an Autologous Stem Cell Transplant (ASCT) for eligible patients, and continuous supportive care.
*Click ▾ for more information
What is multiple myeloma (MM)?
Multiple myeloma (MM) is a chronic and currently incurable hematologic malignancy characterized by the uncontrolled proliferation of abnormal plasma cells in the bone marrow. As the second most common type of blood cancer globally, multiple myeloma (MM) represents a critical area of hematology-oncology research and clinical management.
Epidemiology and Risk Factors
Multiple myeloma (MM) is predominantly a disease of older adults, with the median age at diagnosis typically around 69 years. The disease is slightly more common in men than in women.
A critical epidemiological feature is the significant racial disparity observed in incidence: individuals of Black African descent are approximately twice as likely to develop multiple myeloma (MM) compared to White individuals. While the cause of this disparity is complex and likely multifactorial (involving genetic, environmental, and socio-economic factors), it underscores the importance of risk stratification and screening in diverse populations.
The Spectrum of Plasma Cell Disorders
Multiple myeloma (MM) is not an acute disease but rather the symptomatic end stage of a progression that begins with precursor conditions. Understanding this continuum is vital for early diagnosis and intervention.
- Monoclonal Gammopathy of Undetermined Significance (MGUS): This is the earliest and most common precursor state, present in over 3% of the population aged 50 and older. It is defined by the presence of a low level of M-protein (<3.0 g/dL) and low levels of clonal plasma cells in the bone marrow (<10%), but critically, with no evidence of organ damage. MGUS progresses to active multiple myeloma (MM) at a low rate of approximately 1% per year.
- Smoldering Multiple Myeloma (SMM): SMM represents an intermediate stage between MGUS and multiple myeloma (MM). It is characterized by higher levels of M-protein (≥ 3.0 g/dL) or a higher bone marrow plasma cell percentage (≥ 10%), but, like MGUS, still lacks any end-organ damage. The risk of progression to active MM is significantly higher than MGUS, particularly in the first five years after diagnosis, warranting close monitoring or, in high-risk cases, early therapeutic intervention.
What are plasma cells?
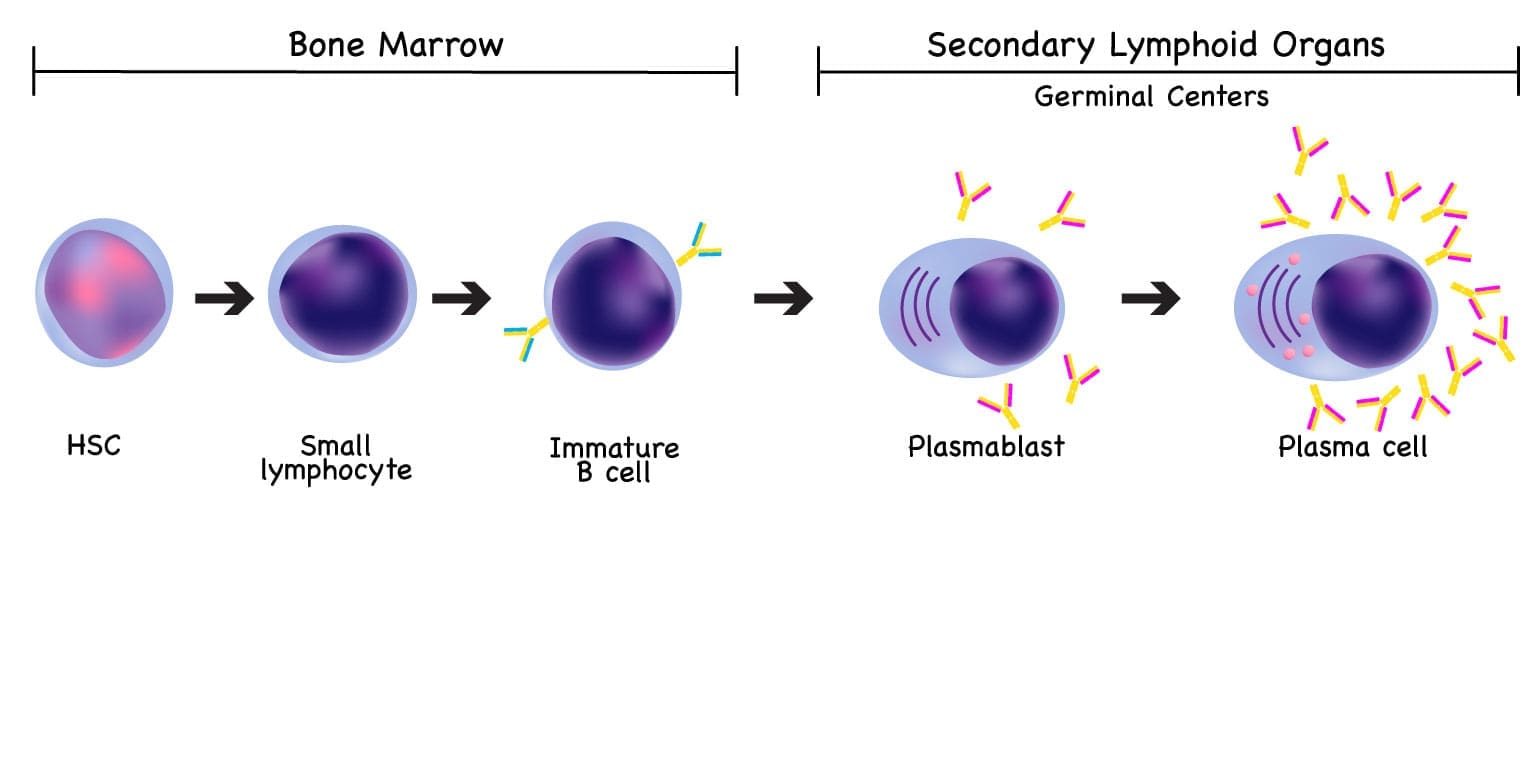
Plasma cells are the dedicated, terminally differentiated cells of the immune system whose sole function is to produce and secrete large quantities of antibodies (immunoglobulins). They are critical components of the humoral (antibody-mediated) arm of adaptive immunity.
While active plasma cells can be found in lymph nodes and spleen during an acute infection, the bone marrow is the primary, long-term niche where they reside and continue their essential role in maintaining protective immunity.
