TL;DR
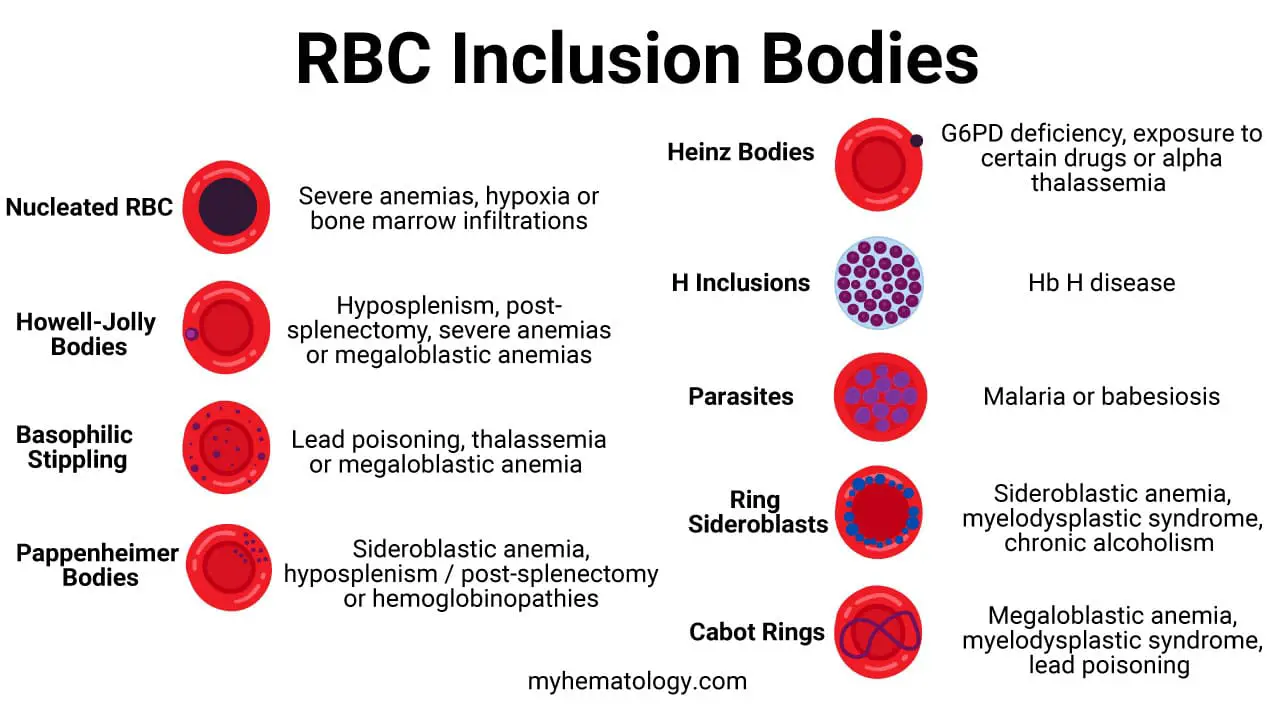
Red blood cell inclusion bodies are abnormal aggregates of material within the cytoplasm of red blood cells, which are visible under a microscope and are indicative of various underlying diseases or conditions.
Certainly. Here is an updated and expanded table that includes nucleated red blood cells, ring sideroblasts, and parasites.
| Inclusion Bodies | Associated Cause or Condition | Composition |
| Howell-Jolly Bodies | Hyposplenism or post-splenectomy (due to a non-functional or absent spleen), severe anemias, or megaloblastic anemia. | Remnants of nuclear DNA that were not expelled during red blood cell maturation. |
| Heinz Bodies | Conditions causing oxidative stress or unstable hemoglobin, such as G6PD deficiency, exposure to certain drugs/toxins (e.g., dapsone), or alpha-thalassemia. | Aggregates of denatured hemoglobin that have precipitated to form the inclusion bodies. |
| Basophilic Stippling | Disturbed red blood cell production (dyserythropoiesis), particularly in lead poisoning, thalassemia, and megaloblastic anemia. | Aggregates of ribosomal RNA and other organelles. |
| Pappenheimer Bodies | Sideroblastic anemia, hyposplenism/post-splenectomy, and hemoglobinopathies. These are often a sign of iron-loading disorders. | Granules containing iron (hemosiderin) that appear at the periphery of the red blood cell. |
| Cabot Rings | Severe dyserythropoiesis, most notably in megaloblastic anemia, myelodysplastic syndromes, and lead poisoning. | Remnants of the mitotic spindle or nuclear membrane. |
| Hemoglobin H (HbH) Inclusions | Hemoglobin H disease, which is a severe form of alpha-thalassemia. | Precipitates of unstable beta-globin chains (β4). They are a specific type of Heinz body. |
| Nucleated Red Blood Cells (NRBCs) | Stressful bone marrow conditions such as severe anemia, hypoxia, or bone marrow infiltration (e.g., leukemia, myelofibrosis). | Immature red blood cells that retain their nucleus. |
| Ring Sideroblasts | Sideroblastic anemia (hereditary or acquired), myelodysplastic syndromes, and chronic alcoholism. | Immature red blood cells with a ring of iron granules encircling the nucleus. This indicates impaired iron utilization. |
| Parasites | Infection with a blood-borne parasite, most commonly malaria (Plasmodium species) or babesiosis. | The foreign organism itself, which invades and reproduces inside the red blood cells. |
Introduction
Red blood cell (RBC) morphology plays a crucial role in diagnosing hematological disorders. By examining the size, shape, and presence of RBC inclusion bodies, clinicians gain valuable insights into the underlying cause of abnormal blood function.
RBC morphology can sometimes detect abnormalities before symptoms even arise. For instance, mild anisocytosis might precede the development of iron deficiency anemia. Evaluating RBC morphology is a relatively inexpensive and readily available test compared to some advanced diagnostics.
