Introduction
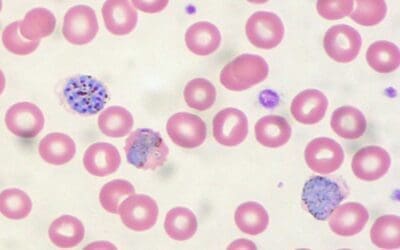
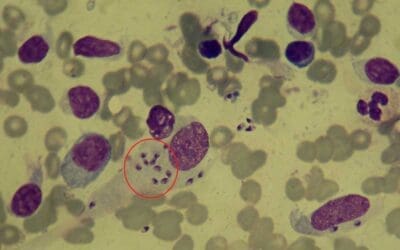
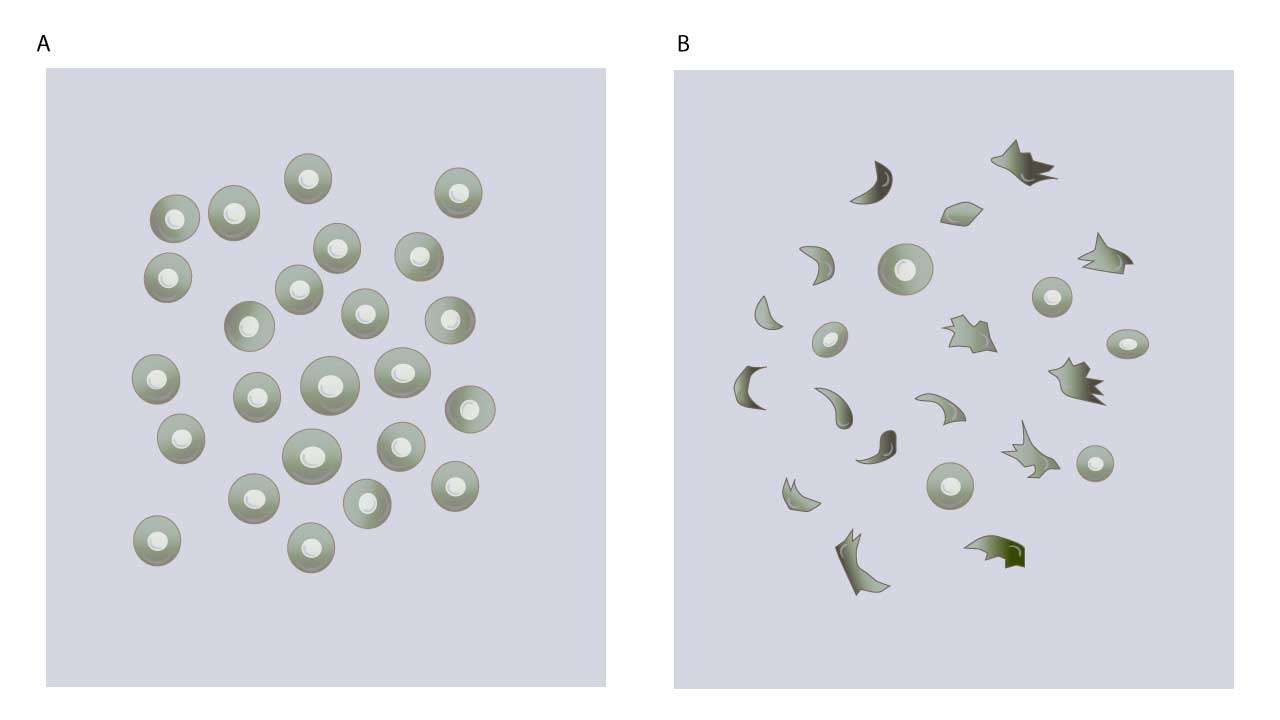
The sickle cell test, also known as the dithionite solubility test, is a simple and rapid method used to detect the presence of sickle cell disease or sickle cell trait. Sickle cell disease (SCD) is a group of inherited blood disorders characterized by the presence of abnormal hemoglobin, Hemoglobin S (HbS). This mutation, caused by a single amino acid substitution in the β-globin chain, distorts the red blood cells into rigid, crescent-shaped “sickle” cells as the deoxygenated hemoglobins form tactoids. These misshapen cells have a shortened lifespan, leading to chronic anemia and a cascade of clinical complications.