Origin and Differentiation
Plasma cells are born from B lymphocytes (B cells), a type of white blood cell that matures in the bone marrow. The journey of a B cell to a plasma cell involves several key steps:
- Antigen Recognition: A mature, naive B cell encounters a specific antigen (e.g., a protein on a virus or bacterium) that matches its B-cell receptor.
- Activation and T-cell Help: The B cell processes the antigen and presents it to a helper T cell. This interaction, often involving communication with professional antigen-presenting cells like Dendritic Cells (DCs), provides the necessary signals for the B cell to fully activate.
- Clonal Expansion and Differentiation: The activated B cell undergoes rapid multiplication (clonal expansion) and then differentiates into two main cell types:
- Memory B cells: Long-lived cells that provide immunological memory, ready to respond quickly to a future infection by the same pathogen.
- Plasma cells: These cells migrate, often to the bone marrow or spleen, where they become long-lived antibody factories.
How do plasma cells produce antibodies?
Normally, the plasma cells, representing the final stage of B-cell maturation produce polyclonal immunoglobulins to fight infections. The maturation of B lymphocytes occurs in the bone marrow and afterwards migrates to secondary lymph nodes, where antigens are presented to B cells.
Immature plasma cells are characteristically short-lived cells and producers of IgM involved in the primary immune response. In some circumstances, plasma cells experience hypermutations of the Ig light and heavy chains variable genes, secreting other Ig isotypes such as IgG and IgA or infrequently IgE and IgD or paraproteins. Later, these cells migrate to the bone marrow to differentiate into long-lived plasma cells, lasting for days or months.
Here are some examples of diseases that can affect plasma cells:
- Multiple myeloma: A cancer of the plasma cells
- Waldenström’s macroglobulinemia: A cancer of the plasma cells that produce a type of antibody called IgM
- Amyloidosis: A disease in which abnormal proteins called amyloid fibrils accumulate in the tissues, including the plasma cells
- Common variable immunodeficiency (CVID): A disorder in which the body does not produce enough antibodies
Pathophysiology of Multiple Myeloma
Multiple myeloma (MM) is a malignancy driven by genetic instability and the profound dependency of the tumor cells on the supportive milieu of the bone marrow. The disease mechanism can be broken down into three interacting elements: the molecular lesion, the microenvironmental support, and the resulting end-organ damage.
The Clonal Evolution and Molecular Drivers
Multiple myeloma (MM) begins with a genetic abnormality, typically occurring in the B-cell line before its terminal differentiation into a plasma cell.
- Primary Lesions: The most common initial genetic events are aneuploidy (abnormal number of chromosomes, usually trisomies of odd-numbered chromosomes) or immunoglobulin heavy chain (IgH) translocations involving chromosome 14. These events often occur during the B-cell development stage where B cells interact with T cells and Dendritic Cells (DCs) (a process critical for B-cell activation), locking the cell into a malignant pathway.
- Secondary Lesions: As the disease progresses from MGUS to SMM and finally to active multiple myeloma (MM), secondary mutations accumulate. These include deletions of tumor suppressor genes (p53 or RB) and activation of oncogenes (like MYC), which confer growth advantages, increase drug resistance, and drive aggressive disease behavior.
- Clonal Plasma Cells: These genetically damaged B-cells mature into malignant plasma cells (CD138+). They are immortal, resistant to apoptosis, and secrete the non-functional M-protein.
The Bone Marrow Microenvironment (BMM)
The malignant plasma cells are highly dependent on the bone marrow microenvironment (BMM) for growth, survival, and drug resistance. This interaction is reciprocal: the tumor cells alter the BMM to create a self-sustaining niche.
Key components in the BMM interaction include:
- Adhesion Molecules: Multiple myeloma (MM) cells attach to bone marrow stromal cells (BMSCs) via adhesion molecules. This binding triggers the release of potent cytokines.
- Cytokine Release: BMSCs and multiple myeloma (MM) cells secrete numerous growth factors, most notably Interleukin-6 (IL-6).
- IL-6 is a crucial survival factor for multiple myeloma (MM) cells, acting in both an autocrine (self-stimulatory) and paracrine (stimulatory to neighboring cells) manner.
- Other factors, such as VEGF (Vascular Endothelial Growth Factor) and IGF-1 (Insulin-like Growth Factor 1), also support tumor growth and blood vessel formation (angiogenesis).
- Immune Suppression: The myeloma cells actively suppress the local immune system (T cells, NK cells), allowing the tumor to escape surveillance and proliferate unchecked.
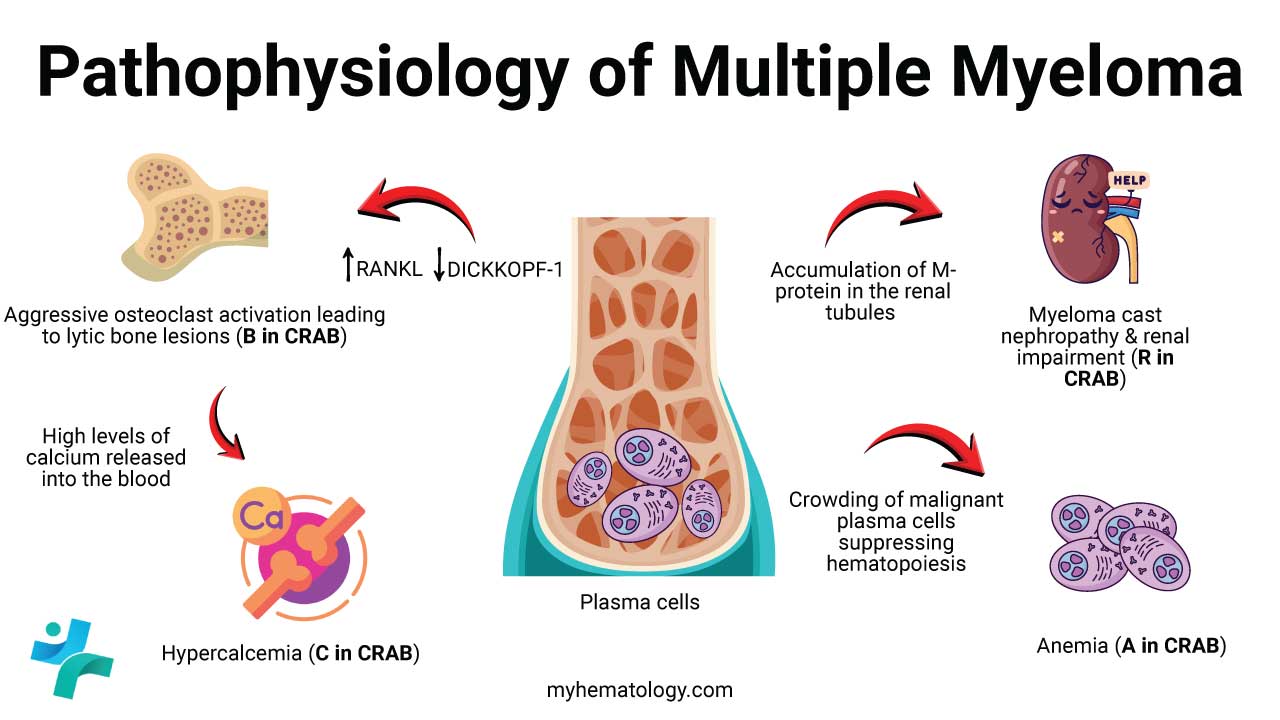
End-Organ Damage: The CRAB Criteria
The proliferation of plasma cells and the resulting cytokine storm directly lead to the four major clinical hallmarks of multiple myeloma (MM) (CRAB):
Hypercalcemia (C for Calcium elevation)
Elevated calcium in the blood is a direct consequence of massive bone resorption driven by osteoclast activation. Hypercalcemia can cause non-specific symptoms like mental confusion, excessive thirst, and gastrointestinal issues.