While valuable, RBC morphology has limitations. Some inclusion bodies can be non-specific, and certain disorders might not cause significant morphological changes. The information gleaned from RBC morphology helps direct further investigations.
In this article we are focusing on the inclusion bodies that can be found in abnormal red blood cells. However, a more detailed explanation on red blood cell morphology based on size, color, shape and distribution can be found in another article here.
Key Morphological RBC Characteristics Assessed in Approach to Anemia
Red blood cell (RBC) morphology provides a wealth of information for diagnosing hematological disorders. By scrutinizing specific characteristics including inclusion bodies, medical professionals can gain valuable insights into the underlying cause of abnormal blood function.
Size (Anisocytosis)
- Normocytosis (normal size): While not a definitive indicator of normalcy, RBCs with a consistent diameter (around 6-8 μm) suggest the absence of major size variations.
- Microcytosis (small cells): Smaller than normal RBCs can point towards:
- Iron deficiency anemia: Insufficient iron impairs hemoglobin production, leading to smaller cells with less hemoglobin content.
- Thalassemia: Genetic disorders affecting hemoglobin synthesis can result in microcytic RBCs.
- Macrocytosis (large cells): Larger than normal RBCs can be associated with:
- Megaloblastic anemia: Vitamin B12 or folate deficiency disrupts DNA synthesis, leading to the production of abnormally large and immature RBCs.
- Liver disease: Impaired liver function can sometimes affect RBC maturation, resulting in macrocytosis.
Shape (Poikilocytosis)
- Normocytosis (normal shape): The ideal RBC shape is a biconcave disc, offering a large surface area for efficient gas exchange.
- Poikilocytosis (abnormal shapes): Deviations from the normal disc shape can indicate various conditions:
- Spherocytosis: Spherically shaped cells suggest a hereditary disorder where the RBC membrane is defective, leading to premature cell destruction (hereditary spherocytosis).
- Elliptocytosis: Elliptical shaped cells can be inherited (hereditary elliptocysosis) or acquired (due to vitamin E or iron deficiency).
- Sickle cell disease: Rigid, sickle-shaped cells are a characteristic finding in this genetic disorder.
- Stomatocytosis: Elliptical cells with a central slit-like indentation can be hereditary or caused by autoimmune disorders.
Color (Hemoglobin Content)
- Normochromasia (normal color): Adequate hemoglobin concentration results in a characteristic pink staining pattern on a blood smear.
- Hypochromasia (pale cells): Lighter than normal staining suggests reduced hemoglobin content, often seen in:
- Iron deficiency anemia: Insufficient iron limits hemoglobin production, leading to pale RBCs.
- Polychromasia: Polychromasia refers to RBCs that stain a slightly bluish hue compared to the normal pink color. This coloration is due to the presence of residual RNA within young, recently matured RBCs. While a small number of polychromatic RBCs might be present in a healthy individual, an increased number can indicate:
- Increased RBC production in response to blood loss or hemolytic anemia.
- Bone marrow dysfunction, where RBC maturation is impaired.
Inclusion Bodies
The presence of abnormal inclusion bodies within RBCs holds significant diagnostic value:
- Howell-Jolly bodies: Howell-Jolly bodies are remnants of the nucleus that are normally expelled during RBC maturation. Their presence suggests impaired nuclear extrusion, often seen in megaloblastic anemia.
- Basophilic stippling: Basophilic stippling are fine, bluish granules composed of aggregated ribosomes. They can indicate:
- Iron deficiency anemia: When iron is limited, ribosome production is disrupted.
- Thalassemia: Abnormal hemoglobin synthesis can lead to basophilic stippling.
- Heinz bodies: Heinz bodies are denatured clumps of hemoglobin precipitated within RBCs due to oxidative stress. They might be associated with:
- G6PD deficiency: An enzyme deficiency that makes RBCs susceptible to oxidative damage.
- Certain medications or toxins.
- H-inclusions (beta globin tetramers): These H-inclusions are aggregates of beta globin protein chains, which are the building blocks of hemoglobin. Their presence suggests an abnormality in hemoglobin structure, potentially indicating a type of thalassemia, a group of inherited blood disorders.
- Parasites: In some cases, blood smears can reveal the presence of parasites that infect red blood cells. Examples include malaria and babesiosis.
Common RBC Inclusion Bodies Seen in Disorders
Common RBC morphology in terms of size, shape, color, and distribution has been covered in the RBC morphology post. This article, we will be focusing on common RBC inclusion bodies seen in hematological disorders.
Nucleated Red Blood Cells (Normoblasts)
Nucleated red blood cells (NRBCs), also known as normoblasts, are immature red blood cells that typically wouldn’t be found circulating in the bloodstream of a healthy adult. Their presence on a peripheral blood smear signifies a potential underlying condition affecting red blood cell production or release.