The consequences of sickling extend far beyond mere oxygen deficiency. Impaired blood flow due to cell clumping and blockages in small blood vessels triggers episodes of severe pain, known as crises, affecting various organs. Additionally, SCD patients are susceptible to infections, strokes, organ damage, and delayed growth and development.
While advancements in management strategies have significantly improved the life expectancy of patients with SCD, early diagnosis remains crucial for prompt intervention and preventative measures. The sodium dithionite solubility test, the focus of this laboratory protocol, is a simple yet valuable tool for detecting the presence of HbS in red blood cells.
Principle of Sickle Cell Test
Understanding the underlying biochemistry of sickling is essential for interpreting the sickle cell test results. Sodium dithionite, a reducing agent, removes oxygen from hemoglobin molecules. Sodium dithionite (Na2S2O4), also known as sodium hydrosulfite, acts as a potent reducing agent. Its magic lies in its ability to snatch electrons from molecules, including hemoglobin. When dithionite encounters hemoglobin, it readily removes an oxygen molecule from the iron atom within the heme group, converting it from oxyhemoglobin (HbO2) to deoxyhemoglobin (Hb). Normally, deoxygenated hemoglobin adopts a different conformation. However, sickled red blood cells will form a typical sickle-shape as dithionite depletion of oxygen triggers HbS to polymerize.
Materials
- Anticoagulated patient peripheral blood sample
- Dithionite solution: 10% sodium dithionite (Na2S2O4) in phosphate buffer, pH 6.8.
- Pasteur pipette
- Microscope slides and coverslips
- Petroleum jelly-paraffin wax or depex
- Test tube
Protocol
- Make a fresh dithionite solution by dissolving 10 mg of sodium dithionite in 1 mL of phosphate buffer, pH 6.8. Mix well and use immediately.
- Place a drop of dithionite solution and a drop of anticoagulated blood into a test tube.
- Mix the blood and dithionite gently to ensure thorough mixing.
- Place a drop of the mixture on a clean microscope slide.
- Cover the slide with a coverslip and avoid trapping air bubbles.
- Cover all edges of the cover slip with petroleum jelly-paraffin wax or depex.
- Observe under the microscope.
Interpretation
In HbS disease, sickling can manifest significantly faster compared to HbS trait. While HbS disease might exhibit immediate or near-immediate sickling, observing clear sickling in HbS trait within an hour at 37°C might not be conclusive and might require additional time or altered conditions like lower oxygen tension.
Several factors can contribute to false-negative results in the sickle cell test.
Pre-analytical factors
- Blood sample age: Sickling is a dynamic process, and red blood cells gradually lose their ability to sickle over time. Using aged blood samples (more than 24 hours old) can decrease the sensitivity of the test and lead to false negatives.
- Sample handling: Improper storage or handling of the blood sample, such as exposure to extreme temperatures or agitation, can damage red blood cells and affect their sickling ability.
- Severely low hemoglobin levels (anemia): In cases of severe anemia, the concentration of red blood cells in the sample is significantly reduced. This can make it difficult to visualize the characteristic clumps of sickled cells, leading to a false negative.
- Recent blood transfusion: If a patient with HbS trait has recently received a blood transfusion with normal red blood cells, the presence of HbA can dilute the HbS concentration in the test sample, potentially masking the sickling phenomenon.
Analytical factors
- Incorrect dithionite concentration or pH: The success of the test relies on the dithionite solution being prepared with the proper concentration and pH. Deviations from the recommended values can affect the deoxygenation process and decrease the test’s sensitivity.
- Technical errors: Improper mixing of the blood and dithionite solution, inadequate observation time, or issues with the microscope itself can lead to misinterpretation of the results and false negatives.
Biological factors
- Presence of certain hemoglobin variants: Some rare hemoglobin variants co-existing with HbS can interfere with the sickling process and mask the typical clumping pattern in the test.
- Fetal hemoglobin (HbF): Newborns and infants have high levels of HbF, which inhibits sickling. Therefore, the dithionite test might not be reliable for diagnosing HbS trait in young children until HbF levels decrease significantly.
It’s crucial to interpret the dithionite (sickle cell) test results with caution and in conjunction with other clinical and laboratory findings.
Note: While the dithionite solubility (sickle cell) test is a rapid and inexpensive screening tool, it is not definitive. A positive result or if a suspicion of HbS exists despite a negative dithionite (sickle cell) test, it should be confirmed with a more specific test like hemoglobin electrophoresis.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.