Renal Failure (R for Renal impairment)
Kidney damage in multiple myeloma (MM) is multifaceted:
- Myeloma Kidney: The primary mechanism is the deposition of excessive monoclonal free light chains (known as Bence-Jones proteins) in the kidney tubules. These light chains precipitate, obstructing the tubules and causing tubulointerstitial nephritis.
- Hypercalcemia: High calcium levels are toxic to the kidneys and cause vasospasm, reducing blood flow.
- Amyloidosis: In some cases, the M-protein fragments misfold and deposit as amyloid fibrils in the kidney (and other organs), further compromising function.
Anemia (A for Anemia)
Anemia (a reduction in red blood cells) occurs due to two main reasons:
- Marrow Infiltration: The sheer volume of malignant plasma cells physically crowds the bone marrow, inhibiting normal blood cell production (hematopoiesis).
- Cytokine-Mediated Inhibition: IL-6 and other inflammatory cytokines produced by the tumor and BMM suppress the production of healthy red blood cells by interfering with the function of erythropoietin.
Bone Disease (B for Bone lesions)
This is the most defining pathological feature. Multiple myeloma (MM) cells disrupt the finely tuned balance between bone breakdown (resorption by osteoclasts) and bone formation (by osteoblasts).
- Osteoclast Activation (Increased Breakdown): Multiple myeloma (MM) cells and BMSCs secrete high levels of factors that stimulate osteoclasts, such as RANKL (Receptor Activator of Nuclear factor Kappa-B Ligand) and MIP-1α (Macrophage Inflammatory Protein-1α). This causes rampant bone destruction.
- Osteoblast Inhibition (Suppressed Formation): Multiple myeloma (MM) cells also secrete inhibitors that block osteoblast activity (e.g., DKK1, sclerostin).
- Result: The net result is a purely lytic process, creating the characteristic “punched-out” lesions seen on imaging. This destruction releases calcium, leading to hypercalcemia.
Multiple Myeloma (MM) Symptoms
The symptoms of active, or symptomatic, multiple myeloma (MM) arise from the accumulation of malignant plasma cells in the bone marrow, the overproduction of monoclonal proteins, and the subsequent damage to specific organs. The classic presentation is often remembered using the CRAB mnemonic.
The CRAB Criteria: Markers of Active Disease
The presence of any of the following four conditions, along with clonal plasma cells, indicates active myeloma requiring treatment.
C: Hypercalcemia (Elevated Calcium Levels)
Massive bone destruction (lysis) by activated osteoclasts releases large amounts of calcium into the bloodstream.
High calcium levels can affect neuromuscular and renal function, leading to:
- Stones: Kidney stones, excessive thirst (polydipsia), and frequent urination (polyuria).
- Groans: Abdominal pain, nausea, vomiting, and constipation.
- Moans: Confusion, depression, fatigue, and muscle weakness.
R: Renal Insufficiency (Kidney Problems)
Primarily due to the precipitation of monoclonal free light chains (Bence-Jones proteins) in the kidney tubules, which obstruct and damage the tissue (Myeloma Kidney). Hypercalcemia and the use of certain drugs can also contribute.
Often asymptomatic in early stages, but progression leads to:
- Fatigue and weakness (due to toxin buildup).
- Swelling, especially in the legs and feet (edema).
- Reduced urine output.
A: Anemia (Low Red Blood Cell Count)
The malignant plasma cells physically crowd out healthy red blood cell production in the bone marrow, and inflammatory cytokines suppress normal blood cell development.
Anemia is responsible for the most common patient complaints:
- Extreme Fatigue and Weakness: Reduced oxygen-carrying capacity.
- Shortness of Breath (Dyspnea).
- Pallor (pale skin).
- Tachycardia (rapid heart rate) as the heart tries to compensate.
B: Bone Disease (Lytic Lesions and Pain)
Unbalanced bone remodeling resulting in uncontrolled bone destruction. This is often the first symptom that prompts a patient to seek medical attention.
- Bone Pain: Most commonly affects the back (spine), ribs, and pelvis. It is typically persistent, localized, and worsens with movement.
- Pathologic Fractures: Fractures that occur spontaneously or from minimal trauma because the bones have been weakened by lytic lesions.
- Spinal Cord Compression: If vertebral damage leads to collapse, it can press on the spinal cord, causing sudden severe back pain, numbness, tingling, and loss of bowel or bladder control (a medical emergency).
Other Common Signs and Symptoms
Beyond the CRAB criteria, multiple myeloma (MM) can cause several other significant clinical problems.
- Recurrent Infections: Despite the massive production of M-protein, these are non-functional. The overall production of diverse, functional antibodies (called polyclonal immunoglobulins) is suppressed. This is a state of “functional hypogammaglobulinemia,” resulting in an increased susceptibility to bacterial infections, particularly encapsulated bacteria (like Streptococcus pneumoniae). Symptoms include frequent or severe pneumonia, urinary tract infections, and sepsis.