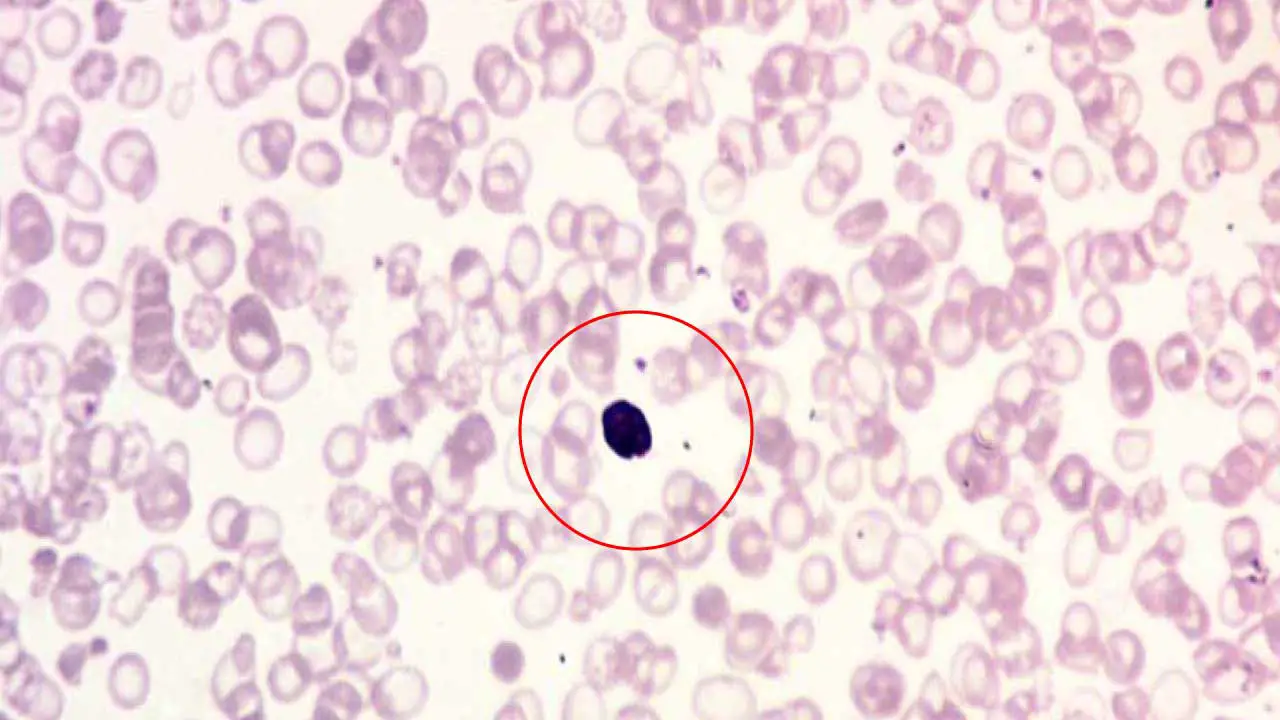
Appearance on Blood Smear
- Large Cells with Dark Centers: NRBCs are larger than mature red blood cells (RBCs). They have a well-defined, round nucleus (inclusion bodies) that stains dark purple with standard blood smear stains.
- Cytoplasm Color: The cytoplasm surrounding the nucleus (inclusion bodies) can vary depending on the stage of development:
- Basophilic Normoblasts: Early NRBCs have a more basophilic (bluish) cytoplasm due to the presence of ribosomes involved in protein synthesis for hemoglobin production.
- Polychromatophilic Normoblasts: As the NRBC matures, the cytoplasm takes on a more polychromatophilic (reddish-blue) hue due to the accumulation of hemoglobin.
- Orthochromatic Normoblasts: Late-stage NRBCs have cytoplasm that appears similar to a mature RBC, ranging from normochromic (normal pink) to slightly hypochromic (pale) depending on hemoglobin content.
Causes of Nucleated Red Blood Cells in Blood Smear
The presence of NRBCs in the blood smear suggests an increased demand for RBC production or a disruption in the normal release process from the bone marrow.
- Severe Anemia: Conditions like iron deficiency anemia, vitamin B12 or folate deficiency, thalassemia major or hemolytic anemia can significantly increase the body’s demand for RBCs. The bone marrow may release immature NRBCs into circulation in an attempt to meet this demand.
- Bone Marrow Infiltration: Diseases that infiltrate or replace bone marrow with abnormal cells, such as myelofibrosis, tumors, or granulomas, can disrupt normal RBC production and release, leading to the presence of NRBCs.
- Blood Loss: Acute or chronic blood loss can trigger the bone marrow to accelerate RBC production. In severe cases, NRBCs may be released prematurely.
Howell-Jolly Bodies
Howell-Jolly bodies are small, round inclusion bodies found within mature red blood cells (RBCs) on a peripheral blood smear. The presence of Howell-Jolly bodies indicates a potential issue with the spleen, the organ responsible for filtering out these cellular remnants.
Appearance of Howell-Jolly Bodies on Blood Smear
- Tiny, Round Inclusion Bodies: Howell-Jolly bodies are quite small, typically ranging from 0.5 to 1.0 μm in diameter. They appear as round, dark purple structured inclusion bodies within the cytoplasm of the RBC.
- Single or Multiple Inclusion Bodies: Usually, only one Howell-Jolly body is present per affected RBC, but occasionally, multiple Howell-Holly bodies might be seen in a single cell.
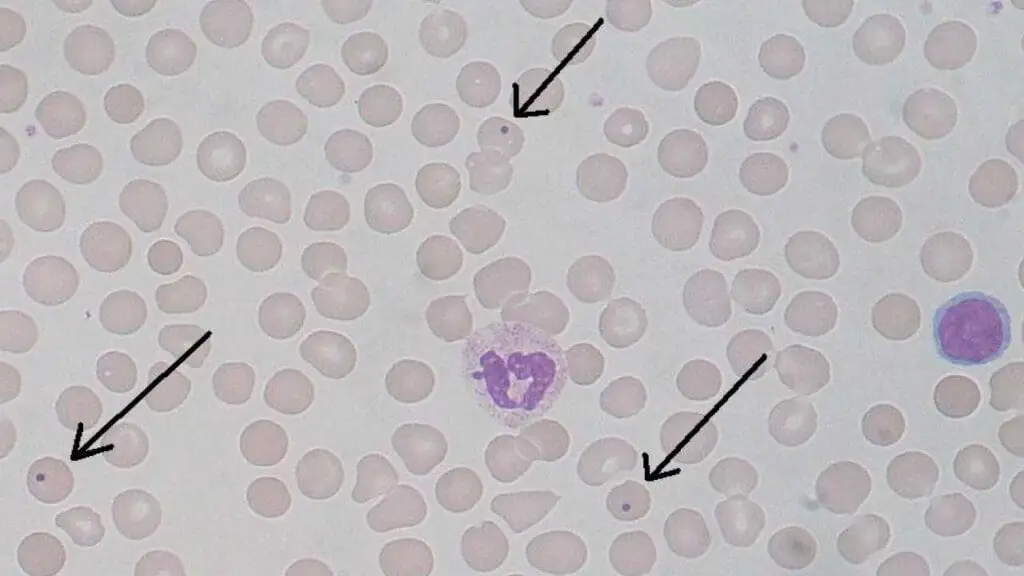
Causes of Howell-Jolly Bodies
The presence of Howell-Jolly bodies suggests a condition where the spleen is unable to efficiently remove these nuclear remnants from mature RBCs before they enter circulation.