- Neurological Symptoms:
- Peripheral Neuropathy: Nerve damage can occur, often presenting as tingling, numbness, or burning pain, especially in the hands and feet. This can be caused by the deposition of M-proteins, pressure from bone lesions, or as a side effect of some treatments.
- Hyperviscosity Syndrome: Less common, but possible with high levels of M-protein (especially gM or IgA). The “thickening” of the blood leads to headaches and dizziness, visual disturbances (retinopathy) and bleeding problems.
- Weight Loss and Constitutional Symptoms: Caused by advanced cancer and chronic inflammatory state due to high cytokine levels. Symptoms include unintentional weight loss, loss of appetite, and general malaise.
Because multiple myeloma (MM) symptoms are so generalized (fatigue, back pain), they are often mistaken for age-related ailments like arthritis or simple anemia, which highlights why a laboratory evaluation for M-protein is essential when these non-specific symptoms are chronic or persistent.
Investigation and Diagnosis
The investigation and diagnosis of Multiple Myeloma (MM) require a systematic approach, combining laboratory analysis, pathological assessment of the bone marrow, and advanced imaging. The goal is not just to detect the malignant plasma cells, but also to confirm that the disease is active and causing end-organ damage (the CRAB criteria).
Initial Screening and Laboratory Workup
Diagnosis typically begins when a patient presents with non-specific symptoms (like chronic fatigue or back pain) or when routine blood work reveals abnormalities.
Blood and Chemistry Tests
- Complete Blood Count (CBC): Used to detect Anemia (A in CRAB), which is the most common finding. It may also reveal low white blood cell or platelet counts (leukopenia or thrombocytopenia) due to marrow crowding. Rouleaux formation is marked in peripheral blood film.
- Blood Chemistry Panel:
- Calcium (C in CRAB): Essential for detecting hypercalcemia.
- Creatinine and BUN (Blood Urea Nitrogen): To assess kidney function and diagnose Renal impairment (R in CRAB).
- Lactate Dehydrogenase (LDH) and Beta-2 Microglobulin (β2M): These are not diagnostic but are crucial prognostic markers used for disease staging.
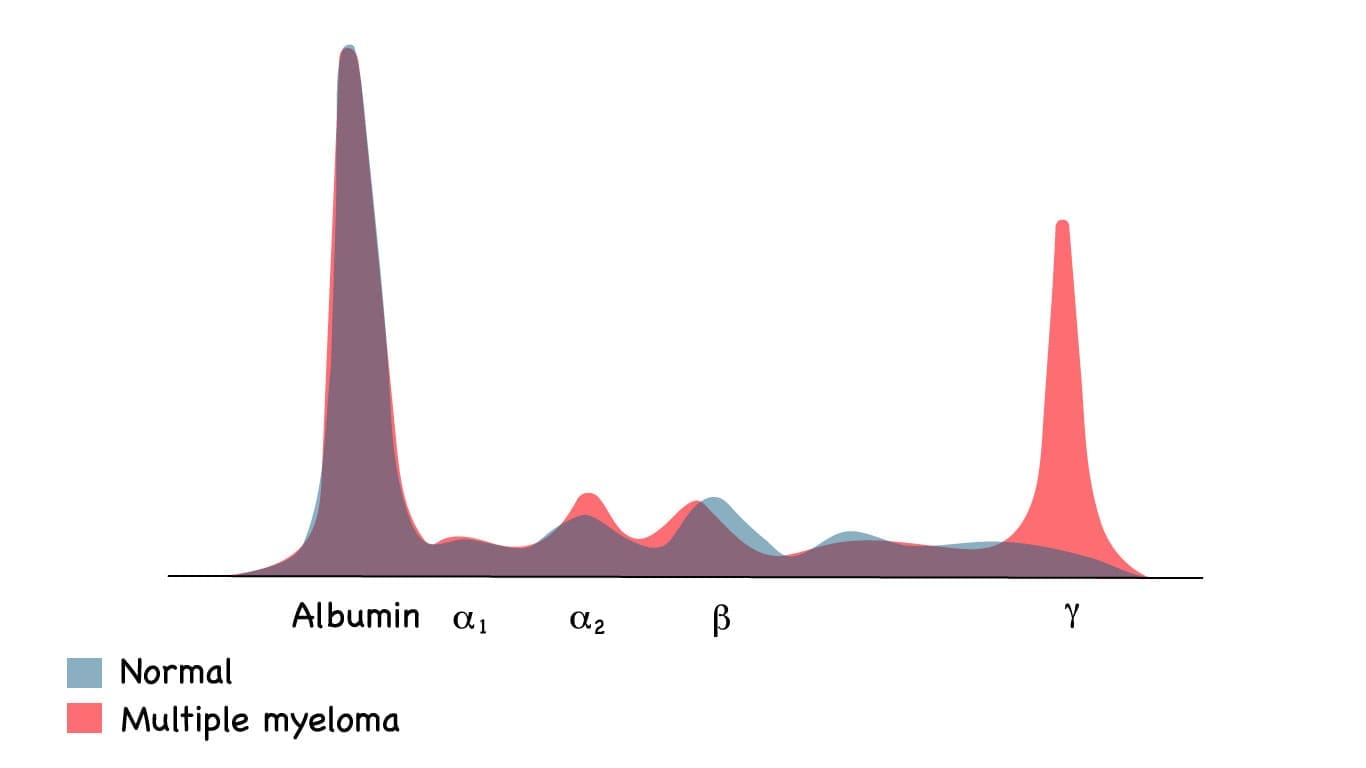
M-Protein Detection (Paraprotein)
These tests are critical for identifying the monoclonal protein (M-protein) produced by the cancerous plasma cell clone.
- Serum Protein Electrophoresis (SPEP): Separates blood proteins to look for an abnormal, sharp spike, known as the M-spike (monoclonal spike). This is the hallmark of plasma cell dyscrasias.
- Serum Free Light Chain (SFLC) Assay: Measures the levels of unbound kappa and lambda light chains in the blood and calculates the kappa/lambda ratio. An abnormal ratio strongly suggests clonal proliferation, even when the M-spike is small.
- Urine Protein Electrophoresis (UPEP): Checks for Bence-Jones proteins (free light chains) being excreted in the urine, which are highly damaging to the kidneys.
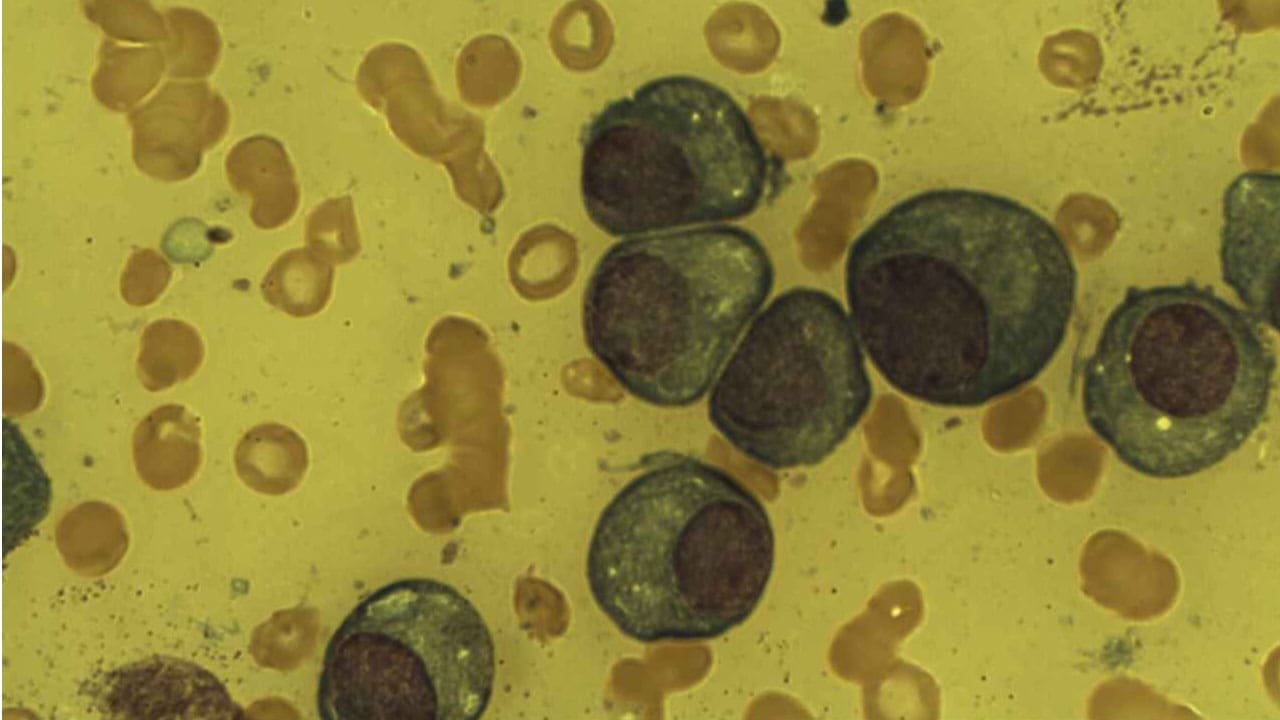
Bone Marrow Evaluation (The Definitive Test)
A bone marrow aspiration and biopsy is mandatory to confirm the cellular diagnosis and determine the percentage and characteristics of the plasma cells.
- Plasma Cell Percentage: The diagnosis of active multiple myeloma (MM) requires the presence of ≥ 10% clonal plasma cells in the bone marrow biopsy specimen, or a biopsy-proven plasmacytoma (a single, localized tumor mass).
- Immunophenotyping: Flow cytometry is used to identify the plasma cells and confirm they are clonal (i.e., arising from a single abnormal parent cell) by examining their surface markers (like CD38 and CD138).
Risk Stratification and Cytogenetics
To determine prognosis and guide therapy, the malignant cells are analyzed for genetic abnormalities, typically using Fluorescence In Situ Hybridization (FISH). This is a critical part of the initial workup.
- High-Risk Markers: Certain genetic changes, such as deletions of chromosome 17p (del(17p)) or specific translocations (e.g., t(4;14) and t(14;16)), indicate a more aggressive disease requiring intensive treatment.
Imaging Studies (Assessing Bone Disease)
Multiple myeloma (MM) is defined by bone destruction, so comprehensive imaging is essential to diagnose Bone disease (B in CRAB).
- Skeletal Survey: This is a series of conventional X-rays (plain films) of the entire skeleton. It is historically the standard but is less sensitive, as it only detects lytic lesions once significant bone mass has been lost. It is good for showing the classic “punched-out” lesions.
- Low-Dose Whole-Body Computed Tomography (LD-WBCT): This is the current preferred method as it is significantly more sensitive than plain X-rays at detecting bone damage and lytic lesions.
- Magnetic Resonance Imaging (MRI) or PET-CT: These are highly sensitive for detecting soft tissue involvement, extramedullary disease (tumor outside the bone marrow), and focal bone lesions that may not be apparent on CT. An MRI of the spine is especially important if spinal cord compression is suspected.
The International Myeloma Working Group (IMWG) Diagnostic Criteria
The IMWG criteria, most recently updated in 2014, serve as the definitive international standard for defining and classifying plasma cell disorders. It is a binary system used to determine whether a patient should be actively treated.
Active Multiple Myeloma is diagnosed when all three conditions are met:
- Presence of clonal plasma cells ≥ 10% in the bone marrow (or a biopsy-proven plasmacytoma).
- Presence of M-protein in the serum or urine.
- Presence of at least one Myeloma Defining Event (MDE). MDEs include the classic CRAB features (hypercalcemia, renal insufficiency, anemia, bone lesions) OR the following “SLiM CRAB” biomarkers of high-risk disease, even if the patient is asymptomatic:
- Sixty: Bone marrow plasma cells ≥ 60%.
- Light chain ratio: SFLC ratio ≥ 100 (with the involved light chain ≥ 100 mg/L).
- MRI lesions: More than one focal lesion found on MRI.