- Asplenia: The complete absence of a spleen, either congenitally (present at birth) or due to surgical removal (splenectomy).
- Hypofunctional Spleen: Conditions that impair spleen function, such as sickle cell disease with functional asplenia or severe liver disease, can also lead to the presence of Howell-Jolly bodies.
Basophilic Stippling
Basophilic stippling refers to the presence of fine, bluish-purple granular inclusion bodies scattered throughout the cytoplasm of red blood cells (RBCs) on a peripheral blood smear. These granular inclusion bodies are aggregates of ribosomes, the protein-building factories within the cell. While a few stippled cells might be seen in healthy individuals, an increased number can be a sign of underlying issues.
Appearance of Basophilic Stippling on Blood Smear
- Size and Number of Inclusion Bodies: Basophilic stippling appears as multiple, small, and evenly distributed dark blue or blue-purple dots within the red blood cell. They are typically smaller in size compared to Howell-Jolly bodies.
- Location of Inclusion Bodies: Basophilic stippling granules are scattered throughout the cytoplasm, not concentrated in any specific area.
- Color of Inclusion Bodies: They have a distinct bluish-purple hue, differentiating them from other inclusions like Heinz bodies (reddish-brown).
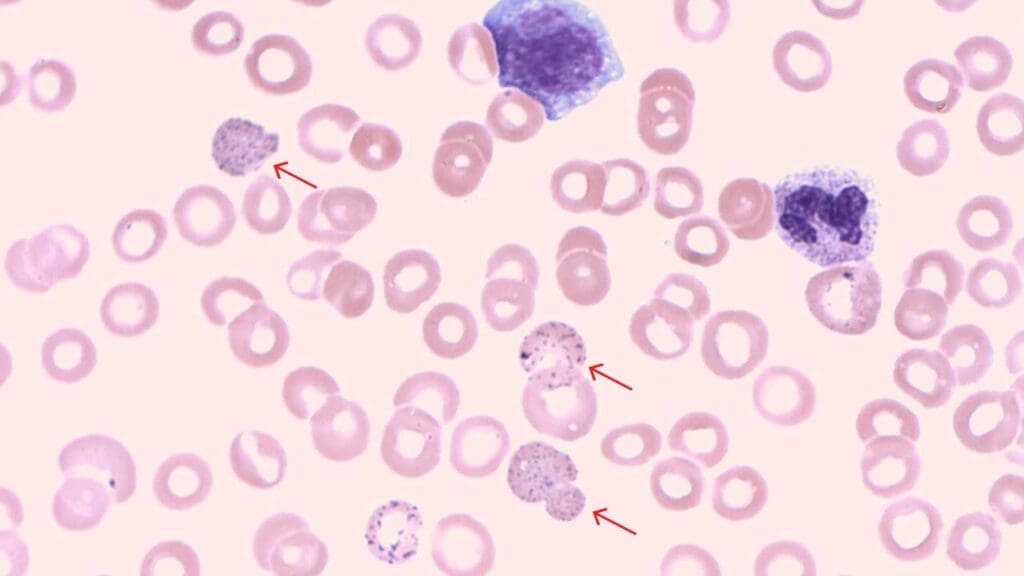
Causes of Basophilic Stippling
Several conditions can cause basophilic stippling, so a detailed clinical evaluation is crucial for diagnosis.
- Iron Deficiency Anemia: When iron is limited, ribosome production is disrupted, leading to the formation of basophilic stippling.
- Thalassemia: Genetic disorders affecting hemoglobin synthesis can also cause basophilic stippling due to abnormal protein production and accumulation of ribosomal aggregates.
- Megaloblastic Anemia (Vitamin B12 or Folate Deficiency): Deficiencies in these vitamins can impair DNA synthesis and RBC maturation, leading to basophilic stippling in some cases.
- Lead Poisoning: Lead disrupts various cellular processes, including ribosome function, and can cause basophilic stippling.
- Sideroblastic Anemias: A group of anemias where iron metabolism is disrupted. In some cases, basophilic stippling might be seen along with ring siderocytes (another type of inclusion bodies).
Siderotic Granules (Pappenheimer Bodies)
Siderotic granules, also known as Pappenheimer bodies, are a type of inclusion bodies found within red blood cells (RBCs) on a peripheral blood smear. These granules signify an abnormal accumulation of iron inside the RBC. While some iron is necessary for hemoglobin production, excessive levels can be detrimental.
Appearance of Pappenheimer Bodies on Blood Smear
- Size and Number: Siderotic granules are typically small, dense, and appear as blue-black or purple inclusion bodies on a Wright-stained blood smear. They are usually one or two per cell, located near the periphery (edge) of the RBC. They are typically smaller than a Howell-Jolly body. While they are visible on a standard smear, their composition of iron can be confirmed with a special stain, such as Prussian blue. Pappenheimer bodies are typically smaller than Howell-Jolly bodies. While they are visible on a standard smear, their composition of iron can be confirmed with a special stain, such as Prussian blue.
- Shape: These granules can be round or slightly irregular in shape.
- Color: These inclusion bodies have distinct blue-black or purple hue that differentiates them from other inclusion bodies like basophilic stippling (blue-purple dots) or Heinz bodies (reddish-brown).