The inclusion of the SLiM biomarkers allows physicians to diagnose and treat patients who have extremely high-risk disease (formerly called “high-risk SMM”) before irreversible CRAB damage occurs.
| Feature | Monoclonal Gammopathy of Undetermined Significance (MGUS) | Smoldering Multiple Myeloma (SMM) | Active Multiple Myeloma (MM) |
| M-Protein | Low (< 3 g/dL) | Higher (≥ 3 g/dL) | Any level |
| Clonal Plasma Cells | Low (< 10%) | Higher (≥ 10% – 60%) | ≥ 10% |
| Myeloma Defining Event (MDE) | Absent | Absent | Present (CRAB or SLiM criteria) |
Differential Diagnosis for Multiple Myeloma
| Condition | Underlying Pathology | Distinguishing Feature (vs. MM) |
| Monoclonal Gammopathy of Undetermined Significance (MGUS) | Small, stable clone of abnormal plasma cells. | Clonal plasma cells < 10% in bone marrow. CRAB/SLiM MDEs are ABSENT. |
| Smoldering Multiple Myeloma (SMM) | Larger clone of abnormal plasma cells. | Clonal plasma cells ≥ 10% (up to 60%), and M-protein ≥ 3 g/dL. CRAB MDEs are ABSENT. (May include SLiM features, which would reclassify it as active MM). |
| Systemic AL Amyloidosis | Small clone of plasma cells produces misfolded light chains that deposit as fibrils. | Bone marrow plasma cells often < 10%. Organ damage is due to protein deposition (amyloid), not tumor mass or lytic bone lesions. |
| Solitary Plasmacytoma | Localized collection of clonal plasma cells (a tumor mass). | The tumor is solitary (confined to one bone site or one soft tissue site). Systemic CRAB features are absent. |
| Monoclonal Gammopathy of Renal Significance (MGRS) | Small clone of plasma cells (often < 10%) that causes severe non-amyloid kidney damage via toxic M-protein. | Kidney failure (R in CRAB) is present, but clonal burden is low, and other CRAB features are absent. |
| Other Hematologic Malignancies | Malignancies of white blood cells (e.g., Lymphoma, Leukemia). | Cells are typically lymphocytes, blast cells, or myeloid cells, NOT terminally differentiated plasma cells (CD138+). M-protein is usually absent. |
What are the different stages of multiple myeloma (MM)?
Multiple myeloma (MM) is staged for treatment purposes to help doctors determine the best treatment plan for each patient.
For myeloma, staging-wise it is important to begin with whether the patient is experiencing symptoms. It is common to classify people with newly diagnosed myeloma as either:
- Asymptomatic. This means the person does not have any multiple myeloma symptoms and signs. This is called smoldering myeloma.
- Symptomatic. This means the person has multiple myeloma symptoms and signs. Patients with multiple myeloma symptoms, or who are about to develop symptoms, need treatment. Multiple myeloma symptoms are described with the mnemonic acronym “CRAB.”
The Revised International Staging System (R-ISS) is now used more commonly to classify multiple myeloma. It defines the factors that affect a person’s survival of the disease. The system has 3 stages based on:
- Serum albumin level: Albumin is a protein that is produced by the liver. It helps to regulate the fluid balance in the blood and tissues. A low albumin level is a sign of advanced multiple myeloma.
- Beta-2 microglobulin (β2-M) level: β2-M is a protein that is produced by all cells in the body. It is removed from the blood by the kidneys. A high β2-M level is a sign of advanced multiple myeloma.
- Lactodehydrogenase (LDH) level: LDH is an enzyme that is found in all cells in the body. It is responsible for converting lactate to pyruvate, which is a source of energy for the cells. Elevated serum LDH levels are associated with a more aggressive form of multiple myeloma and a worse prognosis. Patients with elevated serum LDH levels are more likely to have bone marrow involvement, and they are more likely to develop complications from multiple myeloma, such as hypercalcemia and renal failure.
- Cytogenetic risk: Certain chromosomal abnormalities are associated with a worse prognosis in multiple myeloma. Recent efforts have been made to further classify myeloma based on patterns of gene expression in myeloma cells. Detection of specific chromosomal abnormalities are through fluorescence in situ hybridization (FISH) technique. High-risk cytogenetic abnormalities include:
- del(17p) (deletion of chromosome 17p)
- t(4;14) (translocation between chromosomes 4 and 14)
- t(14;16) (translocation between chromosomes 14 and 16)
Revised International Staging System (R-ISS)
| Stage | Criteria | Prognosis |
|---|---|---|
| R-ISS I | β2-microglobulin < 3.5 mg/L Albumin ≥ 3.5 g/dL Standard-risk cytogenetics (absence of high-risk cytogenetic abnormalities) Normal LDH | Longest survival |
| R-ISS II | Not R-ISS stage I or III | Intermediate survival |
| R-ISS III | β2-microglobulin > 5.5 mg/L And either: High-risk cytogenetics (presence of del(17p) and/or t(4;14) and/or t(14;16)) or elevated LDH | Shortest survival |
- Recurrent or relapsed myeloma is myeloma that returns after a period of being in control after treatment is called recurrent myeloma or relapsed myeloma. If there is a recurrence, the cancer may need to be staged again (called re-staging) using one of the systems above.
The ISS staging system is used to guide treatment decisions in multiple myeloma. Patients with stage I are typically treated with less aggressive regimens, while patients with stage III are typically treated with more aggressive regimens.
In addition to the ISS staging system, there are a number of other staging systems that are used in multiple myeloma. These staging systems are more complex, but they may be more accurate in predicting prognosis.
Treatment and Management
The management of multiple myeloma (MM) is highly individualized, depending primarily on the patient’s age, fitness, disease stage (R-ISS), and risk classification (FISH findings).
The primary goals of MM therapy have evolved from mere symptom palliation to achieving deep, sustained remissions:
- Symptom Control: Addressing CRAB features (bone pain, hypercalcemia, anemia).
- Prolonged Survival: Maximizing the time a patient lives free of disease progression.
- Deep Remission: Achieving a complete response (CR) or better, often monitored using Minimal Residual Disease (MRD) status.
Newly Diagnosed MM (NDMM) Strategy
Treatment for NDMM is bifurcated based on whether the patient is eligible for an Autologous Stem Cell Transplant (ASCT).
1. Transplant-Eligible Patients (Typically younger, fitter patients)
The treatment sequence aims for the deepest possible response before transplant consolidation.
- Induction Therapy: A short, intense regimen (usually 3 to 4 cycles) using triple combinations to reduce the tumor burden drastically. Common Regimen is VRd (Velcade/bortezomib, Revlimid/lenalidomide, dexamethasone).
- Consolidation (ASCT): High-dose chemotherapy (usually Melphalan) is given to kill remaining plasma cells, followed by the re-infusion of the patient’s own previously collected stem cells to “rescue” the bone marrow.