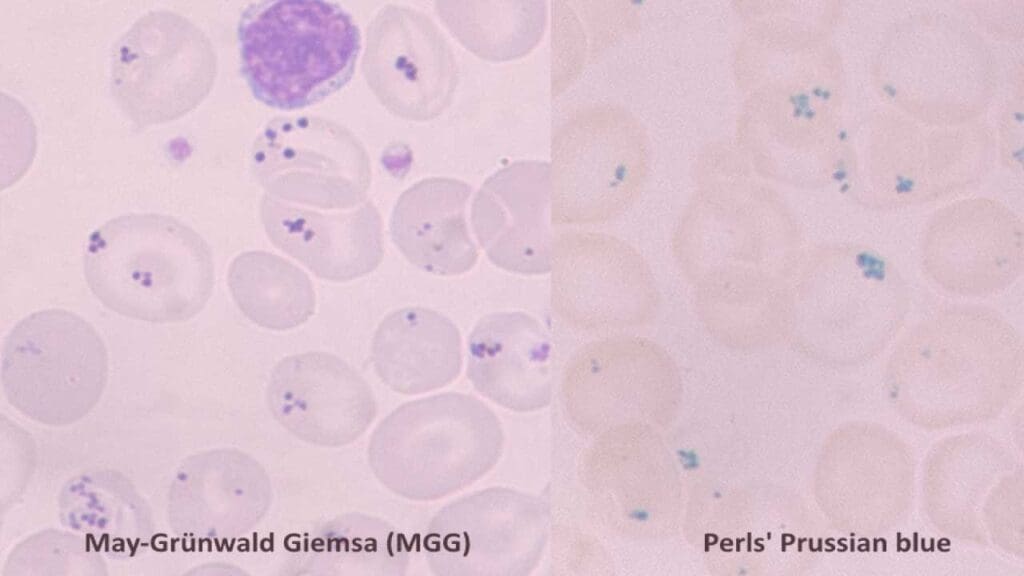
Causes of Siderotic Granules
The presence of Pappenheimer bodies suggests iron overload within the RBCs, but the underlying cause can vary.
- Iron Overload Conditions
- Hemosiderosis: Excessive iron storage in tissues due to increased absorption or repeated blood transfusions.
- Hereditary Hemochromatosis: A genetic disorder causing iron overload throughout the body.
- Ineffective Erythropoiesis: Bone marrow produces abnormal or immature RBCs that cannot effectively utilize iron for hemoglobin production. This can lead to iron accumulation within these cells. Causes include:
- Thalassemia: Genetic disorders affecting hemoglobin synthesis.
- Sideroblastic Anemias: A group of anemias where iron metabolism is disrupted within the developing RBCs, leading to ring siderocytes (another iron-containing inclusion bodies) and sometimes siderotic granules.
Heinz Bodies
Heinz bodies are unique inclusion bodies found within red blood cells (RBCs) on a peripheral blood smear. These telltale signs represent denatured clumps of hemoglobin, the oxygen-carrying protein within RBCs. While the presence of these inclusion bodies doesn’t directly cause symptoms, it indicates underlying cellular damage.
Appearance of Heinz Bodies on Blood Smear
- Size and Number: Heinz bodies are typically small, usually less than 1 micrometer in diameter. They can be single or multiple, appearing in varying numbers within affected RBCs.
- Location: These inclusion bodies are predominantly located near the periphery of the RBC, close to the cell membrane. This is because denatured hemoglobin tends to aggregate at the cell edge.
- Color: Heinz bodies have a distinct reddish-brown or pinkish-gray color on a Wright-Giemsa stained blood smear. This coloration helps differentiate them from other inclusions like basophilic stippling (bluish-purple dots) or siderotic granules (blue-purple or black).
- Detection: Regular blood smear staining techniques might not always reveal faint Heinz bodies. Special stains like supravital staining with methylene blue can enhance their visualization.
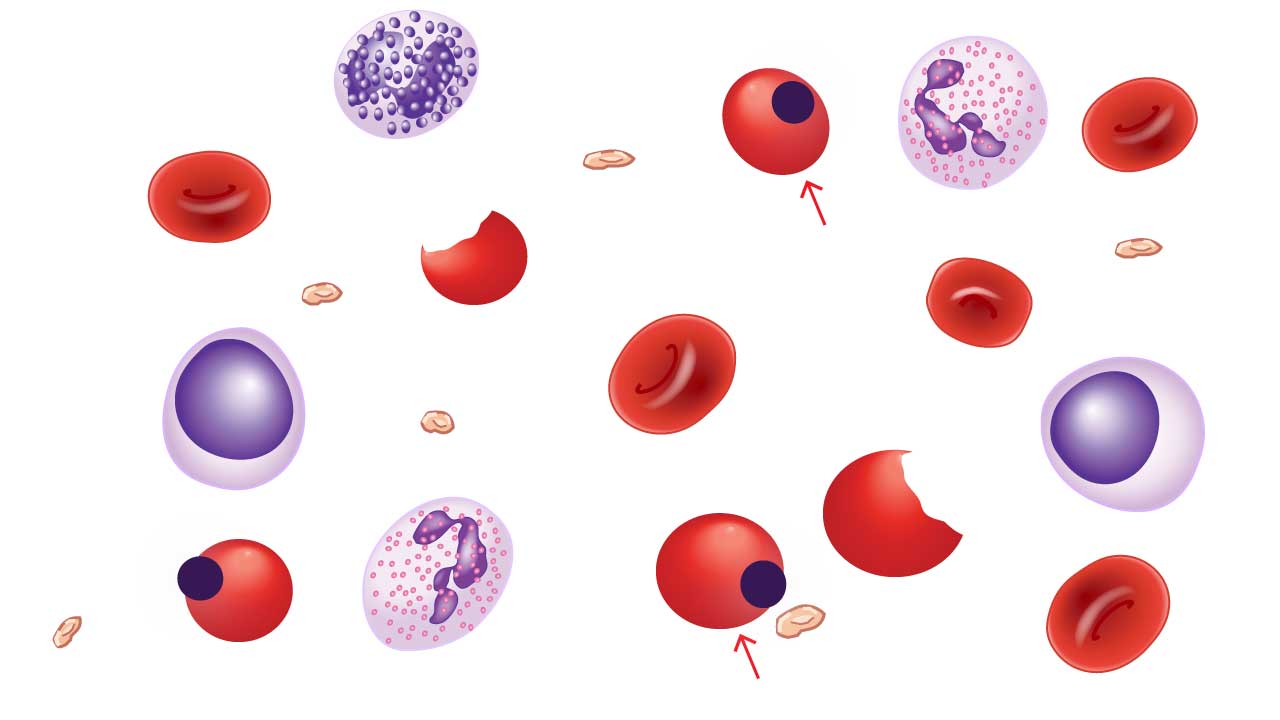
Causes of Heinz Bodies
Several factors can lead to the formation of Heinz bodies and subsequent damage to hemoglobin:
- Oxidative Stress: This occurs when there’s an imbalance between free radicals (damaging molecules) and antioxidants (protective molecules) within the cell. This imbalance can damage hemoglobin, leading to its denaturation and formation of Heinz bodies. Certain medications, environmental toxins, and conditions like G6PD deficiency can increase oxidative stress in RBCs.
- G6PD Deficiency: Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme crucial for maintaining the antioxidant defense system within RBCs. A deficiency in this enzyme can make RBCs more susceptible to oxidative damage from certain medications or fava beans, leading to Heinz body formation.
- Certain Medications and Chemicals: Some medications (e.g., antimalarials, certain antibiotics) and chemicals (e.g., lead, naphthalene) can directly damage hemoglobin or create conditions that promote oxidative stress, ultimately causing Heinz body formation.
Differences between Howell-Jolly Bodies and Heinz Bodies
| Feature | Howell-Jolly Bodies | Heinz Bodies |
| Composition | Remnants of nuclear DNA | Aggregates of denatured hemoglobin |
| Cause | Incomplete expulsion of the nucleus during red blood cell maturation, often due to a non-functional or absent spleen. | Oxidative damage to hemoglobin, often due to drugs, toxins, or genetic conditions like G6PD deficiency. |
| Shape | Small, dense, and spherical | Irregularly shaped, can be single or multiple. Often appear attached to the inner red blood cell membrane. |
| Visibility | Visible with a standard Romanowsky stain (e.g., Wright-Giemsa). They appear as a single, dark purple dot. | Not visible with a standard Romanowsky stain. They require a special supravital stain (e.g., brilliant cresyl blue or crystal violet) to be seen. |
| Location | Typically appears as a single inclusion, located in the red blood cell’s cytoplasm. | Can be single or multiple, often seen as an inclusion pushing against the inner red blood cell membrane. |
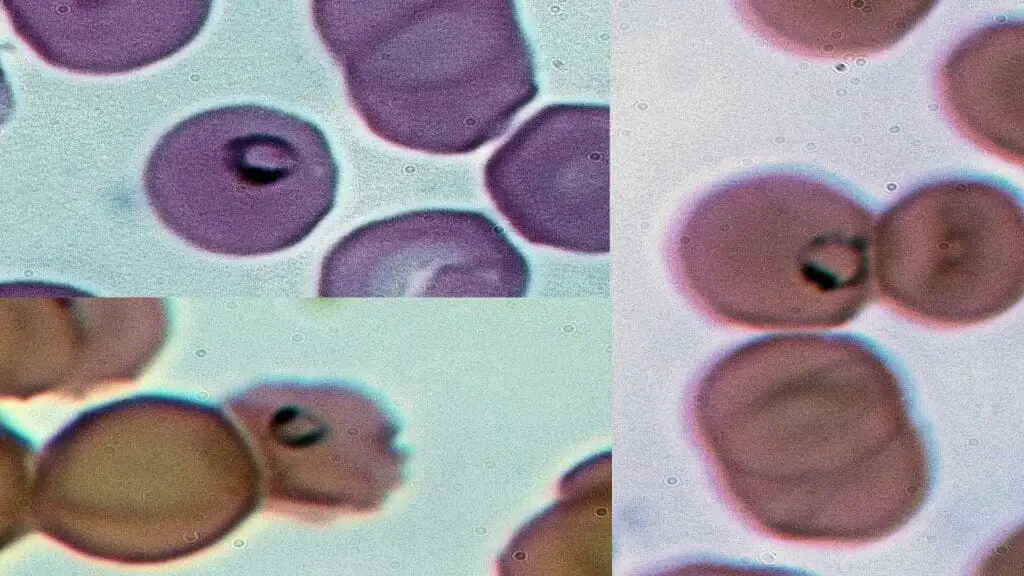
H Inclusions in Hb H Disease
In Hb H disease, H inclusions refer to a specific type of inclusion bodies formed due to the presence of excess beta globin chains.
Appearance of H Inclusions on Blood Smear
- Composition: Hb H inclusions in Hb H disease are composed of aggregates of beta globin tetramers (Hb H). When there’s a deficiency in alpha-globin production, these excess beta globin chains precipitate and clump together within the red blood cell (RBC).
- Visualization: When stained with a supravital stain (like brilliant cresyl blue), HbH inclusions appear as numerous small, fine, dark blue dots scattered throughout the entire red blood cell, giving it a characteristic “golf ball” or “raspberry” appearance.
Causes of H Inclusions in Hb H Disease
Hb H disease itself is the cause of these inclusion bodies. It arises due to a genetic condition where there’s a deficiency in alpha-globin production. This imbalance leads to the formation of excess beta globin tetramers (Hb H) that precipitate and form inclusion bodies within RBCs.
Differences between H Inclusions and Heinz Bodies
| Feature | H Inclusions | Heinz Bodies |
| Composition | Unstable tetramers of beta-globin chains (β4). | Irreversibly denatured hemoglobin due to oxidative stress. |
| Associated Condition | Hemoglobin H disease (a type of alpha-thalassemia). | G6PD deficiency, exposure to oxidizing drugs/toxins, or unstable hemoglobins. |
| Appearance | Numerous, small dots giving a “golf ball” appearance. | Small, rounded inclusions often attached to the cell membrane. |
Parasites
Parasites can invade red blood cells (RBCs), causing various diseases. When examining a blood smear for parasites, medical professionals look for specific features to identify the culprit.