- Maintenance Therapy: Therapy (often single-agent Lenalidomide) given continuously after transplant to prevent relapse and prolong remission.
2. Transplant-Ineligible Patients (Typically older patients or those with significant co-morbidities)
Treatment focuses on continuous, less intense regimens that are well-tolerated over a long period. Regimens combining novel agents and a steroid are given until disease progression or intolerable toxicity. Common Regimen include DRd (Daratumumab, lenalidomide, dexamethasone) or VRd (at lower doses). The addition of monoclonal antibodies (Daratumumab) upfront is a standard for many patients in this group.
Relapsed/Refractory MM (RRMM)
When multiple myeloma (MM) returns after treatment (relapse), subsequent therapy involves sequential combinations of drugs, ideally using agents with a different mechanism of action than the previous lines of treatment.
Novel Agents (The Pillars of Modern MM Therapy)
| Class | Mechanism of Action | Examples | Role in Therapy |
|---|---|---|---|
| Proteasome Inhibitors (PIs) | Block the cell’s waste-disposal system, causing toxic buildup in the cancer cells and triggering cell death. | Bortezomib (Velcade), Carfilzomib (Kyprolis), Ixazomib (Ninlaro). | Used in induction, maintenance, and relapse settings. |
| Immunomodulatory Drugs (IMiDs) | Modify the tumor microenvironment, inhibit growth factors, and stimulate immune cells (T-cells, NK cells) to fight the tumor. | Lenalidomide (Revlimid), Pomalidomide (Pomalyst). | Foundation of many current regimens. |
| Monoclonal Antibodies (MoAbs) | Target specific proteins on the plasma cell surface to trigger destruction via the immune system. | Daratumumab (anti-CD38), Isatuximab (anti-CD38). | Highly effective in combination regimens. |
| ADC (Antibody-Drug Conjugate) | Deliver a potent toxin directly to the myeloma cell by linking it to an antibody that binds a surface protein. | Belantamab Mafodotin (targets BCMA). | Used in later lines of therapy. |
Immunotherapy and Cellular Therapy
These therapies represent the cutting edge, leveraging the immune system’s power to target residual disease.
- CAR T-cell Therapy: T-cells are harvested from the patient, genetically modified to express a Chimeric Antigen Receptor (CAR) that specifically targets a protein on the myeloma cell (most commonly BCMA – B-cell Maturation Antigen). These highly specific, activated T-cells are re-infused to eradicate the tumor.
- Bispecific T-cell Engagers (BiTEs): These are synthetic antibodies designed with two “arms.” One arm grabs a T-cell (via CD3), and the other arm grabs a myeloma cell (via BCMA), physically bridging the two cells. This forces the T-cell to activate and destroy the myeloma cell. These are showing rapid efficacy in RRMM.
Supportive Care and Complications
Supportive care is essential for maintaining quality of life and preventing complications, particularly those related to the CRAB features.
- Bone Health Management: Bisphosphonates (e.g., Zoledronic acid) or Denosumab are given intravenously to inhibit osteoclasts, which helps reduce bone pain and the frequency of Skeletal-Related Events (SREs), such as fractures.
- Infection Prophylaxis: Patients are functionally immunosuppressed (hypogammaglobulinemia) and require vaccinations (flu, pneumonia) and often antiviral prophylaxis (e.g., Acyclovir) during intense therapy to prevent reactivation of viruses like shingles.
- Renal Management: Aggressive hydration, correction of hypercalcemia, and careful monitoring of nephrotoxic drugs are vital to preserve kidney function.
- Pain Management: A multimodal approach using NSAIDs, opioids, and sometimes focal radiation therapy for localized, severe bone pain is employed.
The modern approach to multiple myeloma (MM) involves constantly cycling through these different drug mechanisms to keep the disease at bay. Myeloma treatment is focused on developing new and more effective treatments, as well as on finding ways to improve the quality of life for multiple myeloma patients.
Frequently Asked Questions (FAQs)
What is life expectancy with multiple myeloma?
The life expectancy with multiple myeloma can vary greatly depending on several factors, including:
- Stage of diagnosis: Earlier stages generally have a better prognosis.
- Age and overall health: Younger individuals and those with good overall health tend to have a better prognosis.
- Specific genetic features: Certain genetic mutations can influence the course of the disease.
- Response to treatment: Patients who respond well to treatment typically have a better prognosis.
Some general statistics:
- Overall 5-year survival rate: Around 50-60% of people with multiple myeloma will survive for at least 5 years after diagnosis.
- Early-stage diagnosis: For the 5% of people diagnosed at an early stage, the 5-year survival rate is significantly higher, around 71%.
- Later-stage diagnosis: If the cancer has spread to a distant part of the body, the 5-year survival rate is lower, around 48%.
Can you fully recover from multiple myeloma?
There is currently no cure for multiple myeloma. However, with advancements in treatment, it can be managed effectively, potentially leading to long-term remission.
What is smoldering myeloma?
Smoldering myeloma, also known as asymptomatic myeloma, is a precursor stage to the blood cancer multiple myeloma. In smoldering myeloma, abnormal plasma cells are present in higher numbers than usual, but not high enough to cause symptoms or organ damage. Unlike active multiple myeloma, smoldering myeloma typically doesn’t cause any noticeable symptoms. This is why it’s called “asymptomatic.” Identifying smoldering myeloma early allows doctors to monitor your condition closely and potentially intervene before it progresses to active myeloma. Some individuals may have this stage for a decade or even longer before transitioning to active myeloma.
| Feature | Smoldering Myeloma | Active Multiple Myeloma |
|---|---|---|
| Symptoms | Usually none | Fatigue, bone pain, weakness, frequent infections, anemia |
| Plasma Cells | Elevated in bone marrow, may be detectable in blood/urine | High levels in bone marrow, blood, and urine |
| Bone Damage | No significant damage | May cause bone weakening and fractures |
| Organ Damage | No | May affect kidneys and other organs |
| Treatment | Usually no immediate treatment, only monitoring | Requires treatment to control the disease |
Why is Early Detection Important?
Catching smoldering myeloma early allows for:
- Close monitoring: Doctors can track the progression of the disease and intervene if needed.
- Potential to delay or prevent active myeloma: Early intervention with medications or other therapies may help delay or even prevent the development of symptomatic multiple myeloma.
- Improved long-term outcomes: Early detection and management can significantly improve your overall prognosis and quality of life.