Appearance of Parasites on Blood Smear
- Varies by Parasite: The appearance of parasites within RBCs depends on the specific type of parasite causing the infection. Some common features to look for include:
- Ring Forms: Early-stage malarial parasites (Plasmodium species) appear as signet rings within the RBC. They have a distinctive blue staining cytoplasm and a centrally located red chromatin dot.
- Bands/Trophozoites: As malarial parasites mature, they develop into larger, band-shaped forms or amoeboid trophozoites. These might fill a significant portion of the RBC and contain multiple chromatin dots.
- Schizonts: Mature malarial parasites undergo multiple divisions, forming a rosette-like structure called a schizont within the RBC. This structure contains numerous daughter merozoites, the next generation of infective parasites.
- Babesia: These pear-shaped or round parasites can be single or multiple within an RBC. They have a characteristic pale cytoplasm and centrally located red nucleus.
- Microfilariae: These slender, thread-like larvae of certain filarial worms can sometimes be seen traversing across an RBC on a thick blood smear.
- Location: Parasites can be located anywhere within the RBC cytoplasm, but some, like malarial ring forms, might have a more central location.
- Color: Depending on the parasite and staining technique, the parasite itself might appear blue, red, or purple, while the internal structures like chromatin dots have a distinct red coloration.
Causes of Parasites in Red Blood Cells
Several parasitic infections can cause the presence of parasites within RBCs:
- Malaria: Caused by Plasmodium species transmitted by mosquito bites. Different Plasmodium species have characteristic appearances on a blood smear.
- Babesiosis: A tick-borne illness caused by Babesia parasites.
- Cytauxzoonosis: A tick-borne disease primarily affecting animals, but rare human cases have been reported.
- Filariasis: Caused by various filarial worms, where microfilariae, the larval stage, can sometimes be seen in the blood.
Ring Sideroblasts
Ring sideroblasts are abnormal red blood cell inclusion bodies seen under a microscope during a bone marrow examination. They aren’t typically visible on a peripheral blood smear, which examines blood cells from circulation.
Appearance of Ring Sideroblasts on Bone Marrow Aspirate
- Iron Overload: Ring sideroblasts are characterized by a ring-shaped collection of iron granules inclusion bodies surrounding the nucleus of the red blood cell. This excess iron is what gives them their distinctive appearance.
- Size and Staining: The red blood cell itself might appear slightly smaller than normal and may stain more basophilic (blue) due to immature characteristics.
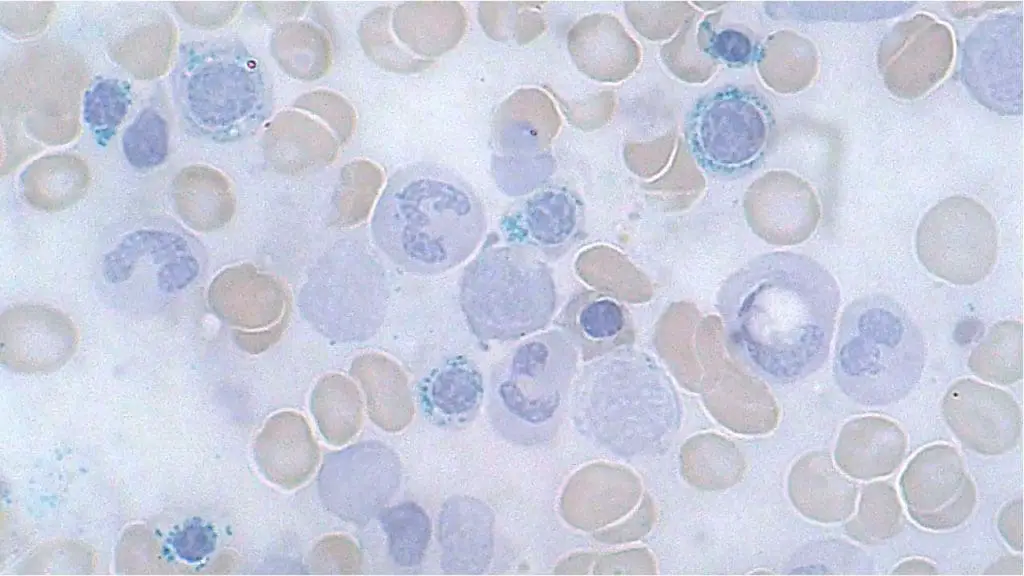
Causes of Ring Sideroblasts
- Defective Iron Utilization: In these cases, the body has sufficient iron stores, but there’s a problem incorporating it into hemoglobin, the oxygen-carrying molecule within red blood cells. This can be due to:
- Genetic Mutations: Mutations in genes involved in iron metabolism or heme synthesis can disrupt the normal process of iron incorporation, leading to ring sideroblasts.
- Vitamin Deficiencies: Deficiencies in vitamin B6 (pyridoxine) or vitamin B12 can also impair iron utilization and contribute to ring sideroblasts.
- Iron Overload: In some cases, the body might have an excessive amount of iron from conditions like hemochromatosis or repeated blood transfusions. While the iron is present, the cells still struggle to effectively incorporate it into hemoglobin, leading to ring sideroblasts.
Diagnostic Importance of Ring Sideroblasts
The presence of ring sideroblasts on a bone marrow biopsy can be a valuable diagnostic clue for various conditions, including:
- Sideroblastic Anemias: This is a group of anemias characterized by the presence of ring sideroblasts. The specific type of sideroblastic anemia can be determined based on additional factors like the cause of iron utilization deficiency.
- Myelodysplastic Syndromes (MDS): These are a group of bone marrow disorders where abnormal blood cells are produced. Ring sideroblasts can be seen in some types of MDS.
Difference between Ring Sideroblasts and Basophilic Stippling
| Feature | Ring Sideroblasts | Basophilic Stippling |
| Composition | Aggregates of iron stored within the mitochondria of immature red blood cells (erythroblasts). | Aggregates of ribosomal RNA that have failed to be degraded. |
| Location | Found only in the bone marrow of nucleated red blood cells, where the iron-laden mitochondria form a ring around the nucleus. | Found in mature red blood cells (erythrocytes) on a peripheral blood smear. |
| Appearance | Visible as a ring of blue-staining granules encircling the nucleus after staining the bone marrow with a special Prussian blue stain. | Visible as numerous fine to coarse blue dots scattered throughout the cytoplasm of the red blood cell with a standard Romanowsky stain (e.g., Wright-Giemsa). |
| Cause | Ineffective iron utilization, often due to impaired heme synthesis. Associated with sideroblastic anemia, lead poisoning, myelodysplastic syndromes, and chronic alcoholism. | Impaired red blood cell maturation and RNA degradation. Associated with lead poisoning, thalassemia, and megaloblastic anemia. |
Cabot Rings
Cabot rings are a rare type of inclusion bodies found within the cytoplasm of red blood cells. They are thought to be remnants of the mitotic spindle from an abnormal cell division process, or possibly remnants of the nuclear membrane. They were first described in 1903 by the American physician Richard Clarke Cabot.
Appearance of Cabot Rings on Blood Smear
Under a microscope, Cabot rings inclusion bodies are characterized by their distinct shape. They appear as thin, threadlike, reddish-violet inclusion bodies that form a ring, loop, or figure-of-eight within the red blood cell. Unlike other inclusions, they are not aggregates of granular material but rather delicate, strand-like structures. These inclusion bodies can be visualized with a standard Wright or Giemsa stain.
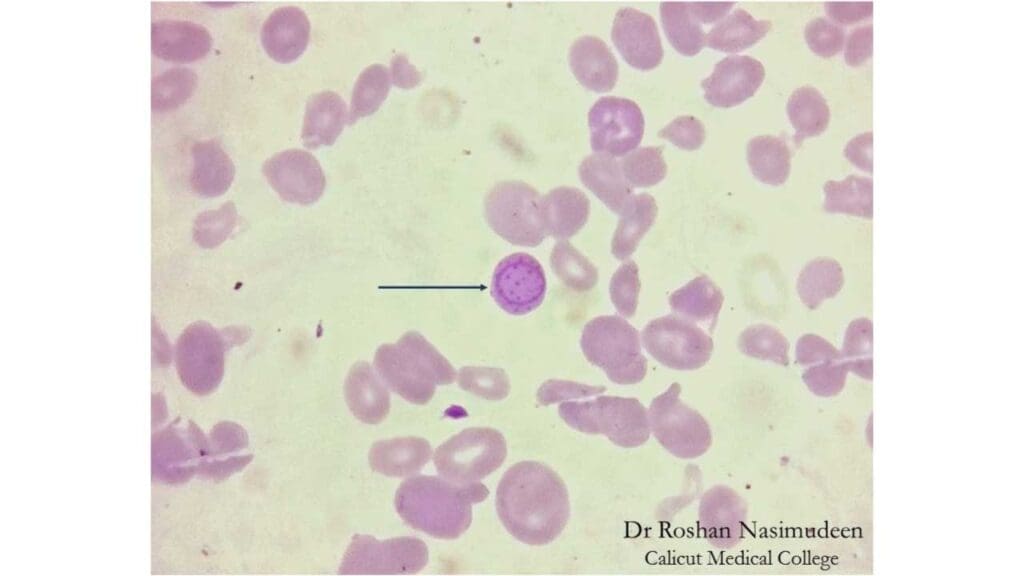
Causes of Cabot Rings
Cabot rings are a sign of abnormal red blood cell production (dyserythropoiesis) and are rarely seen in a normal peripheral blood smear. Their presence suggests an underlying issue, and they are most commonly associated with:
- Megaloblastic anemia: This includes pernicious anemia and vitamin B12 or folate deficiency.
- Lead poisoning
- Myelodysplastic syndromes
- Other severe anemias
Conclusion
Inclusion bodies within red blood cells (RBCs) serve as valuable diagnostic markers in hematology. These unique structures, visible on a peripheral blood smear, offer insights into the underlying cellular abnormalities affecting RBCs.
The type, size, number, and staining characteristics of inclusion bodies can point towards specific disorders. For instance, Howell-Jolly bodies suggest megaloblastic anemia, while basophilic stippling might indicate iron deficiency or thalassemia.
Inclusion bodies can sometimes appear before symptoms arise, allowing for earlier detection and intervention. The information gleaned from inclusions helps direct further investigations. Identifying Heinz bodies might prompt testing for G6PD deficiency to identify a potential cause of oxidative stress.
Importance of Context
However, interpreting inclusion bodies in isolation is insufficient. A comprehensive approach is crucial:
- Clinical Presentation: Symptoms like fatigue, weakness, and jaundice can provide valuable clues.
- Other Laboratory Findings: A complete blood count (CBC) and other tests can offer a more complete picture.
- Medical History: A history of chronic conditions, medications, or exposures (e.g., lead) can be informative.
By considering all these factors alongside the characteristics of inclusion bodies, medical professionals can unlock a deeper understanding of the underlying RBC dysfunction.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Yonder S, Pandey J. Erythrocyte Inclusions. [Updated 2023 Aug 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK594259/
- Tyrrell, L., Rose, G., Shukri, A., & Kahwash, S. B. (2021). Morphologic changes in red blood cells: An illustrated review of clinically important light microscopic findings. The Malaysian journal of pathology, 43(2), 219–239.
- Ford J. (2013). Red blood cell morphology. International journal of laboratory hematology, 35(3), 351–357. https://doi.org/10.1111/ijlh.12082
- Constantino B. T. (2015). Reporting and grading of abnormal red blood cell morphology. International journal of laboratory hematology, 37(1), 1–7. https://doi.org/10.1111/ijlh.12215