What causes death in multiple myeloma?
Multiple myeloma itself, through complications like bone damage, kidney failure, or severe infections, is the most frequent cause of death in patients. However, some individuals may succumb to infections due to weakened immunity, cardiovascular disease which can be more prevalent in myeloma patients, or even other unrelated cancers that can develop during the course of the disease.
What are the first symptoms of myeloma?
While multiple myeloma often has no symptoms in the early stages (smoldering myeloma), initial signs when they do occur can be subtle. Look out for persistent fatigue, unexplained bone pain, especially in the back or ribs, increased susceptibility to infections, or easy bruising. These can be indicative of underlying problems with blood cell production and bone health, and warrant consultation with a healthcare professional for further evaluation.
What is the significance of t(11;14)?
The t(11;14) translocation is considered a standard-risk abnormality but is clinically important as it is associated with Bcl-2 overexpression. This makes patients with t(11;14) particularly susceptible to treatment with Venetoclax, a BCL-2 inhibitor, often used in relapsed settings.
Are multiple myeloma and lymphoma or chronic lymphocytic leukemia related?
Multiple myeloma, lymphoma, and chronic lymphocytic leukemia (CLL) are all blood cancers, but they target different cell types. Myeloma affects plasma cells (antibody producers), while lymphomas originate in lymphocytes (infection fighters) in the lymph nodes or other organs. CLL is a specific type of lymphoma that affects a particular subtype of lymphocyte. While they share some features like abnormal blood cell production, the specific cells involved and their locations within the body distinguish these blood cancers.
Can multiple myeloma spread to other organs or metastasize?
Yes, multiple myeloma can spread beyond the bone marrow, which is where it originates. This spread, sometimes called metastasis, involves abnormal plasma cells traveling through the bloodstream and potentially forming tumors in other organs. While less common than bone involvement, these tumors can occur in places like the skin, lymph nodes, or even the liver. This spread can worsen the disease and requires additional treatment strategies.
Glossary of Medical Terms
- Monoclonal Gammopathy of Undetermined Significance (MGUS): An asymptomatic precursor state defined by the presence of an M-protein, <10% clonal plasma cells in the bone marrow, and no evidence of CRAB/MDE.
- Paraprotein (M-protein): A monoclonal immunoglobulin or light chain (also called M-spike) produced by the clonal population of plasma cells, detectable in the serum or urine.
- Lytic Lesions: Areas of bone destruction (osteolysis) caused by increased osteoclast activity and decreased osteoblast activity, typical of Multiple Myeloma Bone Disease (MBD).
- Autologous Stem Cell Transplantation (ASCT): A procedure where a patient’s own collected stem cells are infused back after high-dose chemotherapy to reconstitute the bone marrow.
- BCMA (B-cell Maturation Antigen): A cell surface receptor highly expressed on plasma cells and an ideal target for novel immunotherapies (CAR T-cells, BiTEs, ADCs).
- Proteasome Inhibitors (PIs): A class of drugs (e.g., Bortezomib) that block the proteasome, disrupting protein homeostasis and triggering apoptosis in myeloma cells.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008 Mar 15;111(6):2962-72. doi: 10.1182/blood-2007-10-078022. PMID: 18332230; PMCID: PMC2265446.
- Wallington-Beddoe, C.T., Mynott, R.L. Prognostic and predictive biomarker developments in multiple myeloma. J Hematol Oncol 14, 151 (2021). https://doi.org/10.1186/s13045-021-01162-7
- Minnie SA, Hill GR. Immunotherapy of multiple myeloma. J Clin Invest. 2020 Apr 1;130(4):1565-1575. doi: 10.1172/JCI129205. PMID: 32149732; PMCID: PMC7108923.
- Rendo MJ, Joseph JJ, Phan LM, DeStefano CB. CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges. Blood Lymphat Cancer. 2022 Aug 29;12:119-136. doi: 10.2147/BLCTT.S327016. PMID: 36060553; PMCID: PMC9439649.
- Avet-Loiseau H, Ludwig H, Landgren O, Paiva B, Morris C, Yang H, Zhou K, Ro S, Mateos MV. Minimal Residual Disease Status as a Surrogate Endpoint for Progression-free Survival in Newly Diagnosed Multiple Myeloma Studies: A Meta-analysis. Clin Lymphoma Myeloma Leuk. 2020 Jan;20(1):e30-e37. doi: 10.1016/j.clml.2019.09.622. Epub 2019 Oct 9. PMID: 31780415; PMCID: PMC7444731.
- Solimando AG, Krebs M, Desantis V, Marziliano D, Caradonna IC, Morizio A, Argentiero A, Shahini E, Bittrich M. Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting. Biomedicines. 2023; 11(7):2087. https://doi.org/10.3390/biomedicines11072087
- Hussain M, Yellapragada S, Al Hadidi S. Differential Diagnosis and Therapeutic Advances in Multiple Myeloma: A Review Article. Blood Lymphat Cancer. 2023 Sep 15;13:33-57. doi: 10.2147/BLCTT.S272703. PMID: 37731771; PMCID: PMC10508231.
- Rees, M. J., & Kumar, S. (2024). High-risk multiple myeloma: Redefining genetic, clinical, and functional high-risk disease in the era of molecular medicine and immunotherapy. American journal of hematology, 99(8), 1560–1575. https://doi.org/10.1002/ajh.27327
- Guzdar, A., & Costello, C. (2020). Supportive Care in Multiple Myeloma. Current hematologic malignancy reports, 15(2), 56–61. https://doi.org/10.1007/s11899-020-00570-9
- Ho, M., Paruzzo, L., Minehart, J., Nabar, N., Noll, J. H., Luo, T., Garfall, A., & Zanwar, S. (2025). Extramedullary Multiple Myeloma: Challenges and Opportunities. Current oncology (Toronto, Ont.), 32(3), 182. https://doi.org/10.3390/curroncol32030182
- Gay, F., Marchetti, E., & Bertuglia, G. (2025). Multiple Myeloma Unpacked. Hematological oncology, 43 Suppl 2(Suppl 2), e70067. https://doi.org/10.1002/hon.70067
- Rodriguez-Otero, P., Paiva, B., & San-Miguel, J. F. (2021). Roadmap to cure multiple myeloma. Cancer treatment reviews, 100, 102284. https://doi.org/10.1016/j.ctrv.2021.102284



